Net Absorption and Metabolism of β-Hydroxy- β-Methyl Butyrate during Late Gestation in a Pig Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Surgical Procedures and Housing
2.2. Diets and Feeding
2.3. Sampling and Data Collection
2.4. Analytical Procedures
2.5. Calculations and Statistical Analysis
(mmol/mL) × urine volume (mL/d) − HMB concentration in CON sow’s urine
(mmol/mL) × urine volume (mL/d)] × 118.13 (g/mol) × 0.001 (mol/mmol)
HMB intake (g/d) × 100
(%)]
(mmol/g) × 118.13 (g/mol) × 0.001 (mol/mmol)
(g/d) × 100
intake (%) − Net portal recovery of HMB (% of intake)
in colostrum/milk (mmol/L) × 118.13 (g/mol) × 1 (kg/L) × 0.001 (mol/mmol)
through colostrum/milk (g/d)/HMB intake (g/d) × 100
118.13 (g/mol) × 0.001 (mol/mmol)
HMB intake (g/d) × 100
3. Results
3.1. Feed intake, Sow Performance and Digestibility
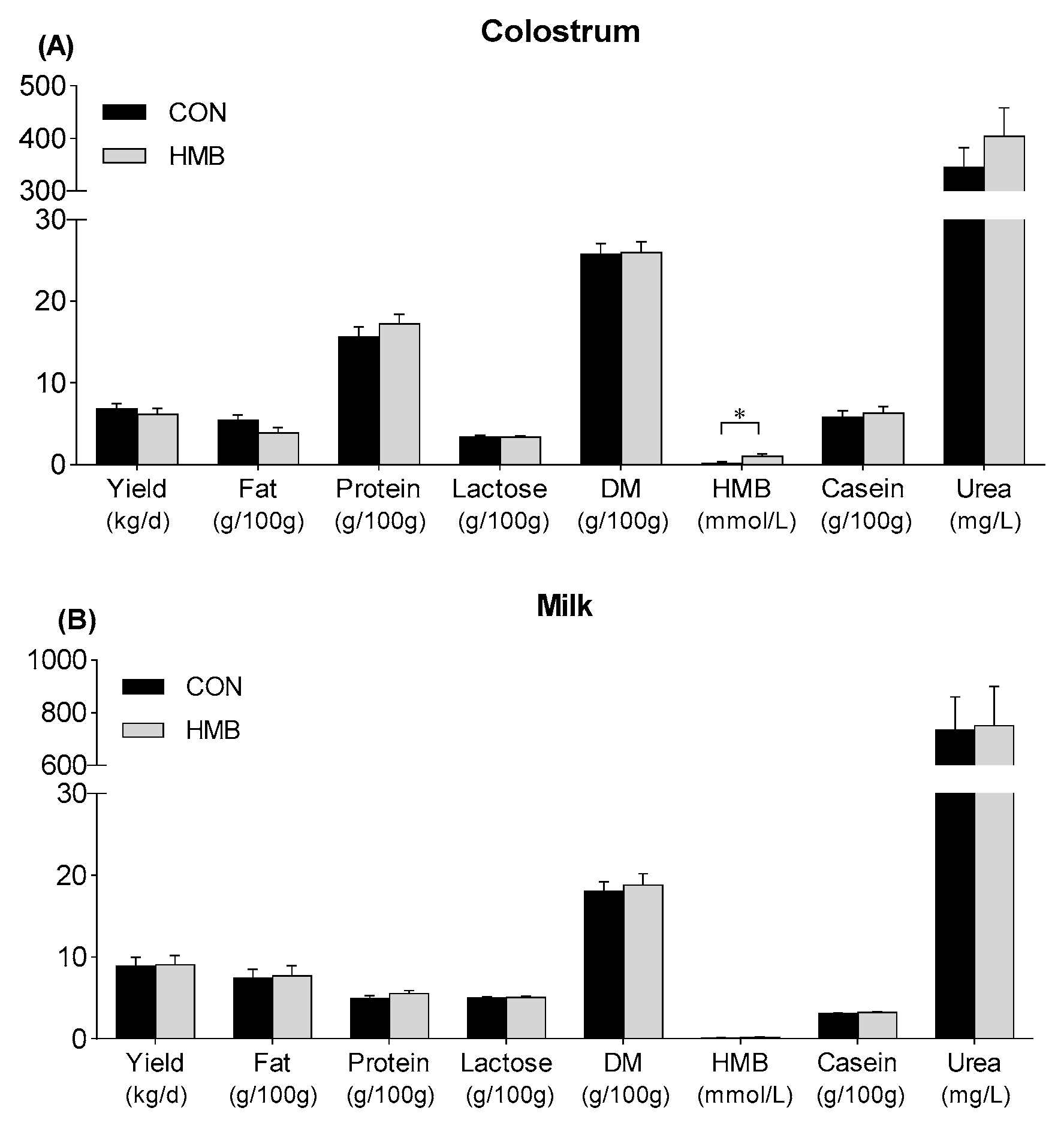
3.2. Yield and Composition of Colostrum and Milk
3.3. Arterial Variables
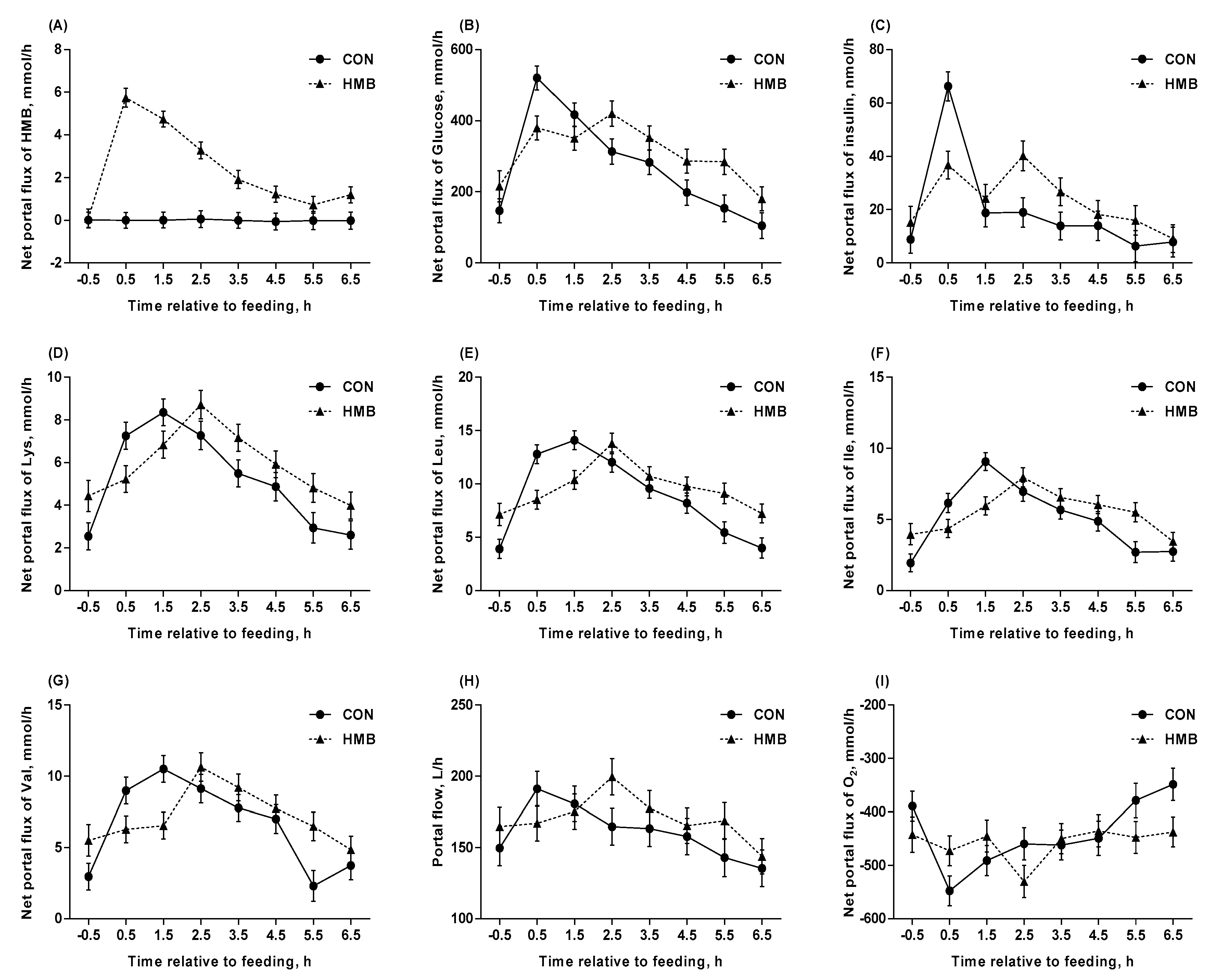
3.4. Blood Flow, Net Portal Flux, and Net Portal Recovery
3.5. Net Hepatic Flux
3.6. Femoral Extraction
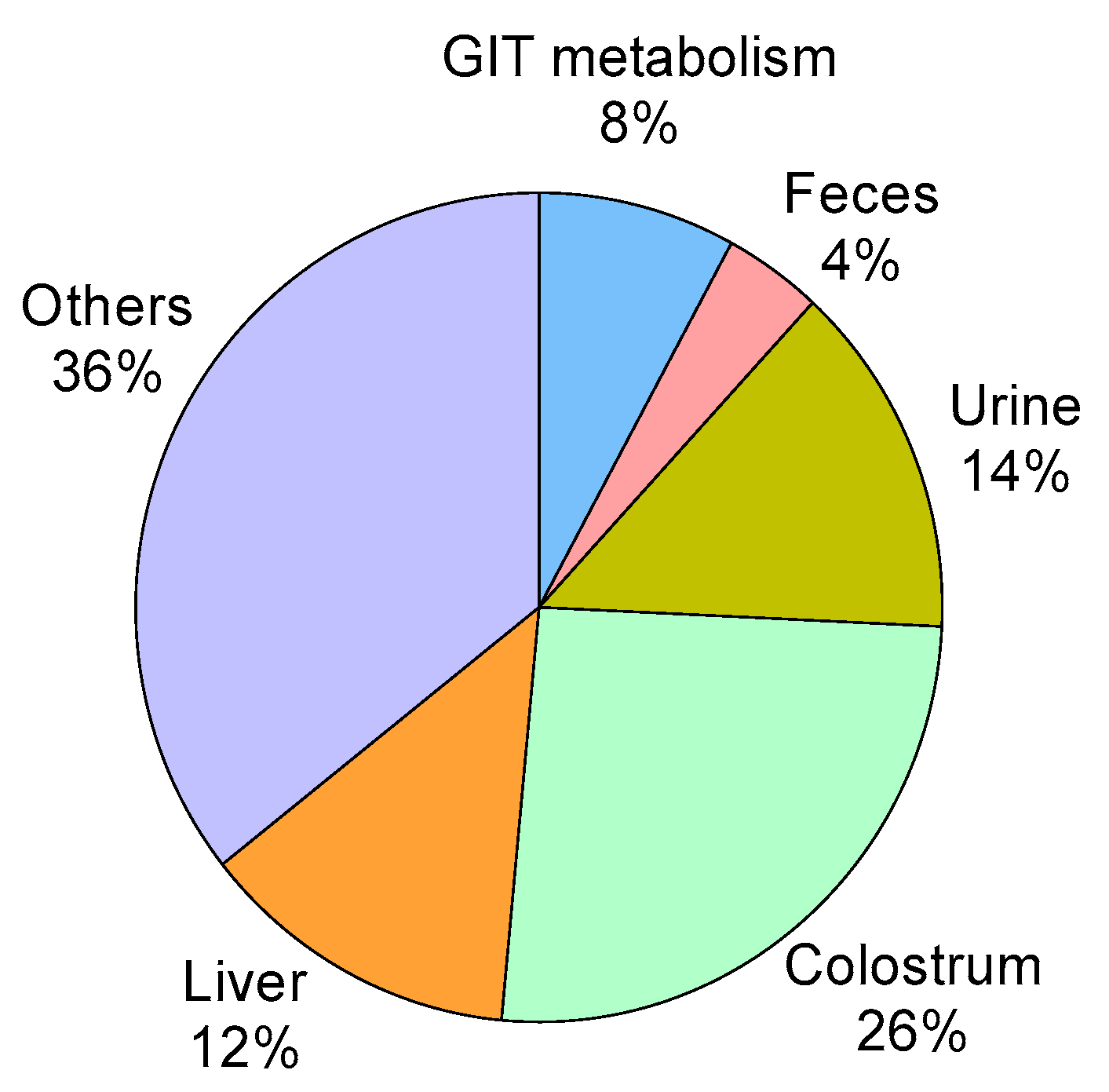
3.7. HMB Metabolism
4. Discussion
4.1. Effects of HMB Supplementation on Reproductive Performance in Late Gestation
4.2. Effects of HMB Supplementation on Blood Flow and O2 Consumption
4.3. Effects of HMB Supplementation on Amino Acids Metabolism
4.4. Metabolic Fate of HMB
4.5. Effects of HMB Supplementation on Glucogenic, Ketogenic Substrates and Insulin Secretion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nissen, S.; Faidley, T.D.; Zimmerman, D.R.; Izard, R.; Fisher, C.T. Colostral milk fat percentage and pig performance are enhanced by feeding the leucine metabolite beta-hydroxy-beta-methyl butyrate to sows. J. Anim. Sci. 1994, 72, 2331–2337. [Google Scholar] [CrossRef]
- Nissen, S.L.; Abumrad, N.N. Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB). J. Nutr. Biochem. 1997, 8, 300–311. [Google Scholar] [CrossRef]
- Wilson, G.J.; Wilson, J.M.; Manninen, A.H. Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: A review. Nutr. Metab. 2008, 5, 1. [Google Scholar] [CrossRef]
- Portal, S.; Eliakim, A.; Nemet, D.; Halevy, O.; Zadik, Z. Effect of HMB supplementation on body composition, fitness, hormonal profile and muscle damage indices. J. Pediatr. Endocrinol. Metab. 2010, 23, 641–650. [Google Scholar] [CrossRef]
