Palmitic Acid Versus Stearic Acid: Effects of Interesterification and Intakes on Cardiometabolic Risk Markers—A Systematic Review
Abstract
1. Introduction
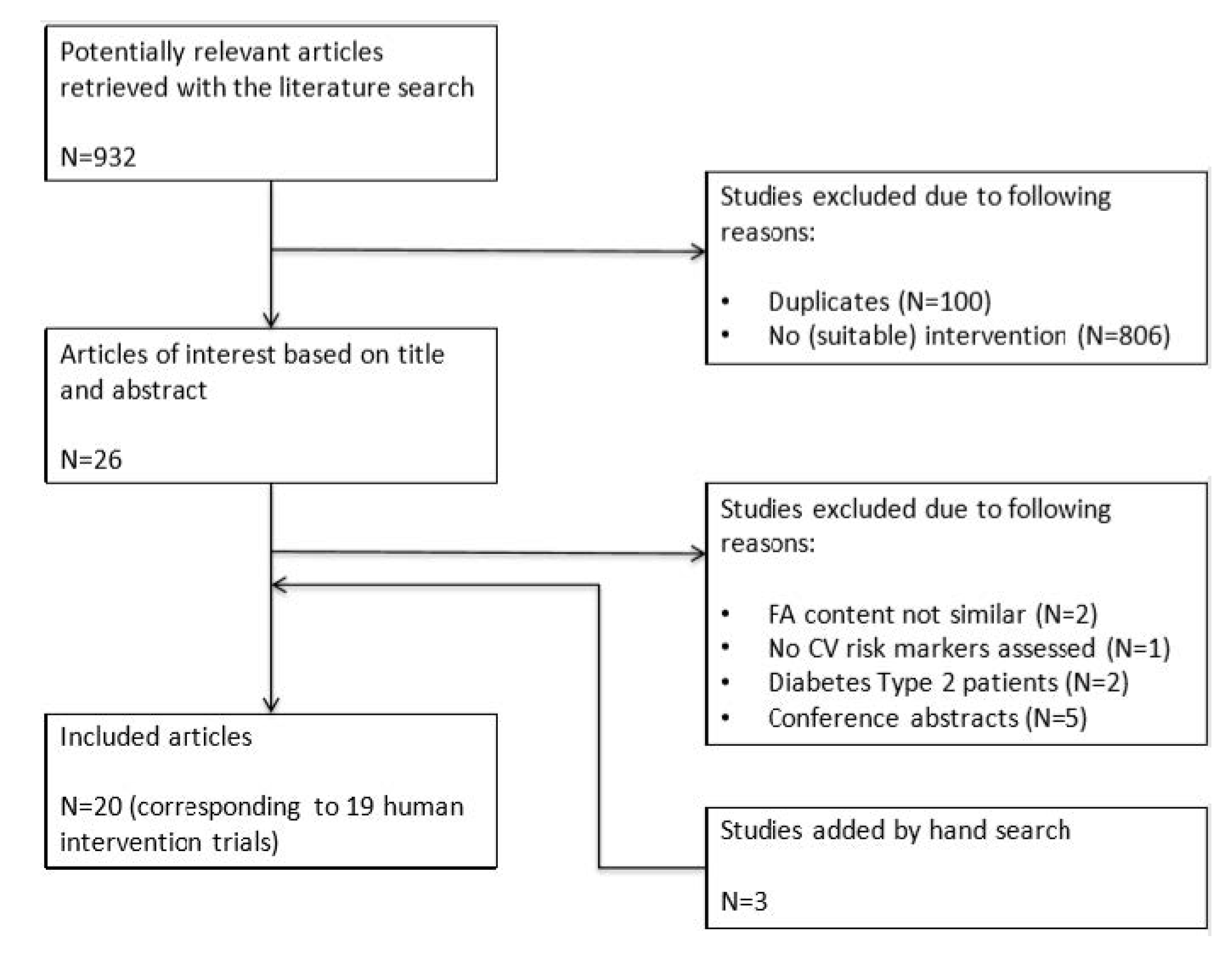
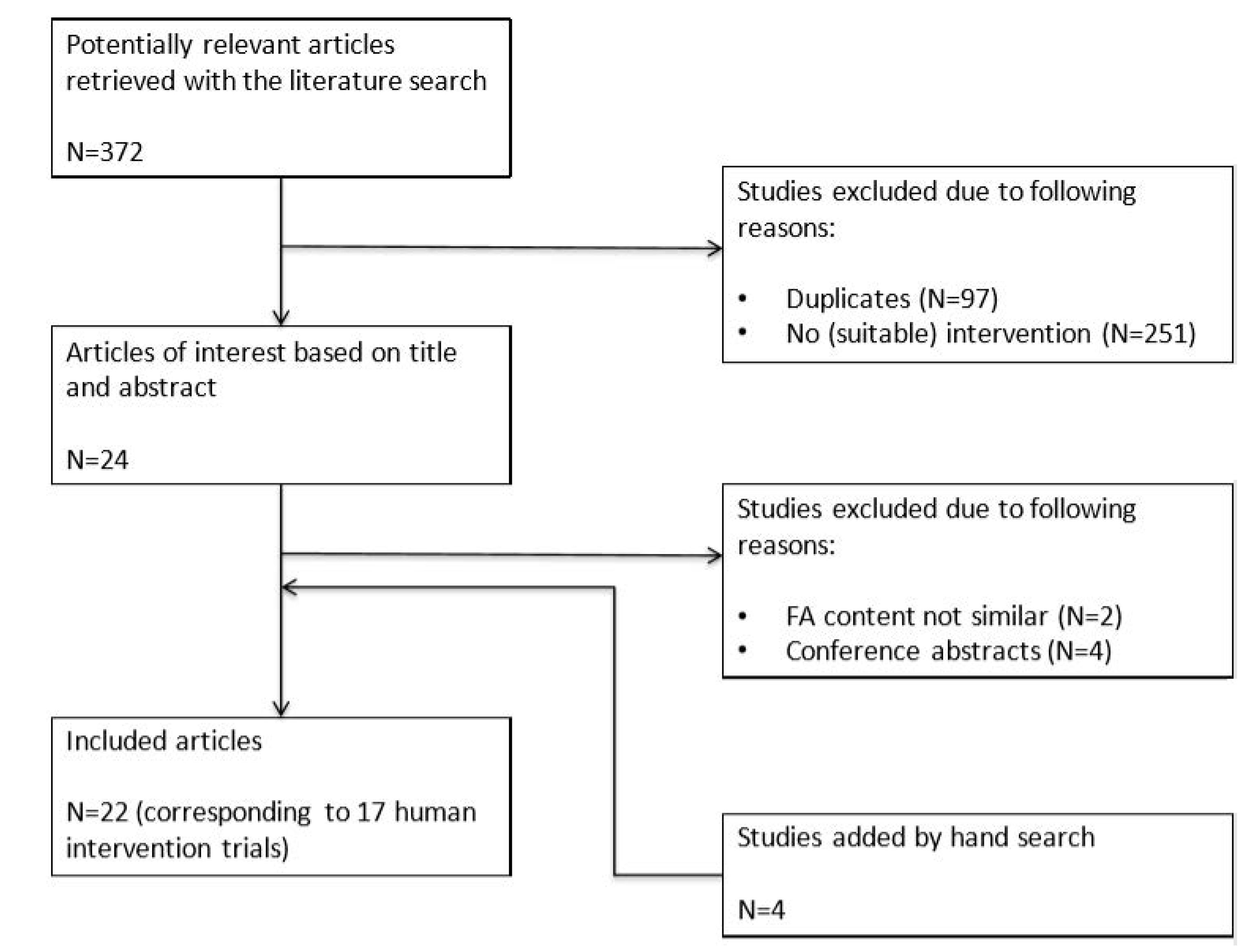
2. Methods
3. Results
3.1. Longer-Term Effects of sn-2 Content of Palmitic Acid or Stearic Acid on Fasting Cardiometabolic Risk Markers
3.1.1. Lipids and (apo) Lipoproteins
3.1.2. Hematological Markers
3.1.3. Other Markers
3.2. Longer-Term Effects of Substituting Palmitic Acid with Stearic Acid on Fasting Cardiometabolic Risk Markers
3.2.1. Lipids and (apo) Lipoproteins
3.2.2. Hematological Markers
3.2.3. Other Markers
3.3. Postprandial Effects of sn-2 Content of Palmitic Acid or Stearic Acid on Cardiometabolic Risk Markers
3.3.1. Lipids and (apo) Lipoproteins
3.3.2. Hematological Markers
3.3.3. Other Markers
3.4. Postprandial Effects of Substituting Palmitic Acid with Stearic Acid on Cardiometabolic Risk Markers
3.4.1. Lipids and (apo) Lipoproteins
3.4.2. Hematological Markers
3.4.3. Other Markers
4. Discussion
4.1. Longer-Term Effects
4.2. Postprandial Effects
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| First author, Year of publication | Study population, Age, BMI | Duration intervention periods, Study design | Total fat (en%) | C16:0 (en%) | Source Low sn-2 High sn-2 | C16:0 sn-2 in fat blends (% 1) | Solid fat at 37 °C (%) | Lipids and lipoproteins | Hematological markers | Other markers |
|---|---|---|---|---|---|---|---|---|---|---|
| Nestel, 1995 [10] | 27 men (mildly hyperchol 2) 49 ± 8 y 26.3 ± 2.5 kg/m2 | 21 days Crossover (no WO) | 31 | 6.7 | Palm oil IE palm oil | 8.7 24.7 wt% | NR | TAG = TC = LDL-C = HDL-C = | ||
| Zock, 1995 [9] | 23 men 37 women 3 29 (19–67) y 22.9 (18.1–30.9) kg/m2 | 21 days Crossover (no WO) | 40 | 11 | Control and IE blend of palm oil blended with sunflower oil | 6.4 66.9 wt% | 0 0 | TAG = TC = 4 LDL-C = 4 HDL-C = | ||
| Meijer, 1997 [13] | 30 men 30 women ± 35.5 y ± 23.8 kg/m2 | 21 days Crossover 5 (no WO) | 34 | 1 or 25 | Control and IE blend that consisted mainly of coconut and palm oils blended with soybean oil | 7.1 18.0 wt% | NR | TAG = NEFA = TC = LDL-C = HDL-C = Lp[a] = | FVIIa = Fibrinogen = PAI-1 antigen = tPA antigen = tPA activity = vWF = | Glucose = CRP = |
| Christophe, 2000 [8] | 32 men 23–53 y 18.1–23.5 kg/m2 | 28 days Parallel | NR ± 131 g | NR ± 5 g | IE butter Butter | NR | NR | TAG = TC = LDL-C= HDL-C= ApoB = ApoA1 = | ||
| Filippou, 2014 [11] | 10 men 31 women ± 29.1 y ± 23.0 kg/m2 | 42 days Crossover (no WO) | 27 | 9 | Palm olein IE palm olein | 9.8 45.9 mol% | 0 5.9 | TAG = TC = LDL-C = HDL-C = ApoB = ApoA1 = Lp[a] = | Glucose = Insulin = C-peptide = | |
| Ng, 2018 [12] | 64 women 21 men 20–60 y 21–30 kg/m2 | 56 days Parallel | 35 | 7 | Palm olein CIE palm olein | 11.1 32.4 wt% | NR | TAG = TC = LDL-C = HDL-C = ApoB = ApoA1 = Lp[a] = | Glucose = Insulin = C-peptide = |
| First author, Year of publication | Study population, Age, BMI | Duration intervention periods, Study design | Total fat (en%) | C18:0 (en%) | Source Low sn-2 High sn-2 | C18:0 sn-2 in fat blends (% 1) | Solid fat at 37 °C (%) | Lipids and lipoproteins | Hematological markers | Other markers |
|---|---|---|---|---|---|---|---|---|---|---|
| Grande, 1970 [15] | 32 men 40–65 y NR | 18 days Latin-square | 34 | 10 | Native or IE cocoa butter 2 blended with safflower oil | NR | NR 3 | TAG = TC = | ||
| Berry, 2007 [14] | 16 men 26.8 ± 8.0 y 23.7 ± 3.7 kg/m2 | 21 days Crossover | 30 g test fat 4 | 74 | Native or IE shea butter blended with sunflower oil | 3.1 22.8 mol% | 22 41 | TAG = TC = LDL-C = HDL-C = | FVIIa = | Glucose = Insulin = |
| First author, Year of publication | Study population, Age, BMI | Duration intervention period, Study design | Total fat (en%) | C16:0 C18:0 (en%) | Difference between diets C16:0 C18:0 (en%) | Main source C16:0 C18:0 | Lipids and lipoproteins | Hematological markers | Other markers |
