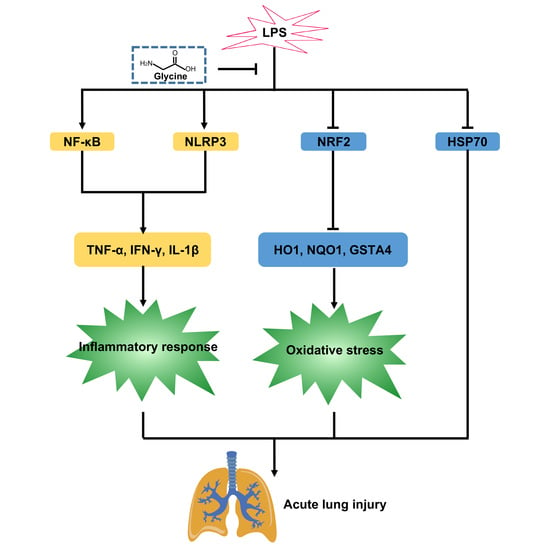
Glycine Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Regulating NLRP3 Inflammasome and NRF2 Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Design
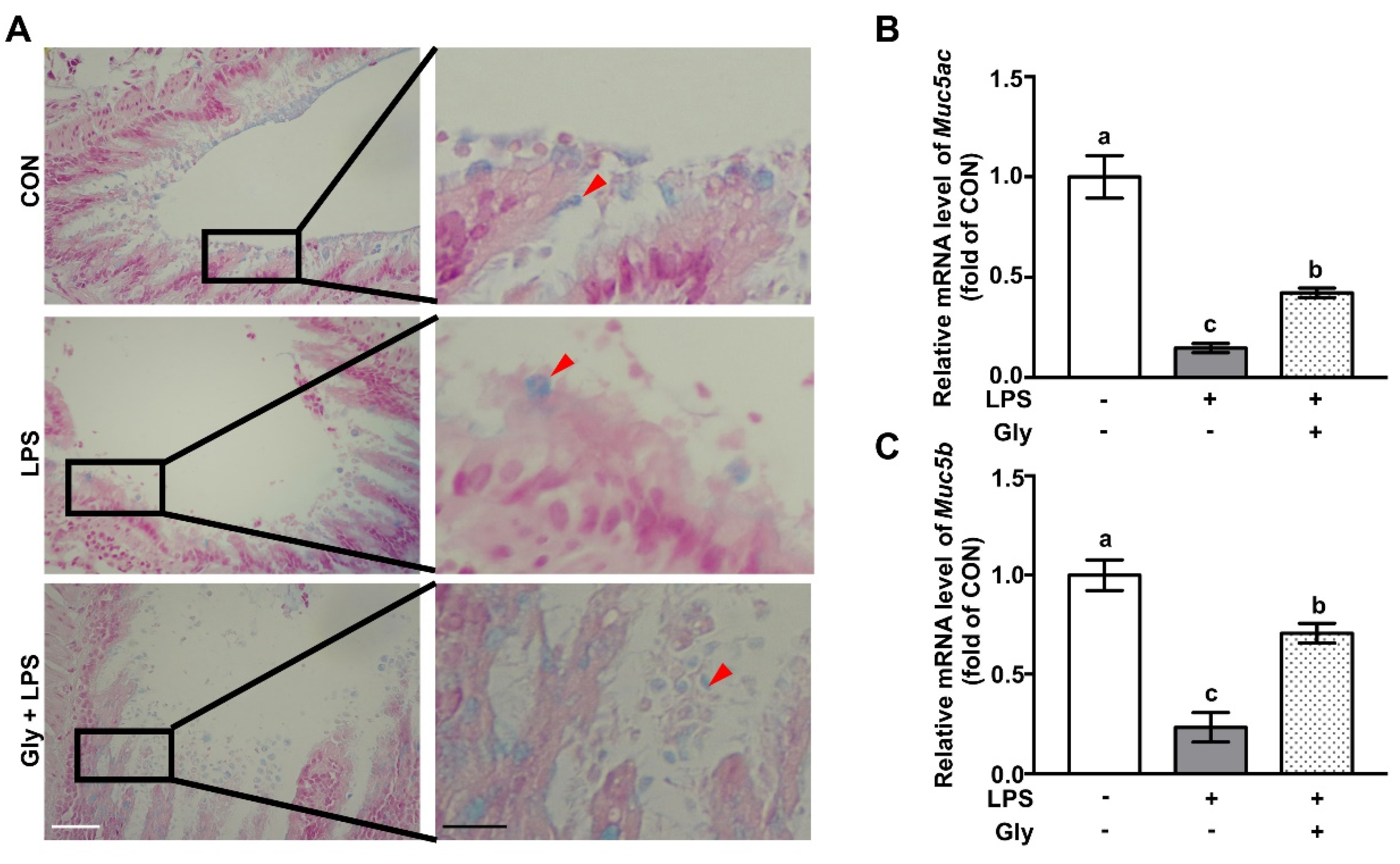
2.3. Alcian Blue Staining
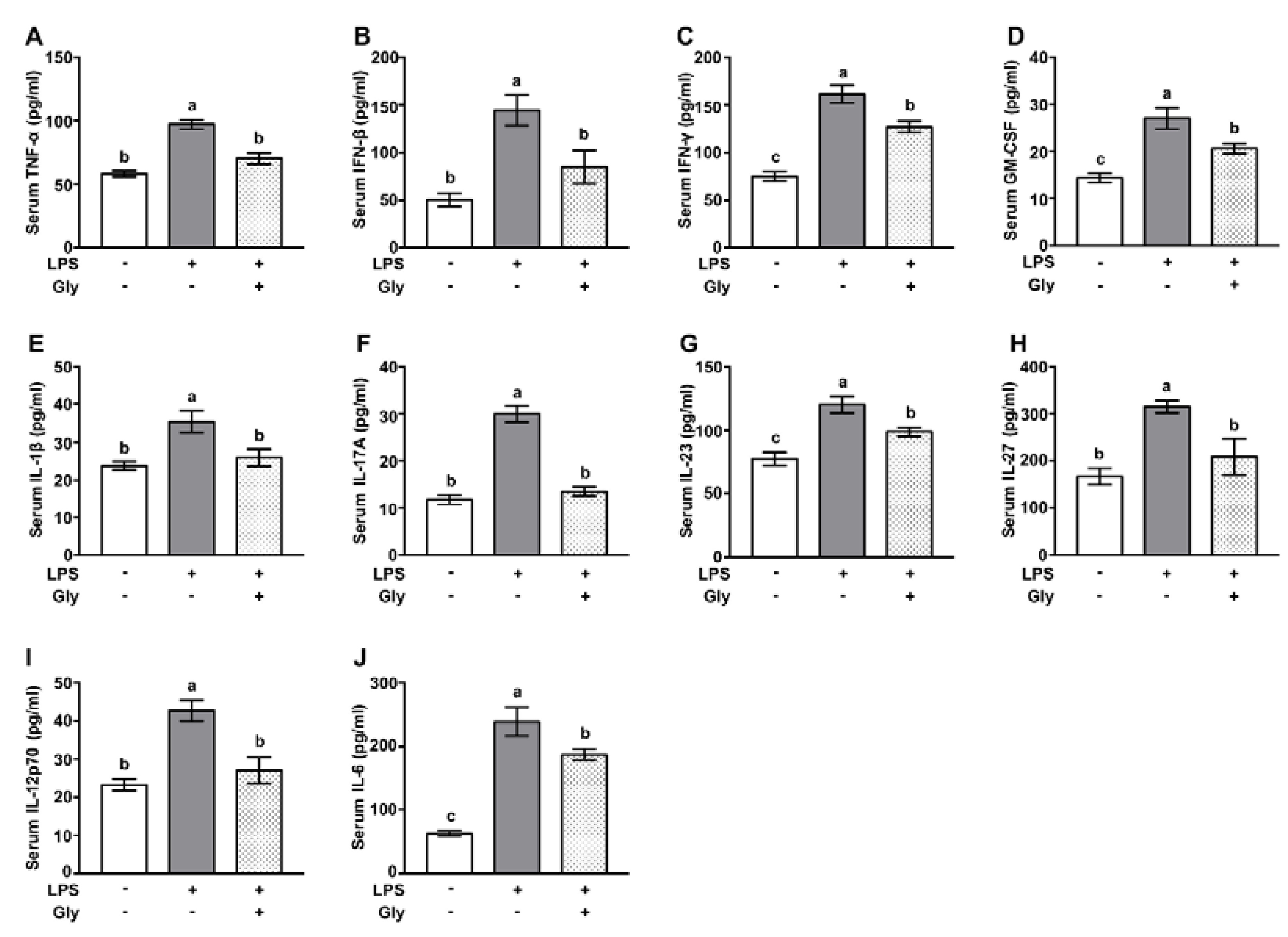
2.4. Serum Inflammatory Cytokine Analysis
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
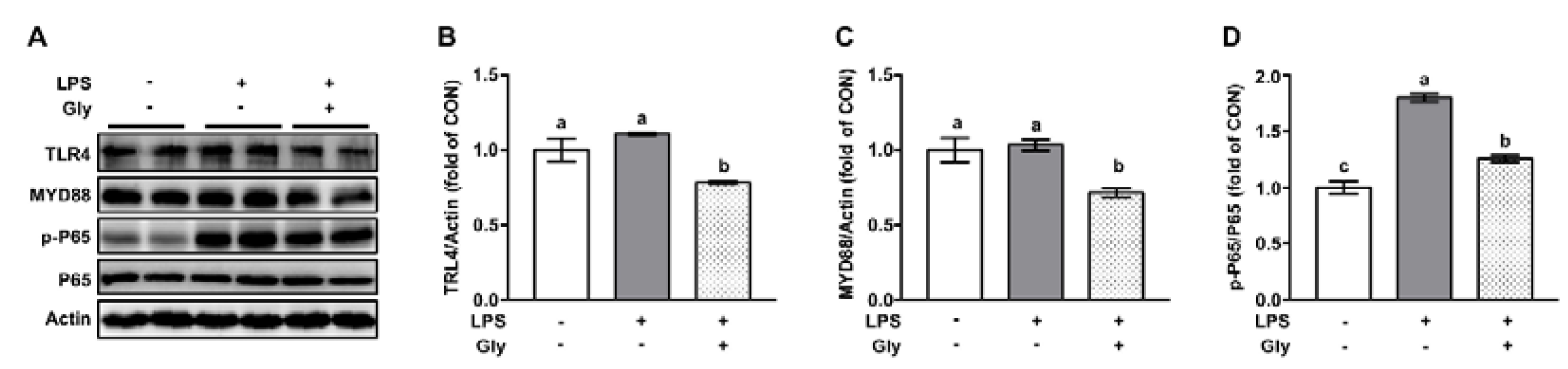
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Glycine Supplementation Restored Mucin Layer in LPS-Treated Mice
3.2. Glycine Pretreatment Suppressed Secretion of Pro-Inflammatory Cytokines
3.3. Glycine Inhibited Activation of NF-κB in LPS-Stimulated Lung
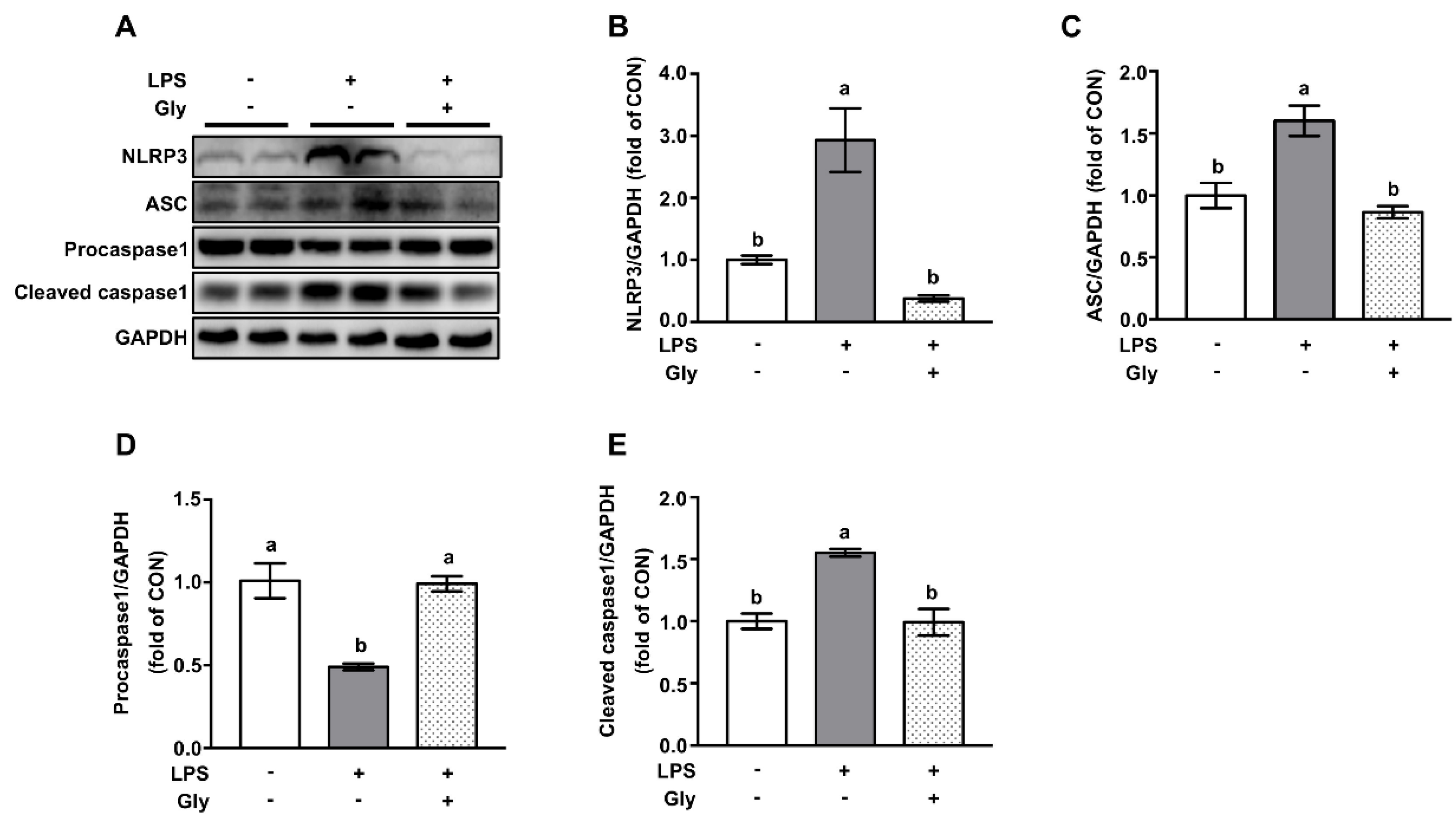
3.4. Glycine Pretreatment Blocked Activation of NLRP3 Inflammasome in Lung Tissues of LPS-Challenged Mice
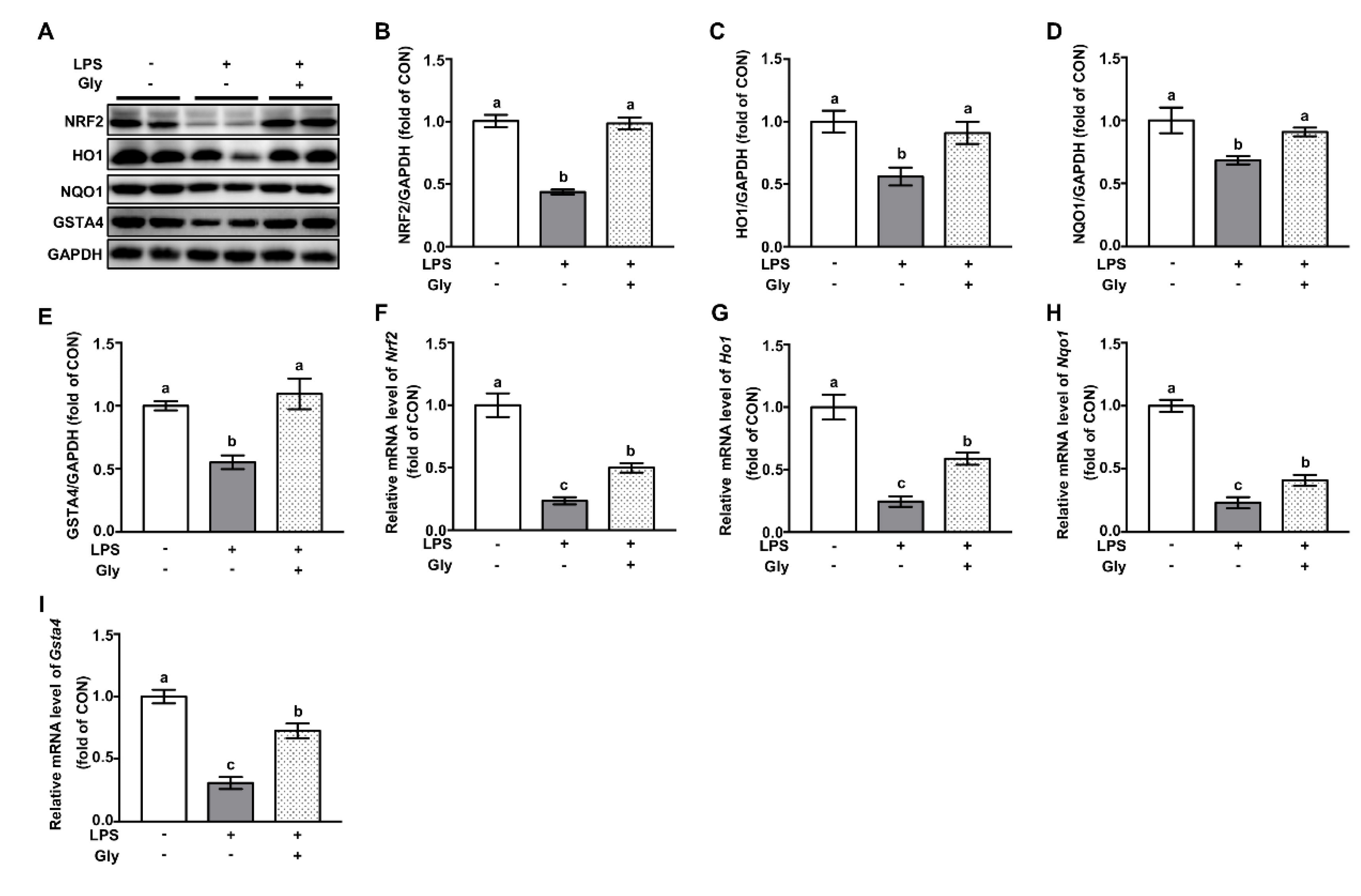
3.5. Glycine Administration Enhanced NRF2 Signaling in Lung Tissues of LPS-Challenged Mice
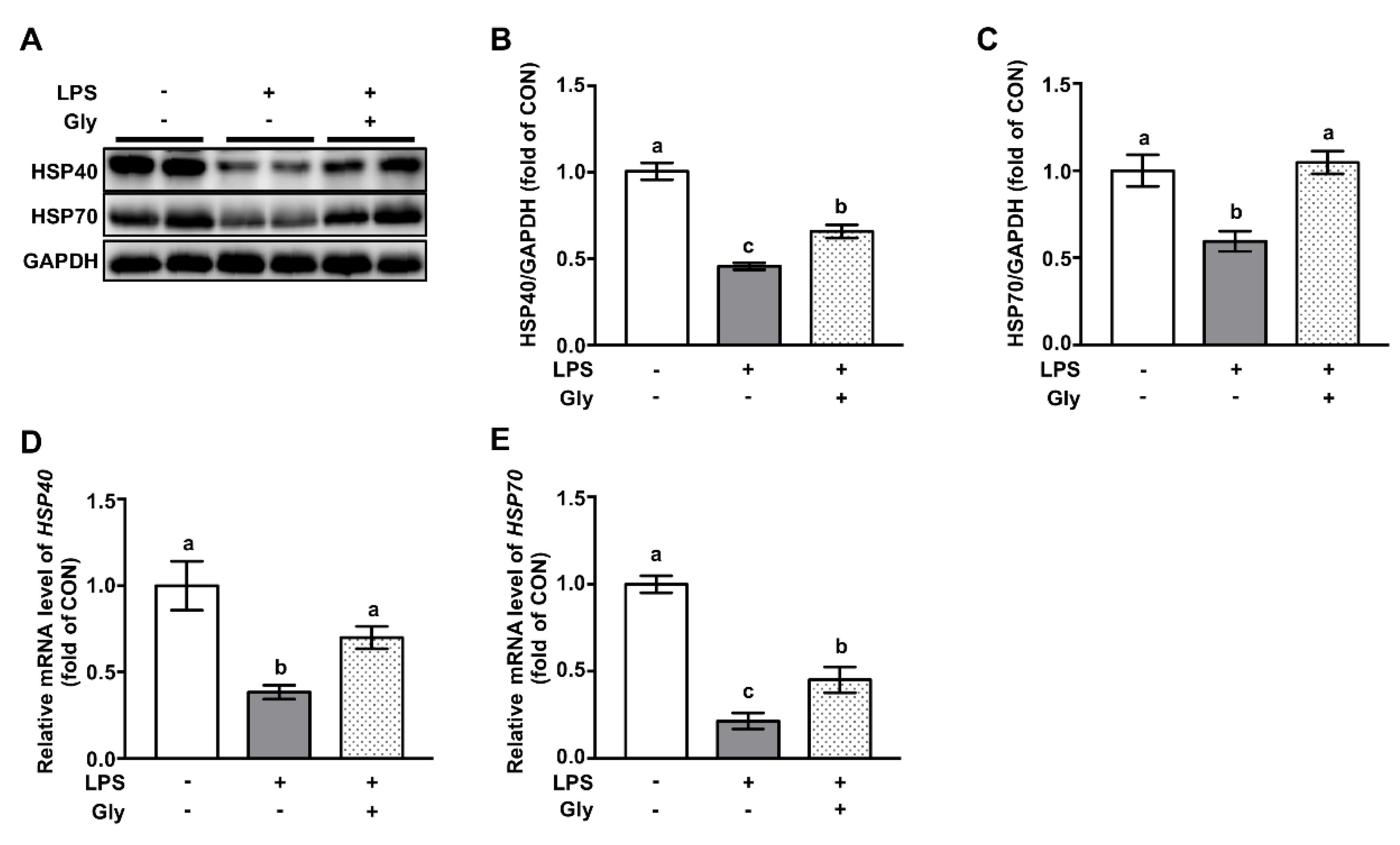
3.6. Glycine Increased Protein Abundance of HSP70 and HSP40 in Lung Tissues of LPS-Challenged Mice
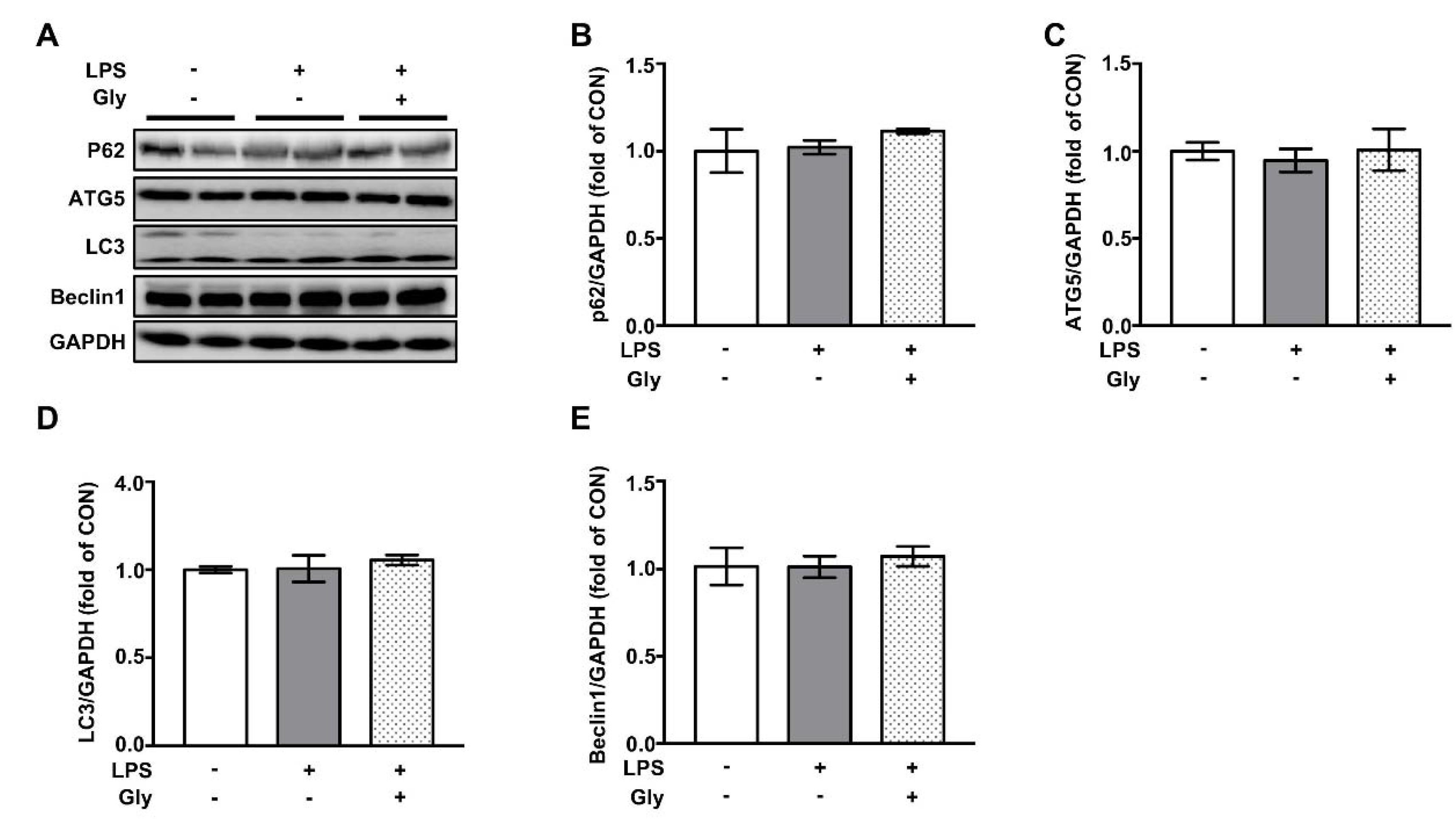
3.7. Autophagy was not Involved in the Beneficial Effect of Glycine on LPS-Induced Acute Lung Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Ridley, C.; Thornton, D.J. Mucins: The frontline defence of the lung. Biochem. Soc. Trans. 2018, 46, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 2011, 31, 379–446. [Google Scholar] [CrossRef]
- Thangavel, J.; Samanta, S.; Rajasingh, S.; Barani, B.; Xuan, Y.T.; Dawn, B.; Rajasingh, J. Epigenetic modifiers reduce inflammation and modulate macrophage phenotype during endotoxemia-induced acute lung injury. J. Cell Sci. 2015, 128, 3094–3105. [Google Scholar] [CrossRef] [PubMed]