- Flummer, C.; Theil, P.K. Effect of beta-hydroxy beta-methyl butyrate supplementation of sows in late gestation and lactation on sow production of colostrum and milk and piglet performance. J. Anim. Sci. 2012, 90, 372–374. [Google Scholar] [CrossRef]
- Van Koevering, M.; Nissen, S. Oxidation of leucine and alpha-ketoisocaproate to beta-hydroxy-beta-methylbutyrate in vivo. Am. J. Physiol. 1992, 262, E27–E31. [Google Scholar] [CrossRef]
- Soumeh, E.A.; van Milgen, J.; Sloth, N.M.; Corrent, E.; Poulsen, H.D.; Nørgaard, J.V. The optimum ratio of standardized ileal digestible isoleucine to lysine for 8–15kg pigs. Anim. Feed Sci. Technol. 2014, 198, 158–165. [Google Scholar] [CrossRef]
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef]
- Soumeh, E.A.; Van Milgen, J.; Sloth, N.M.; Corrent, E.; Poulsen, H.D.; Norgaard, J.V. The optimum ratio of standardized ileal digestible leucine to lysine for 8 to 12 kg female pigs. J. Anim. Sci. 2015, 93, 2218–2224. [Google Scholar] [CrossRef]
- Soumeh, E.A.; van Milgen, J.; Sloth, N.M.; Corrent, E.; Poulsen, H.D.; Norgaard, J.V. Requirement of standardized ileal digestible valine to lysine ratio for 8- to 14-kg pigs. Animal 2015, 9, 1312–1318. [Google Scholar] [CrossRef]
- Tatara, M.R.; Śliwa, E.; Krupski, W. Prenatal programming of skeletal development in the offspring: Effects of maternal treatment with β-hydroxy-β-methylbutyrate (HMB) on femur properties in pigs at slaughter age. Bone 2007, 40, 1615–1622. [Google Scholar] [CrossRef]
- Wan, H.F.; Zhu, J.T.; Shen, Y.; Xiang, X.; Yin, H.J.; Fang, Z.F.; Che, L.Q.; Lin, Y.; Xu, S.Y.; Feng, B.; et al. Effects of Dietary Supplementation of beta-hydroxy-beta-methylbutyrate on Sow Performance and mRNA Expression of Myogenic Markers in Skeletal Muscle of Neonatal Piglets. Reprod. Domest. Anim. 2016, 51, 135–142. [Google Scholar] [CrossRef]
- Sangild, P.T. Gut responses to enteral nutrition in preterm infants and animals. Exp. Biol. Med. 2006, 231, 1695–1711. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Norgaard, J.V.; Wamberg, S.; Engbaek, M.; Fernandez, J.A.; Zacho, H.D.; Poulsen, H.D. Absorption and metabolism of benzoic acid in growing pigs. J. Anim. Sci. 2009, 87, 2815–2822. [Google Scholar] [CrossRef][Green Version]