|---|---|---|---|---|---|---|---|---|---|
| Grande, 1970 [15] | 32 men 40–65 y NR | 18 days Latin-square | 34 | 15 10 | 6 8 | Palm oil Cocoa butter | TAG = TC ↓ | ||
| Bonanome, 1988 [16] | 11 men 64 ± 4.0 y 24 ± 1.7 kg/m2 | 21 days Cross-over (no WO) | 40 | 18 17 | 15 | Palm oil Hydrogenated soybean oil | TAG = TC ↓ VLDL-C = LDL-C ↓ HDL-C = | ||
| Tholstrup, 1994 [17] + 1995 [25] | 15 men 24.9 (22–30) y 23.2 (20.4–26.4) kg/m2 | 21 days Cross-over | 40 | 16 1 14 | 14 | Palm oil Shea butter | TAG = TC ↓ VLDL-C = LDL-C ↓ HDL-C ↓ ApoB ↓ ApoA1 ↓ Lp[a] ↑ | FVIIc ↓ PAI-1 activity = PAI-1 antigen = tPA activity = tPA antigen = EFA = | |
| Dougherty, 1995 [18] | 10 men 37.4 ± 6.6 y 25.2 ± 2.5 kg/m2 | 40 days Cross-over (no WO) | 27-29 | 7 | 5 6 | Palm oil Shea butter | TAG = TC ↓ LDL-C ↓ HDL-C = | ||
| Schwab, 1996 [19] + 1997 [26] | 12 women 2 (premenopausal) 23.5 ± 3.1 y 22.1 ± 2.4 kg/m2 | 28 days Cross-over | 37 | 12 7 | 3 5 | Palm oil, butter Cocoa butter | TAG = NEFA =2 TC ↓ VLDL-C = LDL-C = HDL-C ↓ ApoB = ApoA1 ↓ | CETP activity ↓ Glucose = 2 Insulin = 2 | |
| Nestel, 1998 [21] | 15 subjects (mildly hyperchol men and women 3) 51 ± 7 y 26.2 ± 3.9 kg/m2 | 35 days Cross-over (no WO) | 41-42 | 8 4 | ±5 | Palm olein Fully hydrogenated soybean oil | TAG = TC = LDL-C = HDL-C = | ||
| Snook, 1999 [23] | 16 women (premenopausal) 28 ± 6 y NR | 35 days 3x3 cross-over | 40 | 13 | 10 11 | Tripalmitin Tristearin | TAG = TC ↓ LDL-C ↓ HDL-C = ApoB = ApoA1 = | CETP activity = LCAT activity = | |
| Kelly, 2001 [22] | 13 men 35 ± 12 y 26 ± 3.3 kg/m2 | 28 days Cross-over | 27–28 | 8 7 | 6 5 | Palm stearin and/or palm olein Hydrogenated canola | TAG = TC = LDL-C = HDL-C = | FVIIc = MPV ↓ Fibrinogen = Plasminogen = WBC = RBC = Hb = PLT = APTT = ATIII = | |
| Kelly, 2002 [24] | 9 men 39 ± 10 y 25 ± 2.5 kg/m2 | 21 days Cross-over | 28–29 | 7 4 | 1 2 | Potato crisps, shortbread biscuits, muesli bars Milk chocolate | TAG = TC = LDL-C = HDL-C = | MPV = WBC = RBC = Hb = PLT = | |
| Ng, 2018 [12] | 64 women 21 men 20–60y 21–30 kg/m2 | 56 days Parallel | 35 | 7 8 | 5 7 | IE Palm olein IE hydrogenated soybean oil | TAG ↓ TC = LDL-C = HDL-C = ApoB = ApoA1 = Lp[a] = | Glucose = Insulin = C-peptide = | |
| Meng, 2019 [20] | 20 postmenopausal women (mildly hyperchol 5) 64 ± 7 y 26.4 ± 3.4 kg/m2 | 35 days Cross-over | 30 | 14 10 | 8 9 | Palm oil Cocoa butter | TAG = TC ↓ VLDL-C = LDL-C ↓ HDL-C ↓ ApoB = ApoA1 = Lp[a] = | PT = PTT = | Glucose = Insulin = CRP = TNF-a = IL-6 = SAA-1 = sICAM-1 = sICAM-3 = sVCAM-1 = E-selectin = P-selectin = Thrombomodulin = |
| First author, Year of publication | Population, Age, BMI, Follow-up | Total energy (kcal) | Total fat in grams (en%) | C16:0 content in grams (en%) | Source Low sn-2High sn-2 | C16:0 sn-2 in fat blends (% 1) | Solid fat at 37 °C (%) | Lipids and lipoproteins | Hematological markers | Other markers | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zampelas, 1994 [35] | 16 men 24.8±2.6 y 22.7±2.4 kg/m2 6 h | 662 | 40 (54 en%) | 12 (16 en%) | Palm olein IE blend of palm stearine with sunflower oil | 5.9 72.7 wt% | NR | TAG = NEFA = | Glucose = Insulin = GIP = | ||
| Summers, 1998 [36] | 2 men 6 women (pre- and postmenopausal) 30.5 (18–55) y 24 (19–30) kg/m2 6 h | 932 | 60 (58 en%) | 18 (17 en%) | NR | 5.9 67.8 mol% | NR | TAG = NEFA = | Glucose = Insulin = | ||
| Yli-Jokipii, 2001 [32] | 10 women (premenopausal) 26.9 ± 2.56 y 18.5–25 kg/m2 6 h | NR | 55 g/m2 body surface area | 17 g/m2 body surface area | Palm oil IE palm oil | 9 31 mol% | 0 0 | TAG ↓ NEFA = VLDL-C = CM-C = | Glucose = Insulin = | ||
| Yli-Jokipii, 2003 [27] | 2 men 7 women (premenopausal) 24 ± 3 y 21.5 ± 2.5 kg/m2 8 h | NR | 55 g/m2 body surface area | 17 g/m2 body surface area | IE Lard Lard | 52 69 mol% | 11.0 2 12.5 | TAG = 3 NEFA = TC = | Glucose = Insulin = | ||