- Tartey, S.; Takeuchi, O. Pathogen recognition and Toll-like receptor targeted therapeutics in innate immune cells. Int. Rev. Immunol. 2017, 36, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Dolinay, T.; Kim, Y.S.; Howrylak, J.; Hunninghake, G.M.; An, C.H.; Fredenburgh, L.; Massaro, A.F.; Rogers, A.; Gazourian, L.; Nakahira, K.; et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am. J. Respir. Crit. care Med. 2012, 185, 1225–1234. [Google Scholar] [CrossRef]
- Fukumoto, J.; Fukumoto, I.; Parthasarathy, P.T.; Cox, R.; Huynh, B.; Ramanathan, G.K.; Venugopal, R.B.; Allen-Gipson, D.S.; Lockey, R.F.; Kolliputi, N. NLRP3 deletion protects from hyperoxia-induced acute lung injury. Am. J. Physiol. Cell Physiol. 2013, 305, C182–189. [Google Scholar] [CrossRef]
- Chen, H.; Ding, Y.; Chen, W.; Feng, Y.; Shi, G. Glibenclamide alleviates inflammation in oleic acid model of acute lung injury through NLRP3 inflammasome signaling pathway. Drug Des. Devel. Ther. 2019, 13, 1545–1554. [Google Scholar] [CrossRef]
- Lee, S.; Nakahira, K.; Dalli, J.; Siempos, I.; Norris, P.C.; Colas, R.A.; Moon, J.S.; Shinohara, M.; Hisata, S.; Howrylak, J.A.; et al. NLRP3 inflammasome deficiency protects against microbial sepsis via increased lipoxin B4 synthesis. Am. J. Respir. Crit. care Med. 2017, 196, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wen, Z.; Shi, X.; Fan, J. Inflammasome in the pathogenesis of pulmonary diseases. Exp. Suppl. 2018, 108, 111–151. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Rojo de la Vega, M.; Dodson, M.; Gross, C.; Mansour, H.M.; Lantz, R.C.; Chapman, E.; Wang, T.; Black, S.M.; Garcia, J.G.; Zhang, D.D. Role of Nrf2 and autophagy in acute lung injury. Curr. Pharmacol. Rep. 2016, 2, 91–101. [Google Scholar] [CrossRef]