- Flummer, C.; Lyby, H.; Storli, K.S.; Bjerre-Harpoth, V.; Nielsen, B.M.; Kramer, M.; Rojen, B.A.; Kristensen, N.B.; Theil, P.K. Effects of beta-hydroxy beta-methyl butyrate supplementation to sows in late gestation on absorption and hepatic metabolism of glucose and amino acids during transition. J. Anim. Sci. 2012, 90, 146–148. [Google Scholar] [CrossRef]
- Theil, P.K.; Lauridsen, C.; Quesnel, H. Neonatal piglet survival: Impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal 2014, 8, 1021–1030. [Google Scholar] [CrossRef]
- Stoldt, W. Proposal for simplifying fat determination in food. Fats Soaps 1952, 54, 206–207. [Google Scholar]
- Harvey, R.B.; Brothers, A.J. Renal Extraction of Para-Aminohippurate and Creatinine Measured by Continuous in Vivo Sampling of Arterial and Renal-Vein Blood. Ann. N. Y. Acad. Sci. 1962, 102, 46–54. [Google Scholar] [CrossRef]
- Marsh, W.H.; Fingerhut, B.; Miller, H. Automated and Manual Direct Methods for the Determination of Blood Urea. Clin. Chem. 1965, 11, 624–627. [Google Scholar] [CrossRef]
- Kristensen, N.B. Quantification of whole blood short-chain fatty acids by gas chromatographic determination of plasma 2-chloroethyl derivatives and correction for dilution space in erythrocytes. Acta Agric. Scand. A-Anim. Sci. 2000, 50, 231–236. [Google Scholar] [CrossRef]
- Krogh, U.; Oksbjerg, N.; Storm, A.C.; Feyera, T.; Theil, P.K. Mammary nutrient uptake in multiparous sows fed supplementary arginine during gestation and lactation. J. Anim. Sci. 2017, 95, 2517–2532. [Google Scholar] [CrossRef]
- Theil, P.K.; Nielsen, T.T.; Kristensen, N.B.; Labouriau, R.; Danielsen, V.; Lauridsen, C.; Jakobsen, K.J.A.A.S. Section A-Animal Science. Estimation of milk production in lactating sows by determination of deuterated water turnover in three piglets per litter. Acta Agric. Scand. A-Anim. Sci. 2002, 52, 221–232. [Google Scholar]
- Stein, H.H.; Seve, B.; Fuller, M.F.; Moughan, P.J.; de Lange, C.F.M. Invited review: Amino acid bioavailability and digestibility in pig feed ingredients: Terminology and application. J. Anim. Sci. 2007, 85, 172–180. [Google Scholar] [CrossRef]
- Hu, L.; Kristensen, N.B.; Che, L.; Wu, D.; Theil, P.K. Net absorption and liver metabolism of amino acids and heat production of portal-drained viscera and liver in multiparous sows during transition and lactation. J. Anim. Sci. Biotechnol. 2020, 11. [Google Scholar] [CrossRef]