| Berry, 2007 [33] | 20 men 28.8 ± 10.3 y 23.2 ± 2.6 kg/m2 6 h | 853 | 50 (53 en%) | 14 (15 en%) | Palm oil IE palm oil | 7.2 37.2 mol% | 3.6 15.2 | TAG = TC = LDL-C = HDL-C = | FVIIa = WBC = | Glucose = Insulin = | |
| Sanders, 2011 [7] Filippou, 2014 [37] | 25 men 25 women (premenopausal) ± 24.8 y ± 23.5 kg/m2 8 h | 846 | 50 (53 en%) | 20 (22 en%) | Palm olein IE palm olein | 9.2 39.1 mol% | 0 4.7 | TAG = NEFA = TC = ApoB48 = | Glucose = Insulin = C-peptide = GIP ↓ PYY= | IL-6 = IL-8 = TNF-α = E-selectin = | |
| Hall, 2014 [34] | 11 men 50 ± 7 y 27.6 ± 3.1 kg/m2 6 h | 1047 | 75 (64 en%) | 30 (26 en%) | Palm olein IE palm olein | 9.8 45.9 mol% | NR | TAG = 4 NEFA = TC = | |||
| Hall, 2017 [28] | 12 men 20.5 ± 1.1 y 22.4 ± 2.8 kg/m2 4 h | 832 | 52 56 en% | 26 28 en% | PSt/PK IE PSt/PK | 36.0 54.7 mol% | 24 5 21 | TAG ↑ | Glucose = Insulin = GIP = PYY = |
| First author, Year of publication | Population, Age, BMI, Follow-up | Total energy (kcal) | Total fat in grams (en%) | C18:0 content in grams (en%) | Source Low sn-2 High sn-2 | C18:0 sn-2 in fat blends (% 1) | Solid at 37 °C (%) | Lipids and lipoproteins | Hematological markers | Other markers | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Summers, 1999 [29] | 14 women 49 (29–70) y 27.5 (20.6–52.8) kg/m2 6 h | 932 | 60 (58 en%) | 18 (18 en%) | NR | NR 83.3 | NR | TAG = NEFA = | Glucose = Insulin = | ||
| Sanders, 2003 [30] | 17 men 38.2 ± 11.1 y 24.5 ± 2.9 kg/m2 6 h | 749 | 50 (60 en%) | 17 (20 en%) | Cocoa butter IE cocoa butter | NR | NR 2 | TAG ↓ | TC = LDL-C = | FVIIa ↓ | |
| Berry, 2007 [14] | 16 men 26.8 ± 8.0 y 23.7 ± 3.7 kg/m2 8 h | 853 | 50 (53 en%) | 26 (28 en%) | Native or IE shea butter blended with HOSO | 3.1 22.8 mol% | 22.2 41.2 | TAG = NEFA = | TC = LDL-C = HDL-C = | FVIIa = WBC = | Glucose = Insulin = |
| Robinson, 2009 [31] | 10 non-obese men (55.8 ± 7.0y, 26.6 ± 2.5 kg/m2) 11 obese men (59.3 ± 6.0y, 32.9 ± 4.3 kg/m2), 6 h | NR | 86-102 (76 en%) (1 g/kg body mass) | 25-30 (21 en%) | Canola stearin (EIE, CIE, native) blended with HOSO | 0.5 0.6 25.5 wt% | 5.4 5.6 18.6 | Non-obese: TAG = Obese: TAG ↓ 3 | Both: NEFA = TC = LDL-C = HDL-C | Both: Glucose = Insulin = | |
| First author, year of publication | Population, Age, BMI, Postprandial follow-up | Total energy (kcal) | Total fat in grams (en%) | Content C16:0 C18:0 (g) | Content C16:0 C18:0 (en%) | Source C16:0 C18:0 | Lipids and lipoproteins | Hematological markers | Other markers | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mennen, 1998 [42] | 91 women (postmenopausal) 75.7 ± 5.2 y 27.7 ± 4.1 kg/m2 6–7 h | 948– 889 | 55.7–49.3 (53–50 en%) | 22 19 | 21 19 | NR | TAG = | FVIIa = | ||
| Jensen, 1999 [38] | 15 women (premenopausal) 8 normal-weight (27 ± 2 y, 19.2–23.7 kg/m2) 7 overweight (29 ± 3 y, 28.8–47.5 kg/m2) 8 h | 406kcal/m2 body surface area | 29 g/m2 (65 en%) | 12 g/m2 5 g/m2 | 27 10 | Palm oil Lard | Both: TAG = | Both: Insulin = Leptin = | ||
| Sanders, 2000 [39] | 11 men5 women (premenopausal) 25.5 (18–32) y 23.2 (20.1–27.8) kg/m2 7 h | 1242 | 90 (65 en%) | 37 36 | 27 26 | Palm oil Hydrogenated and IE HOSO | TAG = | FVIIa = FVIIc = | ||
| Tholstrup, 2001 [41] + 2003 [44] + 2004 [43] | 16 men 23.4 ± 2.4 y 23 ± 2 kg/m2 8 h | 1672 1 | 75 1 (50.6 en%2) | 32 1 34 1 | 17 18 | IE blend of tripalmitin or tristearin with HOSO | TAG = NEFA = VLDL-C = LDL-C = HDL-C = ApoB = ApoA1 = Lp[a] = | FVIIa = FVIIc = PAI-1 antigen = tPA activity = | CETP activity = LPL activity = | |
| Teng, 2011 [40] | 10 men 21.9 ± 0.7 y 21.0 ± 1.6 kg/m2 4 h | 754 | 50 (60 en%) | 17 9 | 21 10 | Palm olein Lard | TAG ↓ NEFA = | Glucose = Insulin = Leptin = | IL-6 = TNF-α = IL-1ß = | |
| Sanders, 2011 [7] Filippou, 2014 [37] | 25 men 25 women (premenopausal) ± 24.8y, ± 23.5 kg/m2 8 h | 846 | 50 (53 en%) | 20 9 | 22 9 | Palm olein Lard | TAG ↓ NEFA ↓ TC = ApoB48 = | Glucose = Insulin = C-peptide = GIP ↓ PYY= | IL-6 = IL-8 = TNF-α = E-selectin = |