- Cho, H.Y.; Jedlicka, A.E.; Gladwell, W.; Marzec, J.; McCaw, Z.R.; Bienstock, R.J.; Kleeberger, S.R. Association of Nrf2 polymorphism haplotypes with acute lung injury phenotypes in inbred strains of mice. Antioxidants Redox. Signal. 2015, 22, 325–338. [Google Scholar] [CrossRef]
- Qi, T.; Xu, F.; Yan, X.; Li, S.; Li, H. Sulforaphane exerts anti-inflammatory effects against lipopolysaccharide-induced acute lung injury in mice through the Nrf2/ARE pathway. Int. J. Mol. Med. 2016, 37, 182–188. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Ci, X. Role of Nrf2 and its activators in respiratory diseases. Oxid. Med. Cell Longev. 2019, 2019, 7090534. [Google Scholar] [CrossRef]
- Niu, X.; Wang, Y.; Li, W.; Mu, Q.; Li, H.; Yao, H.; Zhang, H. Protective effects of isofraxidin against lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2015, 24, 432–439. [Google Scholar] [CrossRef]
- Yeh, C.-L.; Pai, M.-H.; Shih, Y.-M.; Shih, J.-M.; Yeh, S.-L. Intravenous arginine administration promotes proangiogenic cells mobilization and attenuates lung injury in mice with polymicrobial sepsis. Nutrients 2017, 9, 507. [Google Scholar] [CrossRef]
- Lai, C.; Liu, W.; Chen, C. Glutamine attenuates acute lung injury caused by acid aspiration. Nutrients 2014, 6, 3101–3116. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Lin, G.; Hu, S.; Wang, B.; Dai, Z.; Wu, G. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J. Nutr. 2014, 144, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wheeler, M.D.; Li, X.; Froh, M.; Schemmer, P.; Yin, M.; Bunzendaul, H.; Bradford, B.; Lemasters, J.J. L-Glycine: A novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, Y.; Jiang, D.; Yang, Y.; Wu, G.; Wu, Z. Protective effects of functional amino acids on apoptosis, inflammatory response, and pulmonary fibrosis in lipopolysaccharide-challenged mice. J. Agric. Food Chem. 2019, 67, 4915–4922. [Google Scholar] [CrossRef] [PubMed]
- Bruck, R.; Wardi, J.; Aeed, H.; Avni, Y.; Shirin, H.; Avinoach, I.; Shahmurov, M.; Hershkoviz, R. Glycine modulates cytokine secretion, inhibits hepatic damage and improves survival in a model of endotoxemia in mice. Liver Int. 2003, 23, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Wu, G.; Sun, Y.; Wang, B.; He, B.; Dai, Z.; Wu, Z. Glutamine enhances tight junction protein expression and modulates corticotropin-releasing factor signaling in the jejunum of weanling piglets. J. Nutr. 2015, 145, 25–31. [Google Scholar] [CrossRef]
- Lillehoj, E.P.; Kato, K.; Lu, W.; Kim, K.C. Cellular and molecular biology of airway mucins. Int. Rev. Cell Mol. Biol. 2013, 303, 139–202. [Google Scholar] [CrossRef]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef]
- Caramori, G.; Di Gregorio, C.; Carlstedt, I.; Casolari, P.; Guzzinati, I.; Adcock, I.M.; Barnes, P.J.; Ciaccia, A.; Cavallesco, G.; Chung, K.F.; et al. Mucin expression in peripheral airways of patients with chronic obstructive pulmonary disease. Histopathology 2004, 45, 477–484. [Google Scholar] [CrossRef]
- Woodruff, P.G.; Modrek, B.; Choy, D.F.; Jia, G.; Abbas, A.R.; Ellwanger, A.; Koth, L.L.; Arron, J.R.; Fahy, J.V. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 388–395. [Google Scholar] [CrossRef]