- Feyera, T.; Zhou, P.; Pedersen, T.F.; Larsen, U.K.; Theil, P.K. Comparison of urine production measured by total collection and para-aminohippuric acid infusion methods. In Proceedings of the 14th International Symposium on Digestive Physiology of Pigs (DPP2018), Brisbane, Australia, 21–24 August 2018. [Google Scholar]
- Feyera, T.; Zhou, P.; Nuntapaitoon, M.; Sorensen, K.U.; Krogh, U.; Bruun, T.S.; Purup, S.; Jorgensen, H.; Poulsen, H.D.; Theil, P.K. Mammary metabolism and colostrogenesis in sows during late gestation and the colostral period. J. Anim. Sci. 2019, 97, 231–245. [Google Scholar] [CrossRef]
- Molfino, A.; Gioia, G.; Rossi Fanelli, F.; Muscaritoli, M. Beta-hydroxy-beta-methylbutyrate supplementation in health and disease: A systematic review of randomized trials. Amino Acids 2013, 45, 1273–1292. [Google Scholar] [CrossRef]
- Holecek, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef]
- Szczesniak, K.A.; Ostaszewski, P.; Fuller, J.C., Jr.; Ciecierska, A.; Sadkowski, T. Dietary supplementation of beta-hydroxy-beta-methylbutyrate in animals—A review. J. Anim. Physiol. Anim. Nutr. 2015, 99, 405–417. [Google Scholar] [CrossRef]
- Tatara, M.R.; Krupski, W.; Tymczyna, B.; Studzinski, T. Effects of combined maternal administration with alpha-ketoglutarate (AKG) and beta-hydroxy-beta-methylbutyrate (HMB) on prenatal programming of skeletal properties in the offspring. Nutr. Metab. 2012, 9, 39. [Google Scholar] [CrossRef]
- Gonzalez-Valero, L.; Rodriguez-Lopez, J.; Lachica, M.; Fernandez-Figares, I. Differences in portal appearance of lysine and methionine in Iberian and Landrace pigs. J. Anim. Sci. 2012, 90, 110–112. [Google Scholar] [CrossRef]
- Tan, B.; Li, X.; Wu, G.; Kong, X.; Liu, Z.; Li, T.; Yin, Y. Dynamic changes in blood flow and oxygen consumption in the portal-drained viscera of growing pigs receiving acute administration of (L)-arginine. Amino Acids 2012, 43, 2481–2489. [Google Scholar] [CrossRef]
- Vukovich, M.D.; Dreifort, G.D. Effect of beta-hydroxy beta-methylbutyrate on the onset of blood lactate accumulation and V (O)(2) peak in endurance-trained cyclists. J. Strength Cond. Res. 2001, 15, 491–497. [Google Scholar]
- Simoes Nunes, C.; Galibois, I.; Rerat, A.; Savoie, L.; Vaugelade, P. Hepatic and portal-drained viscera balances of amino acids, insulin, glucagon and gastrin in the pig after ingestion of casein or rapeseed proteins. Reprod. Nutr. Dev. 1991, 31, 217–231. [Google Scholar] [CrossRef]