References
- FAO. Fats and Fatty Acids in Human Nutrition; Report of an Expert Consultation, FAO: Rome, Italy, 2010; Volume 91. [Google Scholar]
- Ervin, R.B.; Wright, J.D.; Wang, C.Y.; Kennedy-Stephenson, J. Dietary intake of fats and fatty acids for the United States population: 1999–2000. Adv Data 2004, 348, 1–6. [Google Scholar]
- Fattore, E.; Bosetti, C.; Brighenti, F.; Agostoni, C.; Fattore, G. Palm oil and blood lipid-related markers of cardiovascular disease: A systematic review and meta-analysis of dietary intervention trials. Am. J. Clin. Nutr. 2014, 99, 1331–1350. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Berry, S.E. Triacylglycerol structure and interesterification of palmitic and stearic acid-rich fats: An overview and implications for cardiovascular disease. Nutr. Res. Rev. 2009, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, A.; Imperlini, E.; Nigro, E.; Vitucci, D.; Orrù, S.; Daniele, A.; Buono, P.; Mancini, A. Effects of Plant Oil Interesterified Triacylglycerols on Lipemia and Human Health. Int. J. Mol. Sci. 2018, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.; Filippou, A.; Berry, S.E.; Baumgartner, S.; Mensink, R.P. Palmitic acid in the sn-2 position of triacylglycerols acutely influences postprandial lipid metabolism. Am. J. Clin. Nutr. 2011, 94, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Christophe, A.B.; De Greyt, W.F.; Delanghe, J.R.; Huyghebaert, A.D. Substituting enzymatically interesterified butter for native butter has no effect on lipemia or lipoproteinemia in Man. Ann. Nutr. Metab. 2000, 44, 61–67. [Google Scholar] [CrossRef]
- Zock, P.L.; de Vries, J.H.; de Fouw, N.J.; Katan, M.B. Positional distribution of fatty acids in dietary triglycerides: effects on fasting blood lipoprotein concentrations in humans. Am. J. Clin. Nutr. 1995, 61, 48–55. [Google Scholar] [CrossRef]
- Nestel, P.J.; Noakes, M.; Belling, G.B.; McArthur, R.; Clifton, P.M. Effect on plasma lipids of interesterifying a mix of edible oils. Am. J. Clin. Nutr. 1995, 62, 950–955. [Google Scholar] [CrossRef]
- Filippou, A.; Teng, K.T.; Berry, S.E.; Sanders, T.A. Palmitic acid in the sn-2 position of dietary triacylglycerols does not affect insulin secretion or glucose homeostasis in healthy men and women. Eur. J. Clin. Nutr. 2014, 68, 1036–1041. [Google Scholar] [CrossRef]
- Ng, Y.T.; Voon, P.T.; Ng, T.K.W.; Lee, V.K.M.; Mat Sahri, M.; Mohd Esa, N.; Ong, S.H.; Ong, A.S.H. Interesterified palm olein (IEPalm) and interesterified stearic acid-rich fat blend (IEStear) have no adverse effects on insulin resistance: A randomized control trial. Nutrients 2018, 10, 1112. [Google Scholar] [CrossRef]
- Meijer, G.W.; Weststrate, J.A. Interesterification of fats in margarine: Effect on blood lipids, blood enzymes, and hemostasis parameters. Eur. J. Clin. Nutr. 1997, 51, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.E.E.; Miller, G.J.; Sanders, T.A.B. The solid fat content of stearic acid-rich fats determines their postprandial effects. Am. J. Clin. Nutr. 2007, 85, 1486–1494. [Google Scholar] [CrossRef]
- Grande, F.; Anderso, J.T.N.; Keys, A. Comparison of effects of palmitic and stearic acids in the diet on serum cholesterol in man. Am. J. Clin. Nutr. 1970, 23, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Bonanome, A.; Grundy, S.M. Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels. N. Engl. J. Med. 1988, 318, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup, T.; Marckmann, P.; Jespersen, J.; Sandström, B. Fat high in stearic acid favorably affects blood lipids and factor VII coagulant activity in comparison with fats high in palmitic acid or high in myristic and lauric acids. Am. J. Clin. Nutr. 1994, 59, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, R.M.; Allman, M.A.; Iacono, J.M. Effects of diets containing high or low amounts of stearic acid on plasma lipoprotein fractions and fecal fatty acid excretion of men. Am. J. Clin. Nutr. 1995, 61, 1120–1128. [Google Scholar] [CrossRef]