- Kudlak, K.; Demuro, J.P.; Hanna, A.F.; Brem, H. Acute lung injury following the use of granulocyte-macrophage colony-stimulating factor. Int. J. Crit. Illn. Inj. Sci. 2013, 3, 279–281. [Google Scholar] [CrossRef]
- Li, L.; Dong, L.; Zhao, D.; Gao, F.; Yan, J. Classical dendritic cells regulate acute lung inflammation and injury in mice with lipopolysaccharideinduced acute respiratory distress syndrome. Int. J. Mol. Med. 2019, 44, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.B.; Pugin, J.; Lee, J.S.; Matthay, M.A. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003, 14, 523–535. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Olson, C.M.; Hedrick, M.N.; Izadi, H.; Bates, T.C.; Olivera, E.R.; Anguita, J. p38 mitogen-activated protein kinase controls NF-kappaB transcriptional activation and tumor necrosis factor alpha production through RelA phosphorylation mediated by mitogen- and stress-activated protein kinase 1 in response to Borrelia burgdorferi antigens. Infect Immun. 2007, 75, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.B.; Knudtson, K.L.; Monick, M.M.; Hunninghake, G.W. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP). J. Biol. Chem. 1999, 274, 30858–30863. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, H.S.; Chong, Y.H.; Kang, J.L. p38 Mitogen-activated protein kinase up-regulates LPS-induced NF-kappaB activation in the development of lung injury and RAW 264.7 macrophages. Toxicology 2006, 225, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, H.; Jin, Y.; Liu, N.; Chen, J.; Yang, Y.; Dai, Z.; Wang, C.; Wu, G.; Wu, Z. Glycine attenuates lipopolysaccharide-induced apoptosis and inflammatory cell infiltration in mouse liver. J. Nutr. 2020, 150. [Google Scholar]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3beta-Nrf2 signal axis. Redox. Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef]