- Groves, A.M.; Kuschel, C.A.; Knight, D.B.; Skinner, J.R. Relationship between blood pressure and blood flow in newborn preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F29–F32. [Google Scholar] [CrossRef]
- Schouten, W.R.; Briel, J.W.; Auwerda, J.J.A. Relationship between Anal Pressure and Anodermal Blood-Flow—The Vascular Pathogenesis of Anal Fissures. Dis. Colon Rectum 1994, 37, 664–669. [Google Scholar] [CrossRef]
- Nissen, S.; Sharp, R.L.; Panton, L.; Vukovich, M.; Trappe, S.; Fuller, J.C. beta-hydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J. Nutr. 2000, 130, 1937–1945. [Google Scholar] [CrossRef]
- McCarron, D.A.; Reusser, M.E. Finding consensus in the dietary calcium-blood pressure debate. J. Am. Coll. Nutr. 1999, 18, 398S–405S. [Google Scholar] [CrossRef]
- Birkett, N.J. Comments on a meta-analysis of the relation between dietary calcium intake and blood pressure. Am. J. Epidemiol. 1998, 148, 223–228. [Google Scholar] [CrossRef]
- Tatara, M.R. Effect of beta-hydroxy-beta-methylbutyrate (HMB) administration on volumetric bone mineral density, and morphometric and mechanical properties of tibia in male turkeys. J. Anim. Physiol. Anim. Nutr. 2009, 93, 669–677. [Google Scholar] [CrossRef]
- Tatara, M.R.; Sliwa, E.; Krupski, W.; Worzakowska, M. 3-Hydroxy-3-methylbutyrate administration diminishes fundectomy-induced osteopenia of the lumbar spine in pigs. Nutrition 2008, 24, 753–760. [Google Scholar] [CrossRef]
- Stoll, B.; Henry, J.; Reeds, P.J.; Yu, H.; Jahoor, F.; Burrin, D.G. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J. Nutr. 1998, 128, 606–614. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef]
- Stoll, B.; Burrin, D.G. Measuring splanchnic amino acid metabolism in vivo using stable isotopic tracers. J. Anim. Sci. 2006, 84, E60–E72. [Google Scholar] [CrossRef]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef]
- Holecek, M.; Muthny, T.; Kovarik, M.; Sispera, L. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem. Toxicol. 2009, 47, 255–259. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Wu, G. Metabolic functions of the porcine liver. In Nutritional Physiology of Pigs; SEGES: Copenhagen, Denmark, 2012; pp. 1–17. Available online: https://svineproduktion.dk/Services/Undervisningsmateriale2 (accessed on 20 February 2020).