- Schwab, U.S.; Maliranta, H.M.; Sarkkinen, E.S.; Savolainen, M.J.; Kesäniemi, Y.A.; Uusitupa, M.I. Different effects of palmitic and stearic acid-enriched diets on serum lipids and lipoproteins and plasma cholesteryl ester transfer protein activity in healthy young women. Metab. Clin. Exp. 1996, 45, 143–149. [Google Scholar] [CrossRef]
- Meng, H.; Matthan, N.R.; Wu, D.; Li, L.; Rodríguez-Morató, J.; Cohen, R.; Galluccio, J.M.; Dolnikowski, G.G.; Lichtenstein, A.H. Comparison of diets enriched in stearic, oleic, and palmitic acids on inflammation, immune response, cardiometabolic risk factors, and fecal bile acid concentrations in mildly hypercholesterolemic postmenopausal women-randomized crossover trial. Am. J. Clin. Nutr. 2019, 110, 305–315. [Google Scholar] [CrossRef]
- Nestel, P.J.; Pomeroy, S.; Kay, S.; Sasahara, T.; Yamashita, T. Effect of a stearic acid-rich, structured triacylglycerol on plasma lipid concentrations. Am. J. Clin. Nutr. 1998, 68, 1196–1201. [Google Scholar] [CrossRef]
- Kelly, F.D.; Sinclair, A.J.; Mann, N.J.; Turner, A.H.; Abedin, L.; Li, D. A stearic acid-rich diet improves thrombogenic and atherogenic risk factor profiles in healthy males. Eur. J. Clin. Nutr. 2001, 55, 88–96. [Google Scholar] [CrossRef]
- Snook, J.T.; Park, S.; Williams, G.; Tsai, Y.H.; Lee, N. Effect of synthetic triglycerides of myristic, palmitic, and stearic acid on serum lipoprotein metabolism. Eur. J. Clin. Nutr. 1999, 53, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.D.; Sinclair, A.J.; Mann, N.J.; Turner, A.H.; Raffin, F.L.; Blandford, M.V.; Pike, M.J. Short-term diets enriched in stearic or palmitic acids do not alter plasma lipids, platelet aggregation or platelet activation status. Eur. J. Clin. Nutr. 2002, 56, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup, T.; Marckmann, P.; Vessby, B.; Sandström, B. Effect of fats high in individual saturated fatty acids on plasma lipoprotein[a] levels in young healthy men. J. Lipid Res. 1995, 36, 1447–1552. [Google Scholar] [PubMed]
- Schwab, U.; Niskanen, L.; Uusitupa, M. Palmitic and stearic acid enriched diets have similar effects on glucose metabolism in healthy young females. Nutr. Metab. Cardiovasc. Dis. 1997, 7, 315. [Google Scholar]
- Yli-Jokipii, K.M.; Schwab, U.S.; Tahvonen, R.L.; Kurvinen, J.P.; Mykkänen, H.M.; Kallio, H.P.T. Chylomicron and VLDL TAG structures and postprandial lipid response induced by lard and modified lard. Lipids 2003, 38, 693–703. [Google Scholar] [CrossRef]
- Hall, W.L.; Iqbal, S.; Li, H.; Gray, R.; Berry, S.E.E. Modulation of postprandial lipaemia by a single meal containing a commonly consumed interesterified palmitic acid-rich fat blend compared to a non-interesterified equivalent. Eur. J. Nutr. 2017, 56, 2487–2495. [Google Scholar] [CrossRef]
- Summers, L.K.M.; Fielding, B.A.; Herd, S.L.; Ilic, V.; Clark, M.L.; Quinlan, P.T.; Frayn, K.N. Use of structured triacylglycerols containing predominantly stearic and oleic acids to probe early events in metabolic processing of dietary fat. J. Lipid Res. 1999, 40, 1890–1898. [Google Scholar]
- Sanders, T.A.; Berry, S.E.; Miller, G.J. Influence of triacylglycerol structure on the postprandial response of factor VII to stearic acid-rich fats. Am. J. Clin. Nutr. 2003, 77, 777–782. [Google Scholar] [CrossRef]
- Robinson, D.M.; Martin, N.C.; Robinson, L.E.; Ahmadi, L.; Marangoni, A.G.; Wright, A.J. Influence of interesterification of a stearic acid-rich spreadable fat on acute metabolic risk factors. Lipids 2009, 44, 17–26. [Google Scholar] [CrossRef]
- Yli-Jokipii, K.; Kallio, H.; Schwab, U.; Mykkänen, H.; Kurvinen, J.P.; Savolainen, M.J.; Tahvonen, R. Effects of palm oil and transesterified palm oil on chylomicron and VLDL triacylglycerol structures and postprandial lipid response. J. Lipid Res. 2001, 42, 1618–1625. [Google Scholar]
