A 12-Week-Long Intake of Bilberry Extract (Vaccinium myrtillus L.) Improved Objective Findings of Ciliary Muscle Contraction of the Eye: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial
Abstract
1. Introduction
2. Methods and Subjects
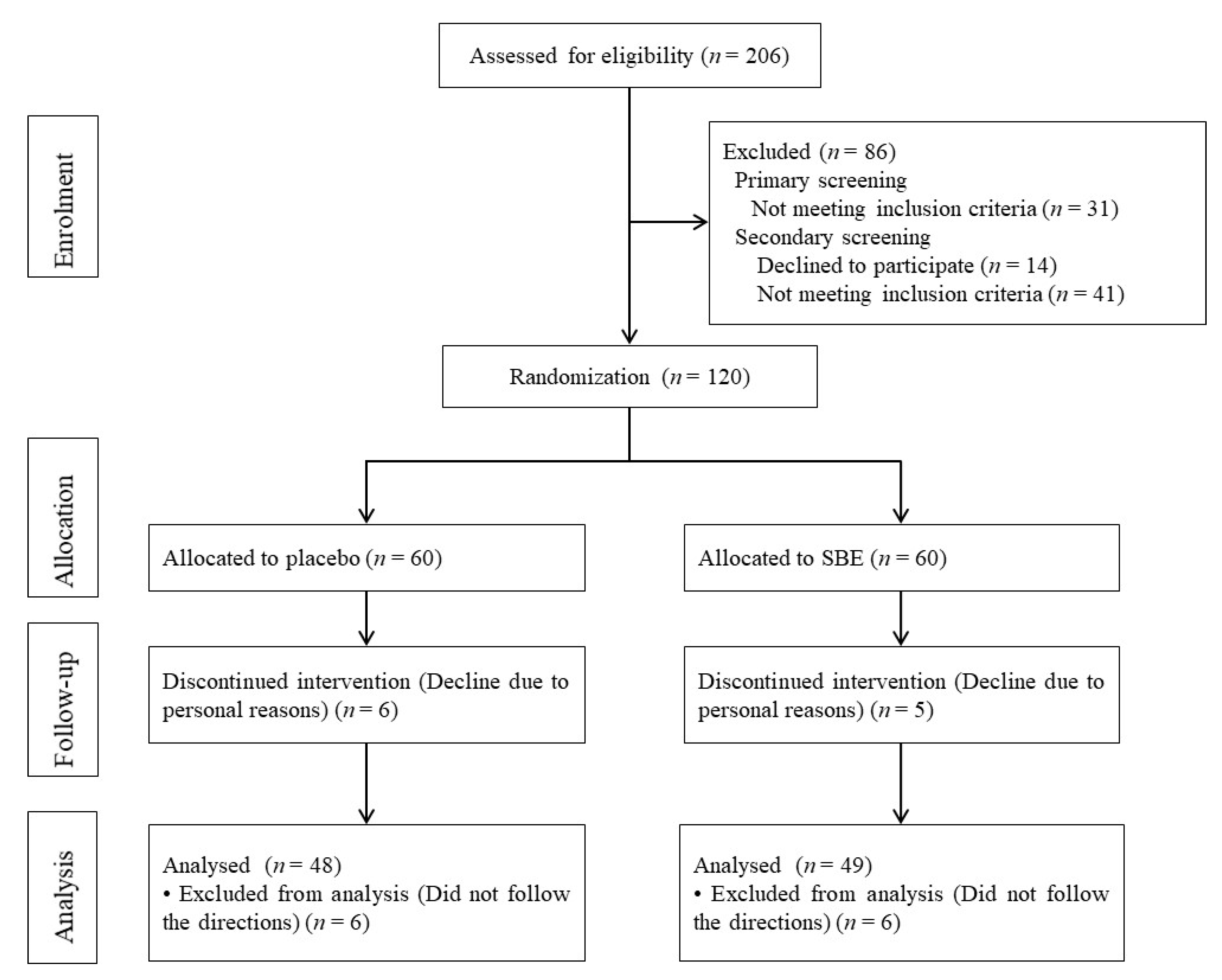
2.1. Trial Design and Subjects
- Serious diseases of the digestive system, liver, pancreas, heart, or kidney, or a history of such a disease
- currently under treatment with pharmaceuticals
- ocular diseases other than ametropia (hyperopia, myopia, or astigmatism)
- existing drug and food allergies
- history of, or under treatment for, drug dependence and substance abuse
- nightshift or irregular work hours