- Nissen, S.; Sharp, R.; Ray, M.; Rathmacher, J.; Rice, D.; Fuller, J., Jr.; Connelly, A.; Abumrad, N.J.J.O.A.P. Effect of leucine metabolite β-hydroxy-β-methylbutyrate on muscle metabolism during resistance-exercise training. J. Appl. Physiol. 1996, 81, 2095–2104. [Google Scholar] [CrossRef]
- Vukovich, M.D.; Slater, G.; Macchi, M.B.; Turner, M.J.; Fallon, K.; Boston, T.; Rathmacher, J. beta-hydroxy-beta-methylbutyrate (HMB) kinetics and the influence of glucose ingestion in humans. J. Nutr. Biochem. 2001, 12, 631–639. [Google Scholar] [CrossRef]
- Fuller, J.C., Jr.; Sharp, R.L.; Angus, H.F.; Baier, S.M.; Rathmacher, J.A. Free acid gel form of beta-hydroxy-beta-methylbutyrate (HMB) improves HMB clearance from plasma in human subjects compared with the calcium HMB salt. Br. J. Nutr. 2011, 105, 367–372. [Google Scholar] [CrossRef]
- Yonamine, C.Y.; Teixeira, S.S.; Campello, R.S.; Gerlinger-Romero, F.; Rodrigues, C.F., Jr.; Guimaraes-Ferreira, L.; Machado, U.F.; Nunes, M.T. Beta hydroxy beta methylbutyrate supplementation impairs peripheral insulin sensitivity in healthy sedentary Wistar rats. Acta Physiol. 2014, 212, 62–74. [Google Scholar] [CrossRef]
- Sharawy, M.H.; El-Awady, M.S.; Megahed, N.; Gameil, N.M.J.C.J.O.P. The ergogenic supplement β-hydroxy-β-methylbutyrate (HMB) attenuates insulin resistance through suppressing GLUT-2 in rat liver. Can. J. Physiol. Pharm. 2015, 94, 488–497. [Google Scholar] [CrossRef]
- Bruckbauer, A.; Zemel, M.B.; Thorpe, T.; Akula, M.R.; Stuckey, A.C.; Osborne, D.; Martin, E.B.; Kennel, S.; Wall, J.S. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr. Metab. 2012, 9, 77. [Google Scholar] [CrossRef]
- Cheng, W.; Phillips, B.; Abumrad, N. Beta-hydroxy-beta-methyl butyrate increases fatty acid oxidation by muscle cells. FASEB J. 1997, 11, 2204. [Google Scholar]
- Cottrell, E.C.; Ozanne, S.E. Developmental programming of energy balance and the metabolic syndrome. Proc. Nutr. Soc. 2007, 66, 198–206. [Google Scholar] [CrossRef]
- Rittig, N.; Bach, E.; Thomsen, H.H.; Moller, A.B.; Hansen, J.; Johannsen, M.; Jensen, E.; Serena, A.; Jorgensen, J.O.; Richelsen, B.; et al. Anabolic effects of leucine-rich whey protein, carbohydrate, and soy protein with and without beta-hydroxy-beta-methylbutyrate (HMB) during fasting-induced catabolism: A human randomized crossover trial. Clin. Nutr. 2017, 36, 697–705. [Google Scholar] [CrossRef]
- Bruckbauer, A.; Zemel, M.B. Synergistic effects of metformin, resveratrol, and hydroxymethylbutyrate on insulin sensitivity. Diabetes Metab. Syndr. Obes. 2013, 6, 93–102. [Google Scholar] [CrossRef][Green Version]


| CON | HMB | SEM | p-Values | |
|---|---|---|---|---|
| Sows | ||||