- Berry, S.E.; Woodward, R.; Yeoh, C.; Miller, G.J.; Sanders, T.A. Effect of interesterification of palmitic acid-rich triacylglycerol on postprandial lipid and factor VII response. Lipids 2007, 42, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.L.; Brito, M.F.; Huang, J.; Wood, L.V.; Filippou, A.; Sanders, T.A.; Berry, S.E. An interesterified palm olein test meal decreases early-phase postprandial lipemia compared to palm olein: A randomized controlled trial. Lipids 2014, 49, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Zampelas, A.; Williams, C.M.; Morgan, L.M.; Wright, J.; Quinlan, P.T. The effect of triacylglycerol fatty acid positional distribution on postprandial plasma metabolite and hormone responses in normal adult men. Br. J. Nutr. 1994, 71, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Summers, L.K.; Fielding, B.A.; Ilic, V.; Quinlan, P.T.; Frayn, K.N. The effect of triacylglycerol-fatty acid positional distribution on postprandial metabolism in subcutaneous adipose tissue. Br. J. Nutr. 1998, 79, 141–147. [Google Scholar] [CrossRef][Green Version]
- Filippou, A.; Berry, S.E.; Baumgartner, S.; Mensink, R.P. Sanders, T.A. Palmitic acid in the sn-2 position decreases glucose-dependent insulinotropic polypeptide secretion in healthy adults. Eur. J. Clin. Nutr. 2014, 68, 549–554. [Google Scholar] [CrossRef]
- Jensen, J.; Bysted, A.; Dawids, S.; Hermansen, K.; Hølmer, G. The effect of palm oil, lard, and puff-pastry margarine on postprandial lipid and hormone responses in normal-weight and obese young women. Br. J. Nutr. 1999, 82, 469–479. [Google Scholar] [CrossRef][Green Version]
- Sanders, T.A.; de Grassi, T.; Miller, G.J.; Morrissey, J.H. Influence of fatty acid chain length and cis/trans isomerization on postprandial lipemia and factor VII in healthy subjects (postprandial lipids and factor VII). Atherosclerosis 2000, 149, 413–420. [Google Scholar] [CrossRef]
- Teng, K.T.; Nagapan, G.; Cheng, H.M.; Nesaretnam, K. Palm olein and olive oil cause a higher increase in postprandial lipemia compared with lard but had no effect on plasma glucose, insulin and adipocytokines. Lipids 2011, 46, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup, T.; Sandström, B.; Bysted, A.; Hølmer, G. Effect of 6 dietary fatty acids on the postprandial lipid profile, plasma fatty acids, lipoprotein lipase, and cholesterol ester transfer activities in healthy young men. Am. J. Clin. Nutr. 2001, 73, 198–208. [Google Scholar] [CrossRef]
- Mennen, L.; de Maat, M.; Meijer, G.; Zock, P.; Grobbee, D.; Kok, F.; Kluft, C.; Schouten, E. Factor VIIa response to a fat-rich meal does not depend on fatty acid composition: a randomized controlled trial. Arter. Thromb Vasc Biol. 1998, 18, 599–603. [Google Scholar] [CrossRef][Green Version]
- Tholstrup, T.; Samman, S. Postprandial lipoprotein(a) is affected differently by specific individual dietary fatty acids in healthy young men. J. Nutr. 2004, 134, 2550–2555. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup, T.; Miller, G.J.; Bysted, A.; Sandström, B. Effect of individual dietary fatty acids on postprandial activation of blood coagulation factor VII and fibrinolysis in healthy young men. Am. J. Clin. Nutr. 2003, 77, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Mensink, R.P.; Kris-Etherton, P.M.; Petersen, B.; Smith, K.; Flickinger, B.D. Predicted changes in fatty acid intakes, plasma lipids, and cardiovascular disease risk following replacement of trans fatty acid-containing soybean oil with application-appropriate alternatives. Lipids 2012, 47, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Bots, S.H.; Peters, S.A.E.; Woodward, M. Sex differences in coronary heart disease and stroke mortality: A global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health 2017, 2, e000298. [Google Scholar] [CrossRef]