- history of refractive surgeries; LASIK (laser-assisted in situ keratomileusis) or others
- users of reading glasses
- current consumption of BE, pharmaceuticals, or supplements that may improve ocular fatigue
- participated in another clinical trial within the last 1 month
- pregnant, breastfeeding, or planning for pregnancy during the trial period
- blood sampling of ≥ 200 mL within the last 1 month, and
- deemed illegible by the trial supervisor or trial attending physician
2.2. Test Foods
2.3. Consumption Methods and Schedule
2.4. Test Items
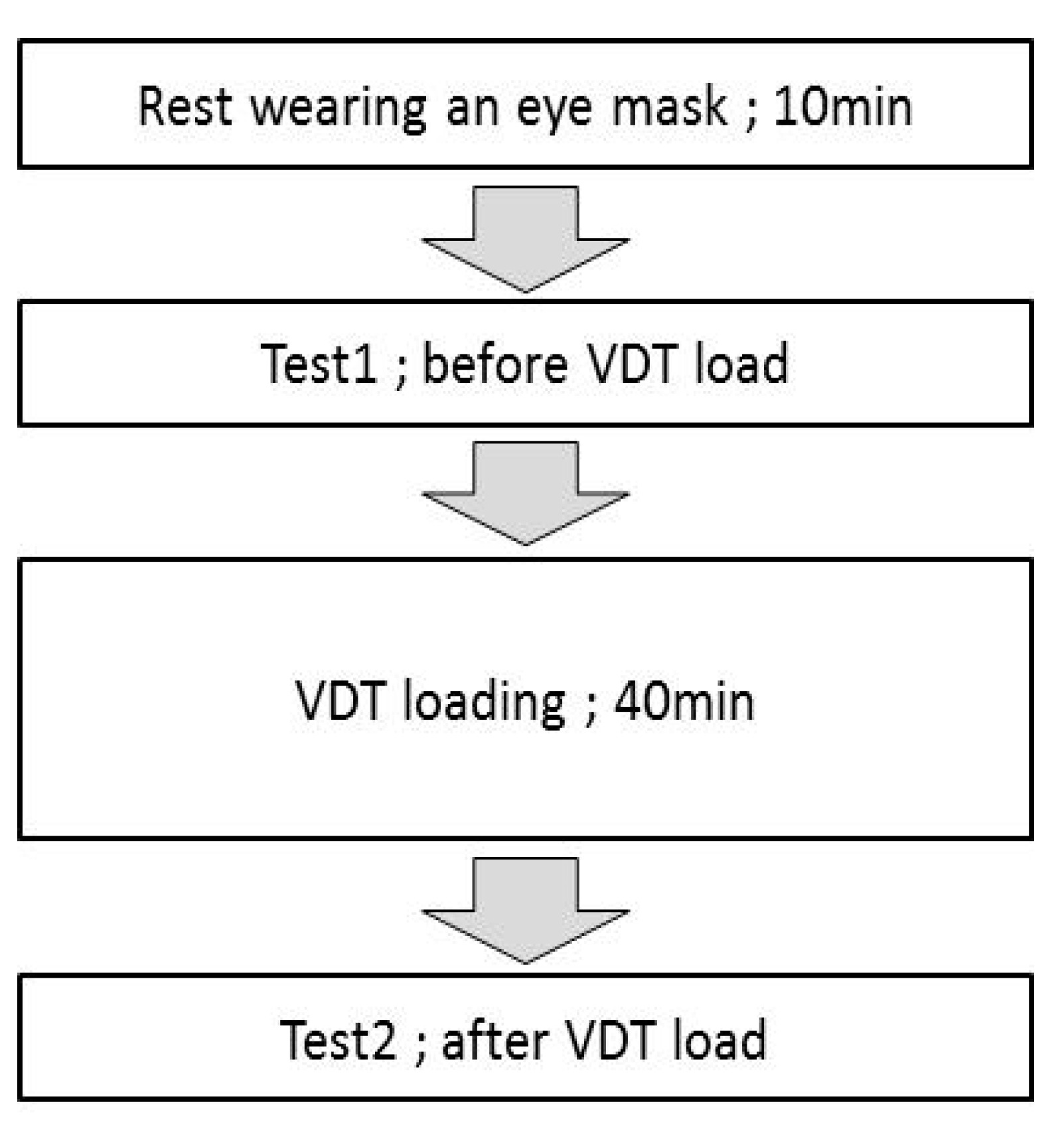
2.4.1. Ocular Fatigue Test
2.4.2. Safety Evaluation
2.5. Statistical Processing
3. Results
3.1. Subject Background
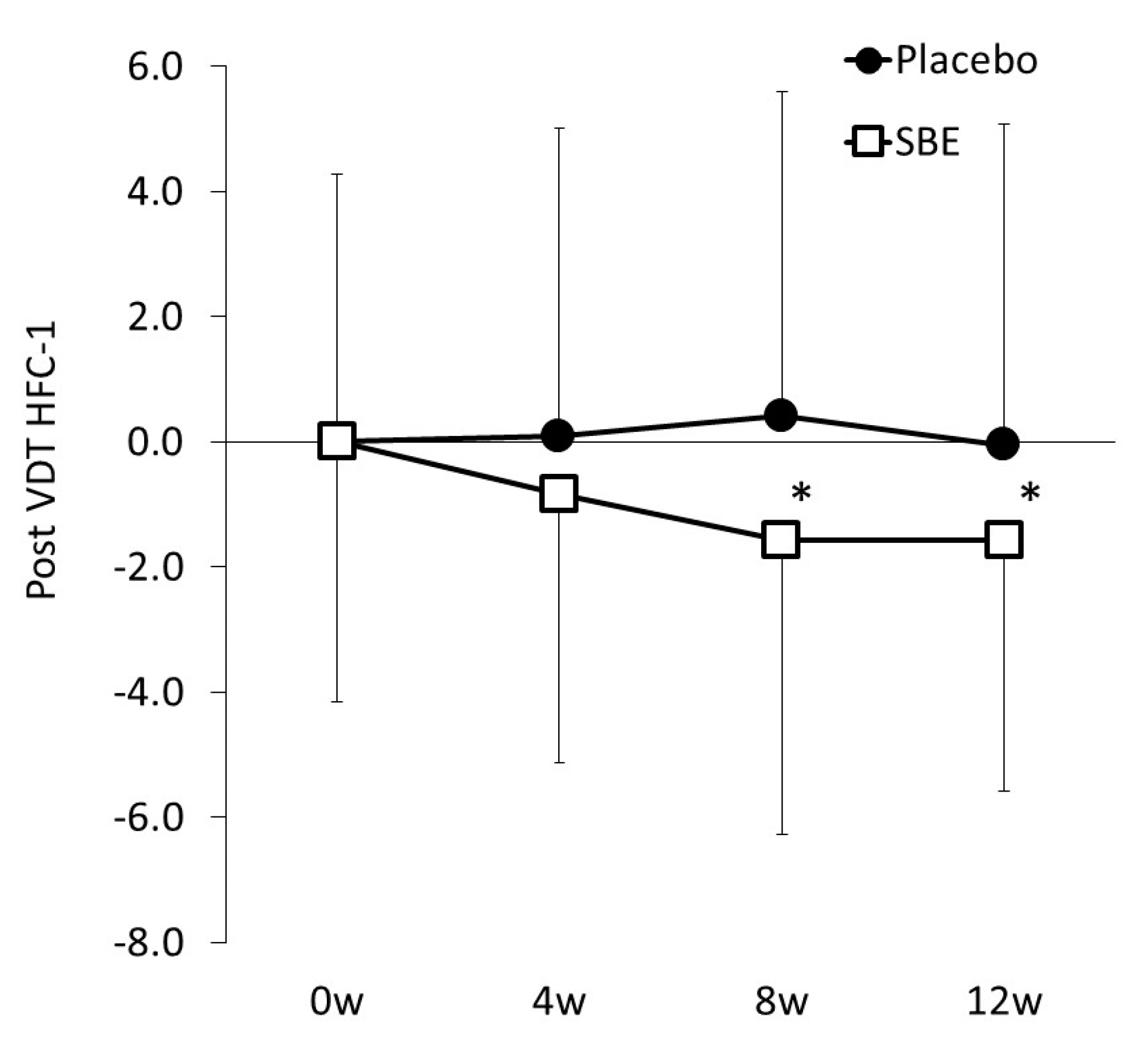
3.2. Impact on Ocular Fatigue Based on HFC-1 Values
3.3. Safety
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HFC | high-frequency component |
| SBE | standardized bilberry extract |
| VDT | visual display terminals |
| VMA | Vaccinium myrtillus anthocyanin |
References
- The Outline of the 2008 Survey on Technological Innovations and Labor. Ministry of Health, Labour and Welfare. Available online: https://www.mhlw.go.jp/toukei/list/48-20.html (accessed on 19 December 2019).
- The 2018 Survey on Usage Time and Information Behavior of Information and Communication Media. Ministry of Internal Affairs and Communications. Available online: https://www.soumu.go.jp/iicp/research/results/media_usage-time.html (accessed on 12 February 2020).