| HMB intake from d –10 to farrowing, g/d | 0.0 | 3.2 | 0.03 | <0.001 |
| Overall feed intake, kg/d | 3.80 | 3.85 | 0.37 | 0.92 |
| Water intake, L/d | 26.0 | 24.8 | 6.9 | 0.89 |
| Sow BW at d –10, kg | 265 | 259 | 7.3 | 0.58 |
| Sow BW loss from d –10 to 2, kg | 20.3 | 15.0 | 2.9 | 0.23 |
| Sow BW loss from d 2 to 28, kg | 21.0 | 19.0 | 5.8 | 0.81 |
| ATTD, % | ||||
| DM | 83.5 | 82.2 | 1.0 | 0.28 |
| GE | 83.0 | 81.6 | 1.1 | 0.31 |
| Nitrogen | 84.2 | 83.5 | 1.3 | 0.63 |
| Fat | 62.7 | 58.4 | 2.2 | 0.13 |
| Crude Fiber | 68.2 | 65.9 | 2.4 | 0.45 |
| Piglets | ||||
| Mean Piglet BW at birth, kg | 1.24 | 1.20 | 0.04 | 0.45 |
| Litter gain, kg/d | 2.38 | 2.36 | 0.17 | 0.92 |
| Litter size at birth | 18.8 | 15.5 | 1.8 | 0.25 |
| Litter size at d 2 (After cross-foster) | 12.0 | 12.3 | 0.2 | 0.29 |
| Litter size at d 28 | 11.7 | 11.0 | 0.7 | 0.56 |
| Item | Treatment | SEM | DIM | SEM | p-Values | ||||
|---|---|---|---|---|---|---|---|---|---|
| CON | HMB | 3 | 17 | Trt | DIM | Trt×DIM | |||
| Essential AA, g/kg milk | |||||||||
| Lysine | 3.65 | 3.86 | 0.12 | 4.20 | 3.31 | 0.11 | 0.20 | <0.001 | 0.80 |
| Methionine | 0.93 | 1.01 | 0.03 | 1.07 | 0.87 | 0.02 | 0.047 | <0.001 | 0.75 |
| Threonine | 2.06 | 2.17 | 0.08 | 2.41 | 1.83 | 0.07 | 0.31 | <0.001 | 0.71 |
| Isoleucine | 2.12 | 2.24 | 0.06 | 2.45 | 1.91 | 0.06 | 0.17 | <0.001 | 0.72 |
| Leucine | 4.19 | 4.43 | 0.13 | 4.85 | 3.78 | 0.12 | 0.19 | <0.001 | 0.70 |
| Valine | 2.74 | 2.89 | 0.09 | 3.18 | 2.45 | 0.09 | 0.24 | <0.001 | 0.68 |
| Histidine | 1.33 | 1.41 | 0.04 | 1.54 | 1.20 | 0.04 | 0.17 | <0.001 | 0.86 |
| Phenylalanine | 1.98 | 2.07 | 0.07 | 2.27 | 1.77 | 0.06 | 0.32 | <0.001 | 0.62 |
| Nonessential AA, g/kg milk | |||||||||
| Alanine | 1.80 | 1.89 | 0.06 | 2.12 | 1.57 | 0.06 | 0.26 | <0.001 | 0.60 |
| Aspartate | 4.11 | 4.39 | 0.12 | 4.77 | 3.73 | 0.12 | 0.12 | <0.001 | 0.72 |
| Cysteine | 0.72 | 0.74 | 0.03 | 0.84 | 0.62 | 0.03 | 0.63 | <0.001 | 0.31 |
| Glutamate | 9.80 | 10.55 | 0.28 | 11.00 | 9.36 | 0.26 | 0.07 | 0.002 | 0.99 |
| Glycine | 1.60 | 1.71 | 0.05 | 1.81 | 1.50 | 0.04 | 0.12 | <0.001 | 0.94 |
| Proline | 5.32 | 5.78 | 0.17 | 5.92 | 5.18 | 0.16 | 0.06 | 0.008 | 0.90 |
| Serine | 2.68 | 2.88 | 0.10 | 3.10 | 2.47 | 0.09 | 0.16 | <0.001 | 0.85 |
| Arginine | 2.37 | 2.52 | 0.08 | 2.76 | 2.14 | 0.08 | 0.18 | <0.001 | 0.76 |
| Item | Treatment | SEM | DIM | SEM | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | HMB | –10 | –3 | Trt | DIM | Trt×DIM | ST | Trt×ST | |||
| Whole blood | |||||||||||
| Hematocrit, % | 27.6 | 28.0 | 0.7 | 27.5 | 28.1 | 0.5 | 0.65 | 0.30 | 0.004 | <0.001 | 0.04 |
| O2, mmol/L | 5.9 | 5.9 | 0.1 | 6.0 | 5.9 | 0.1 | 0.76 | 0.21 | 0.005 | 0.002 | 0.15 |