- Mensink, R.P.; Katan, M.B. Effect of monounsaturated fatty acids versus complex carbohydrates on high-density lipoproteins in healthy men and women. Lancet 1987, 1, 122–125. [Google Scholar] [CrossRef]
- Ginsberg, H.N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 2000, 106, 453–458. [Google Scholar] [CrossRef]
- Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’Acierno, L.; Giordano, R.; et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr. Atheroscler Rep. 2014, 16, 435. [Google Scholar] [CrossRef]
- Lowe, G.; Rumley, A. The relevance of coagulation in cardiovascular disease: What do the biomarkers tell us? Thromb. Haemost. 2014, 112, 860–867. [Google Scholar]
- Kris-Etherton, P.M.; Griel, A.E.; Psota, T.L.; Gebauer, S.K.; Zhang, J.; Etherton, T.D. Dietary stearic acid and risk of cardiovascular disease: intake, sources, digestion, and absorption. Lipids 2005, 40, 1193–1200. [Google Scholar] [CrossRef]
- Mensink, R.P.; Sanders, T.A.; Baer, D.J.; Hayes, K.C.; Howles, P.N.; Marangoni, A. The Increasing Use of Interesterified Lipids in the Food Supply and Their Effects on Health Parameters. Adv. Nutr. 2016, 7, 719–729. [Google Scholar] [CrossRef]
- Kido, T.; Kurata, H.; Kondo, K.; Itakura, H.; Okazaki, M.; Urata, T.; Yokoyama, S. Bioinformatic Analysis of Plasma Apolipoproteins A-I and A-II Revealed Unique Features of A-I/A-II HDL Particles in Human Plasma. Sci. Rep. 2016, 6, 31532. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.G.; Becker, R.C.; Berger, P.B.; Bhatt, D.L.; Eikelboom, J.W.; Konkle, B.; Mohler, E.R.; Reilly, M.P.; Berger, J.S. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J. Thromb. Haemost. 2010, 8, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.G.; Poppitt, S.D.; Minihane, A.M. Postprandial lipemia and cardiovascular disease risk: Interrelationships between dietary, physiological and genetic determinants. Atherosclerosis 2012, 220, 22–33. [Google Scholar] [CrossRef]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. Postprandial lipemia as a cardiometabolic risk factor. Curr. Med. Res. Opin. 2014, 30, 1489–1503. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.G.; Robertson, M.D.; Fielding, B.A.; Frayn, K.N.; Williams, C.M. Olive oil increases the number of triacylglycerol-rich chylomicron particles compared with other oils: an effect retained when a second standard meal is fed. Am. J. Clin. Nutr. 2002, 76, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Tushuizen, M.E.; Nieuwland, R.; Scheffer, P.G.; Sturk, A.; Heine, R.J.; Diamant, M. Two consecutive high-fat meals affect endothelial-dependent vasodilation, oxidative stress and cellular microparticles in healthy men. J. Thromb. Haemost. 2006, 4, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, K.; Holst, J.J.; Baller, B.; Ellrichmann, M.; Nauck, M.A.; Schmidt, W.E.; Meier, J.J. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008, 57, 678–687. [Google Scholar] [CrossRef]
| Fasted Lipids and lipoproteins | High vs low C16:0 sn-2 | High vs low C18:0 sn-2 | Hematological markers | High vs low C16:0 sn-2 | High vs low C18:0 sn-2 | Other markers | High vs low C16:0 sn-2 | High vs low C18:0 sn-2 |
|---|---|---|---|---|---|---|---|---|
| Triacyl- glycerol | 0 ↓ 6 = 0 ↑ | 0 ↓ 2 = 0 ↑ | FVIIa | 0 ↓ 1 = 0 ↑ | 0 ↓ 1 = 0 ↑ | Glucose | 0 ↓ 3 = 0 ↑ | 0 ↓ 1 = 0 ↑ |
| Non-esterified fatty acids | 0 ↓ 1 = 0 ↑ | NA | Fibrinogen | 0 ↓ 1 = 0 ↑ | NA | Insulin | 0 ↓ 2 = 0 ↑ | 0 ↓ 1 = 0 ↑ |
| Total cholesterol | 0 ↓ 6 = * 0 ↑ | 0 ↓ 2 = 0 ↑ | PAI-1 | 0 ↓ 1 = 0 ↑ | NA | C-peptide | 0 ↓ 2 = 0 ↑ | NA |
| LDL- cholesterol | 0 ↓ 6 = * 0 ↑ | 0 ↓ 1 = 0 ↑ | tPA | 0 ↓ 1 = 0 ↑ | NA | C-reactive protein | 0 ↓ 1 = 0 ↑ | NA |
| HDL- cholesterol | 0 ↓ 6 = 0 ↑ | 0 ↓ 1 = 0 ↑ | vWF | 0 ↓ 1 = 0 ↑ | NA | |||
| ApoB | 0 ↓ 3 = 0 ↑ | NA | ||||||
| ApoA1 | 0 ↓ 3 = 0 ↑ | NA | ||||||
| Lp[a] | 0 ↓ 3 = 0 ↑ | NA |