- The 2017 White Paper on Information Communications in Japan. Ministry of Internal Affairs and Communications. Available online: http://www.soumu.go.jp/johotsusintokei/whitepaper/ja/h29/pdf/index.html (accessed on 19 December 2019).
- Arai, S. Studies on Functional Foods in Japan—State of the Art. Biosci. Biotechnol. Biochem. 1996, 60, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Juadjur, A.; Mohn, C.; Schantz, M.; Baum, M.; Winterhalter, P.; Richling, E. Fractionation of an Anthocyanin-Rich Bilberry Extract and in Vitro Antioxidative Activity Testing. Food Chem. 2015, 167, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Gizzi, C.; Belcaro, G.; Gizzi, G.; Feragalli, B.; Dugall, M.; Luzzi, R.; Cornelli, U. Bilberry extracts are not created equal: The role of non anthocyanin fraction. Discovering the "dark side of the force" in a preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2418–2424. [Google Scholar] [PubMed]
- Kosehira, M.; Kitaichi, N. Ocular fatigue improvement by standard bilberry extract. Jpn. Pharmacol. Ther. 2015, 43, 397–403. [Google Scholar]
- Kosehira, M.; Takao, H.; Hayama, R.; Horie, Y.; Kitaichi, N. Recovery from VDT-load ocular fatigue by consumption of specific anthocyanin derived from bilberry fruits. Jpn. Pharmacol. Ther. 2015, 43, 1339–1346. [Google Scholar]
- Kosehira, M.; Kageyama, M.; Kamohara, S.; Kitaichi, N. Suppression of ocular fatigue by consumption of food containing standard bilberry extract: A randomized, double-blind, placebo-controlled, crossover trial. Jpn. Pharmacol. Ther. 2015, 43, 1741–1749. [Google Scholar]
- Kosehira, M.; Kitaichi, N. Clinical effects of evidence-based bilberry extract on asthenopia. In Annals of Nutrition and Metabolism IUNS 21st International Congress of Nutrition, Buenos Aires, Argentina, 15–20 October 2017; Carrera, M., Gil, A., Martinez, J.A., Eds.; The Nutrition Society: London, UK, 2017; 144/987; p. 1184. [Google Scholar]
- Campbell, F.W.; Robson, J.G.; Westheimer, G. Fluctuations of Accommodation under Steady Viewing Conditions. J. Physiol. 1959, 145, 579–594. [Google Scholar] [CrossRef]
- Kajita, M.; Umigai, N.; Nakano, T.; Amano, H.; Takeno, R.; Kajimoto, O. Effect on asthenopia of high-crocetin-content gardenia jasminoides ellis extraction. Jpn. J. Vis. Sci. 2007, 28, 77–84. [Google Scholar]
- Winn, B.; Pugh, J.R.; Gilmartin, B.; Owens, H. Arterial pulse modulates steady-state ocular accommodation. Curr. Eye Res. 1990, 9, 971–975. [Google Scholar] [CrossRef]
- Gray, L.S.; Winn, B.; Gilmartin, B. Effect of target luminance on microfluctuations of accommodation. Ophthalmic Physiol. Opt. 1993, 13, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Kajita, M. Clinical application of accommodation analyzer AA-2. J. Eye 2016, 33, 467–476. [Google Scholar]
- Kajita, M. Accommodative Response and Microfluctuation. Ophthalmology 1998, 40, 169–177. [Google Scholar]
- Kajita, M.; Ono, M.; Suzuki, S.; Kato, K. Accommodative microfluctuation in asthenopia caused by accommodative spasm. Fukushima J. Med. Sci. 2001, 47, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Kajita, M.; Shinohara, M.; Suzuki, S. An examination of changes in accommodative function by the use of IT devices. In Japan Ophthalmologists Association Collection of Achievements by the Computer Vision Syndrome and Environmental Factors Research Group 2002–2004; Japan Ophthalmologists Association: Japan, Tokyo, 2005; pp. 100–103. [Google Scholar]
- Kajita, M. The role of accommodative function in refractive correction: Aspects of asthenopia that could be understood through medical treatment. Jpn. J. Vis. Sci. 2012, 33, 138–146. [Google Scholar]