- Wei, J.; Chen, G.; Shi, X.; Zhou, H.; Liu, M.; Chen, Y.; Feng, D.; Zhang, P.; Wu, L.; Lv, X. Nrf2 activation protects against intratracheal LPS induced mouse/murine acute respiratory distress syndrome by regulating macrophage polarization. Biochem. Biophys. Res. Commun. 2018, 500, 790–796. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, T.; Liu, J.; Gu, L. Vitexin attenuates lipopolysaccharide-induced acute lung injury by controlling the Nrf2 pathway. PLoS ONE 2018, 13, e0196405. [Google Scholar] [CrossRef]
- Huang, X.T.; Liu, W.; Zhou, Y.; Hao, C.X.; Zhou, Y.; Zhang, C.Y.; Sun, C.C.; Luo, Z.Q.; Tang, S.Y. Dihydroartemisinin attenuates lipopolysaccharideinduced acute lung injury in mice by suppressing NFkappaB signaling in an Nrf2 dependent manner. Int. J. Mol. Med. 2019, 44, 2213–2222. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Deng, J.-S.; Chang, Y.-S.; Huang, G.-J. Ginsenoside Rh2 Ameliorates Lipopolysaccharide-Induced Acute Lung Injury by Regulating the TLR4/PI3K/Akt/mTOR, Raf-1/MEK/ERK, and Keap1/Nrf2/HO-1 Signaling Pathways in Mice. Nutrients 2018, 10, 1208. [Google Scholar] [CrossRef]
- Liu, X.; Lu, J.; Liao, Y.; Liu, S.; Chen, Y.; He, R.; Men, L.; Lu, C.; Chen, Z.; Li, S.; et al. Dihydroartemisinin attenuates lipopolysaccharide-induced acute kidney injury by inhibiting inflammation and oxidative stress. Biomed. Pharmacothe. 2019, 117, 109070. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, Y.; Gorshkov, B.; Haigh, S.; Bordan, Z.; Weintraub, D.; Rudic, R.D.; Chakraborty, T.; Barman, S.A.; Verin, A.D.; et al. Hsp70 Suppresses Mitochondrial Reactive oxygen species and preserves pulmonary microvascular barrier integrity following exposure to bacterial toxins. Front. Immunol. 2018, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Singleton, K.D.; Wischmeyer, P.E. Effects of HSP70.1/3 gene knockout on acute respiratory distress syndrome and the inflammatory response following sepsis. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L956–961. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Yang, L.; Zhang, X.; Chen, Y.; Cai, J. Dioscin prevents LPS-induced acute lung injury through inhibiting the TLR4/MyD88 signaling pathway via upregulation of HSP70. Mol. Med. Rep. 2018, 17, 6752–6758. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino Acids: Biochemistry and Nutrition; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Wang, W.; Dai, Z.; Wu, Z.; Lin, G.; Jia, S.; Hu, S.; Dahanayaka, S.; Wu, G. Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino acids 2014, 46, 2037–2045. [Google Scholar] [CrossRef]
- Qu, W.; Ikejima, K.; Zhong, Z.; Waalkes, M.P.; Thurman, R.G. Glycine blocks the increase in intracellular free Ca2+ due to vasoactive mediators in hepatic parenchymal cells. Am. J. Physiol. Gastrointest Liver Physiol. 2002, 283, G1249–G1256. [Google Scholar] [CrossRef]
- Wheeler, M.D.; Ikejema, K.; Enomoto, N.; Stacklewitz, R.F.; Seabra, V.; Zhong, Z.; Yin, M.; Schemmer, P.; Rose, M.L.; Rusyn, I.; et al. Glycine: A new anti-inflammatory immunonutrient. Cell Mol. Life Sci. 1999, 56, 843–856. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M. Autophagy in lung disease pathogenesis and therapeutics. Redox. Biol. 2015, 4, 215–225. [Google Scholar] [CrossRef]