| Fasted Lipids and lipoproteins | C18:0 vs C16:0 | Hematological markers | C18:0 vs C16:0 | C18:0 vs C16:0 | Other markers | C18:0 vs C16:0 | |
|---|---|---|---|---|---|---|---|
| Triacyl- glycerol | 1 ↓ 10 = 0 ↑ | FVIIc | 1 ↓ 1 = 0 ↑ | Fibrinogen | 0 ↓ 1 = 0 ↑ | CETP activity | 1 ↓ 1 = 0 ↑ |
| Total cholesterol | 7 ↓ 4 = 0 ↑ | Mean platelet volume | 1 ↓ 1 = 0 ↑ | Plasminogen | 0 ↓ 1 = 0 ↑ | LCAT activity | 0 ↓ 1 = 0 ↑ |
| VLDL- cholesterol | 0 ↓ 4 = 0 ↑ | PAI-1 activity | 0 ↓ 1 = 0 ↑ | White blood cells | 0 ↓ 2 = 0 ↑ | Glucose | 0 ↓ 2 = 0 ↑ |
| LDL- cholesterol | 5 ↓ 5 = 0 ↑ | PAI-1 antigen | 0 ↓ 1 = 0 ↑ | Red blood cells | 0 ↓ 2 = 0 ↑ | Insulin | 0 ↓ 2 = 0 ↑ |
| HDL- cholesterol | 3 ↓ 7 = 0 ↑ | tPA activity | 0 ↓ 1 = 0 ↑ | Hemoglobin | 0 ↓ 2 = 0 ↑ | C-peptide | 0 ↓ 1 = 0 ↑ |
| ApoB | 1 ↓ 4 = 0 ↑ | tPA antigen | 0 ↓ 1 = 0 ↑ | Platelets | 0 ↓ 2 = 0 ↑ | Various inflammation markers | 0 ↓ 1 = 0 ↑ |
| ApoA1 | 2 ↓ 3 = 0 ↑ | EFA | 0 ↓ 1 = 0 ↑ | Antithrombin III | 0 ↓ 1 = 0 ↑ | ||
| Lp[a] | 0 ↓ 1 = 1 ↑ | Thrombomodulin | 0 ↓ 1 = 0 ↑ | PTT | 0 ↓ 1 = 0 ↑ | ||
| Prothrombin time | 0 ↓ 1 = 0 ↑ | APTT | 0 ↓ 1 = 0 ↑ |
| Postprandial Lipids and lipoproteins | High vs low C16:0 sn-2 | High vs low C18:0 sn-2 | Hemato-logical markers | High vs low C16:0 sn-2 | High vs low C18:0 sn-2 | Other markers | High vs low C16:0 sn-2 | High vs low C18:0 sn-2 |
|---|---|---|---|---|---|---|---|---|
| Triacylglycerol | 1 ↓ 6 = 1 ↑ | 2 ↓ 3 = 0 ↑ | FVIIa | 0 ↓ 1 = 0 ↑ | 1 ↓ 1 = 0 ↑ | Glucose | 0 ↓ 7 = 0 ↑ | 0 ↓ 3 = 0 ↑ |
| Non-esterified fatty acids | 0 ↓ 6 = 0 ↑ | 0 ↓ 3 = 0 ↑ | White blood cells | 0 ↓ 1 = 0 ↑ | 0 ↓ 1 = 0 ↑ | Insulin | 0 ↓ 7 = 0 ↑ | 0 ↓ 3 = 0 ↑ |
| Total cholesterol | 0 ↓ 4 = 0 ↑ | 0 ↓ 3 = 0 ↑ | C-peptide | 0 ↓ 1 = 0 ↑ | NA | |||
| VLDL-cholesterol | 0 ↓ 2 = 0 ↑ | NA | GIP | 1 ↓ 2 = 0 ↑ | NA | |||
| LDL-cholesterol | 0 ↓ 1 = 0 ↑ | 0 ↓ 3 = 0 ↑ | Peptide YY | 0 ↓ 2 = 0 ↑ | NA | |||
| HDL-cholesterol | 0 ↓ 1 = 0 ↑ | 0 ↓ 2 = 0 ↑ | IL-6 | 0 ↓ 1 = 0 ↑ | NA | |||
| Chylomicron- cholesterol | 0 ↓ 2 = 0 ↑ | NA | IL-8 | 0 ↓ 1 = 0 ↑ | NA | |||
| ApoB48 | 0 ↓ 1 = 0 ↑ | NA | TNF-α | 0 ↓ 1 = 0 ↑ | NA | |||
| E-selectin | 0 ↓ 1 = 0 ↑ | NA |
| Postprandial Lipids and lipoproteins | C18:0 vs C16:0 | Hematological markers | C18:0 vs C16:0 | Other markers | C18:0 vs C16:0 |
|---|---|---|---|---|---|
| Triacylglycerol | 1 ↓ 4 = 0 ↑ | FVIIa | 0 ↓ 3 = 0 ↑ | Glucose | 0 ↓ 1 = 0 ↑ |
| Non-esterified fatty acids | 0 ↓ 2 = 0 ↑ | FVIIc | 0 ↓ 2 = 0 ↑ | Insulin | 0 ↓ 2 = 0 ↑ |
| Total cholesterol | 0 ↓ 1 = 0 ↑ | PAI-1 antigen | 0 ↓ 1 = 0 ↑ | GIP | 1 ↓ 0 = 0 ↑ |
| VLDL-cholesterol | 0 ↓ 1 = 0 ↑ | tPA activity | 0 ↓ 1 = 0 ↑ | Peptide YY | 0 ↓ 1 = 0 ↑ |
| LDL-cholesterol | 0 ↓ 1 = 0 ↑ | Leptin | 0 ↓ 1 = 0 ↑ | ||
| HDL-cholesterol | 0 ↓ 1 = 0 ↑ | CETP activity | 0 ↓ 1 = 0 ↑ | ||
| ApoB | 0 ↓ 1 = 0 ↑ | LPL activity | 0 ↓ 1 = 0 ↑ | ||
| ApoA1 | 0 ↓ 1 = 0 ↑ | IL-6 | 0 ↓ 1 = 0 ↑ | ||
| Lp[a] | 0 ↓ 1 = 0 ↑ | TNF-α | 0 ↓ 1 = 0 ↑ | ||
| IL-1β | 0 ↓ 1 = 0 ↑ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Rooijen, M.A.; Mensink, R.P. Palmitic Acid Versus Stearic Acid: Effects of Interesterification and Intakes on Cardiometabolic Risk Markers—A Systematic Review. Nutrients 2020, 12, 615. https://doi.org/10.3390/nu12030615
van Rooijen MA, Mensink RP. Palmitic Acid Versus Stearic Acid: Effects of Interesterification and Intakes on Cardiometabolic Risk Markers—A Systematic Review. Nutrients. 2020; 12(3):615. https://doi.org/10.3390/nu12030615
Chicago/Turabian Stylevan Rooijen, Merel A., and Ronald P. Mensink. 2020. "Palmitic Acid Versus Stearic Acid: Effects of Interesterification and Intakes on Cardiometabolic Risk Markers—A Systematic Review" Nutrients 12, no. 3: 615. https://doi.org/10.3390/nu12030615
APA Stylevan Rooijen, M. A., & Mensink, R. P. (2020). Palmitic Acid Versus Stearic Acid: Effects of Interesterification and Intakes on Cardiometabolic Risk Markers—A Systematic Review. Nutrients, 12(3), 615. https://doi.org/10.3390/nu12030615