- Okita, Y.; Kimura, A.; Masuda, A.; Kasai, I.; Mimura, O. Influence of a glasses-type wearable device on visual function and eye fatigue. Neuro-Ophthalmol. Jpn. 2017, 34, 435–442. [Google Scholar]
- Terahara, N. The structure and properties of anthocyanin. In The Science of Anthocyanin, 1st ed.; Tsuda, T., Suda, I., Tsushida, T., Eds.; Kanpakusha: Tokyo, Japan, 2009; pp. 9–31. [Google Scholar]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef]
- Luo, H.; Lv, X.D.; Wang, G.E.; Li, Y.F.; Kurihara, H.; He, R.R. Anti-inflammatory effects of anthocyanins-rich extract from bilberry (Vaccinium myrtillus L.) on croton oil-induced ear edema and Propionibacterium acnes plus LPS-induced liver damage in mice. Int. J. Food Sci. Nutr. 2014, 65, 594–601. [Google Scholar] [CrossRef] [PubMed]
| SBE Food | Placebo Food | |
|---|---|---|
| SBE * | 240.0 | 0 |
| Dextrin | 27.2 | 51.0 |
| Starch | 45.6 | 193.8 |
| Calcium stearate | 22.1 | 22.1 |
| Silicon dioxide (fine) | 5.1 | 5.1 |
| Caramel color | 0 | 68 |
| Examination period | Screening | 0 week | 4 weeks | 8 weeks | 12 weeks |
|---|---|---|---|---|---|
| Ophthalmic examination (HFC) | ● | ● | ● | ● | ● |
| Anthropometric | ● | ● | |||
| Doctor’s questions | ● | ● | |||
| Blood test/Urinary test | ● | ● | |||
| Diary |  | ||||
 .
.| Variables | Placebo | SBE |
|---|---|---|
| Gender (male/female) (n) | 54 (16/38) | 55 (18/37) |
| Age (years) | 35.46 ± 6.96 | 36.18 ± 7.14 |
| Height (cm) | 163.72 ± 8.03 | 163.11 ± 8.71 |
| Body weight (kg) | 56.78 ± 8.71 | 57.77 ± 10.65 |
| Body mass index (kg/m2) | 21.06 ± 2.3 | 21.58 ± 3.13 |
| Placebo (n = 48) | SBE (n = 49) | p Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | ± | SD | Mean | ± | SD | |||
| 0w | Before VDT load | 50.60 | ± | 4.13 | 51.08 | ± | 3.84 | |
| Post VDT load | 50.72 | ± | 4.28 | 52.21 | ± | 4.16 | ||
| 4w | Before VDT load | 49.51 | ± | 4.13 | 50.90 | ± | 5.04 | |
| Post VDT load | 50.81 | ± | 4.92 | 51.37 | ± | 4.29 | 0.225 | |
| 8w | Before VDT load | 50.94 | ± | 4.93 | 50.08 | ± | 4.68 | |
| Post VDT load | 51.13 | ± | 5.19 | 50.64 | ± | 4.70 | * 0.014 | |
| 12w | Before VDT load | 50.32 | ± | 4.33 | 50.59 | ± | 4.20 | |
| Post VDT load | 50.67 | ± | 5.13 | 50.65 | ± | 4.03 | * 0.017 | |
| Adverse Events | Placebo (n = 54) | SBE (n = 55) |
|---|---|---|
| Common cold | 12 | 10 |
| Headaches | 11 | 18 |
| Digestive symptoms (stomachache or diarrhea) | 6 | 6 |
| Menstrual pain | 3 | 4 |
| Other | 17 | 24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosehira, M.; Machida, N.; Kitaichi, N. A 12-Week-Long Intake of Bilberry Extract (Vaccinium myrtillus L.) Improved Objective Findings of Ciliary Muscle Contraction of the Eye: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial. Nutrients 2020, 12, 600. https://doi.org/10.3390/nu12030600
Kosehira M, Machida N, Kitaichi N. A 12-Week-Long Intake of Bilberry Extract (Vaccinium myrtillus L.) Improved Objective Findings of Ciliary Muscle Contraction of the Eye: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial. Nutrients. 2020; 12(3):600. https://doi.org/10.3390/nu12030600
Chicago/Turabian StyleKosehira, Marie, Naomichi Machida, and Nobuyoshi Kitaichi. 2020. "A 12-Week-Long Intake of Bilberry Extract (Vaccinium myrtillus L.) Improved Objective Findings of Ciliary Muscle Contraction of the Eye: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial" Nutrients 12, no. 3: 600. https://doi.org/10.3390/nu12030600
APA StyleKosehira, M., Machida, N., & Kitaichi, N. (2020). A 12-Week-Long Intake of Bilberry Extract (Vaccinium myrtillus L.) Improved Objective Findings of Ciliary Muscle Contraction of the Eye: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial. Nutrients, 12(3), 600. https://doi.org/10.3390/nu12030600