| Gene | Forward Primer (5’ to 3’) | Reverse Primer (5’ to 3’) |
|---|---|---|
| Muc5ac | GCAATCCCCTTTCCGATGTC | AAAAGGGCAGGTCTTCGGTA |
| Muc5b | GGTTGGCTACATCTTCTGCG | ATCAGCCCAAATCGCACATC |
| Nrf2 | TCCATTTACGGAGACCCACC | GGCCGTTCTGTTTGACACTT |
| Ho1 | CAGGTGTCCAGAGAAGGCTT | GCTTGTTGCGCTCTATCTCC |
| Nqo1 | GTAGCGGCTCCATGTACTCT | AGGATGCCACTCTGAATCGG |
| Gsta4 | TTTAATGGCAGGGGACGGAT | TGTCAGCATCATCCCATCGA |
| Hsp40 | TACACATTCCACGGAGACCC | TGAAGCCACCCATACCCATT |
| Hsp70 | CAACGTGCTCATCTTCGACC | GGCTGATGTCCTTCTTGTGC |
| Gapdh | AAGCCCATCACCATCTTCCA | CACCAGTAGACTCCACGACA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ma, X.; Jiang, D.; Chen, J.; Jia, H.; Wu, Z.; Kim, I.H.; Yang, Y. Glycine Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Regulating NLRP3 Inflammasome and NRF2 Signaling. Nutrients 2020, 12, 611. https://doi.org/10.3390/nu12030611
Zhang Y, Ma X, Jiang D, Chen J, Jia H, Wu Z, Kim IH, Yang Y. Glycine Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Regulating NLRP3 Inflammasome and NRF2 Signaling. Nutrients. 2020; 12(3):611. https://doi.org/10.3390/nu12030611
Chicago/Turabian StyleZhang, Yunchang, Xiaoshi Ma, Da Jiang, Jingqing Chen, Hai Jia, Zhenlong Wu, In Ho Kim, and Ying Yang. 2020. "Glycine Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Regulating NLRP3 Inflammasome and NRF2 Signaling" Nutrients 12, no. 3: 611. https://doi.org/10.3390/nu12030611
APA StyleZhang, Y., Ma, X., Jiang, D., Chen, J., Jia, H., Wu, Z., Kim, I. H., & Yang, Y. (2020). Glycine Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Regulating NLRP3 Inflammasome and NRF2 Signaling. Nutrients, 12(3), 611. https://doi.org/10.3390/nu12030611