| CO2, mmol/L | 26.7 | 27.0 | 0.3 | 27.2 | 26.5 | 0.2 | 0.52 | <0.001 | 0.24 | <0.001 | 0.49 |
| Plasma | |||||||||||
| HMB, µmol/L | 1.6 | 82.7 | 3.8 | 40.4 | 43.9 | 3.8 | <0.001 | 0.53 | 0.47 | <0.001 | <0.001 |
| Insulin, pmol/L | 57.1 | 58.4 | 7.1 | 66.6 | 48.8 | 6.4 | 0.91 | 0.03 | 0.66 | <0.001 | <0.001 |
| Glucose, mmol/L | 5.4 | 5.2 | 0.1 | 5.2 | 5.3 | 0.1 | 0.28 | 0.24 | 0.13 | <0.001 | <0.001 |
| Lactate, mmol/L | 1.3 | 1.2 | 0.1 | 1.2 | 1.3 | 0.1 | 0.78 | 0.44 | 0.42 | <0.001 | 0.002 |
| Urea, mmol/L | 4.0 | 3.7 | 0.4 | 3.8 | 3.9 | 0.3 | 0.64 | 0.47 | 0.21 | <0.001 | 0.18 |
| NEFA, µmol/L | 167 | 114 | 21 | 121 | 160 | 19 | 0.13 | 0.08 | 0.86 | 0.006 | 0.71 |
| TG, µmol/L | 699 | 604 | 28 | 630 | 672 | 25 | 0.046 | 0.21 | 0.06 | 0.03 | 0.06 |
| Essential AA, µmol/L | |||||||||||
| Lysine | 82 | 106 | 8.7 | 98 | 90 | 6.8 | 0.09 | 0.21 | 0.27 | <0.001 | 0.003 |
| Methionine | 30 | 26 | 1.9 | 28 | 27 | 1.5 | 0.22 | 0.44 | 0.07 | <0.001 | 0.17 |
| Threonine | 180 | 184 | 19.1 | 174 | 190 | 14.2 | 0.90 | 0.11 | 0.83 | <0.001 | 0.008 |
| Tryptophan | 66 | 72 | 4.4 | 69 | 69 | 3.2 | 0.42 | 0.49 | 0.86 | <0.001 | 0.009 |
| Isoleucine | 163 | 161 | 7.7 | 166 | 159 | 5.8 | 0.86 | 0.06 | 0.03 | <0.001 | 0.006 |
| Leucine | 198 | 188 | 13.6 | 205 | 180 | 9.8 | 0.63 | <0.001 | 0.01 | <0.001 | 0.02 |
| Valine | 319 | 309 | 19.9 | 340 | 288 | 14.4 | 0.72 | <0.001 | 0.06 | <0.001 | 0.006 |
| Histidine | 86 | 78 | 5.5 | 82 | 82 | 4.0 | 0.32 | 0.70 | 0.71 | <0.001 | 0.30 |
| Phenylalanine | 111 | 100 | 7.7 | 107 | 103 | 5.5 | 0.34 | 0.06 | 0.73 | <0.001 | 0.003 |
| Nonessential AA, µmol/L | |||||||||||
| Alanine | 573 | 570 | 48.6 | 563 | 581 | 35.8 | 0.97 | 0.40 | 0.71 | <0.001 | <0.001 |
| Asparagine | 25 | 20 | 2.4 | 24 | 21 | 1.8 | 0.26 | 0.009 | 0.02 | <0.001 | <0.001 |
| Aspartate | 41 | 41 | 2.4 | 42 | 40 | 1.8 | 0.91 | 0.20 | 0.88 | <0.001 | 0.02 |
| Cysteine | 190 | 220 | 3.1 | 206 | 205 | 2.7 | <0.001 | 0.68 | 0.17 | 0.001 | 0.98 |
| Glutamate | 240 | 249 | 11.2 | 254 | 235 | 9.3 | 0.56 | 0.08 | 0.40 | <0.001 | 0.07 |
| Glycine | 917 | 886 | 39.8 | 875 | 928 | 32.6 | 0.60 | 0.15 | 0.71 | <0.001 | 0.43 |
| Proline | 483 | 503 | 39.3 | 511 | 476 | 30.3 | 0.74 | 0.18 | 0.10 | <0.001 | 0.14 |
| Serine | 151 | 138 | 9.2 | 148 | 142 | 6.7 | 0.35 | 0.08 | 0.02 | <0.001 | 0.003 |
| Tyrosine | 119 | 116 | 8.4 | 121 | 114 | 6.1 | 0.82 | 0.03 | 0.86 | <0.001 | 0.009 |
| Glutamine | 276 | 241 | 14.2 | 276 | 241 | 11.4 | 0.13 | 0.009 | 0.20 | <0.001 | 0.04 |
| VFA, µmol/L | |||||||||||
| Acetate | 400 | 376 | 18.0 | 401 | 375 | 14.0 | 0.38 | 0.04 | 0.83 | <0.001 | 0.06 |
| Propionate | 2.5 | 3.7 | 0.8 | 4.6 | 1.6 | 0.8 | 0.28 | 0.008 | 0.94 | 0.54 | 0.65 |
| Butyrate | 6.7 | 7.1 | 1.1 | 6.5 | 7.4 | 0.8 | 0.82 | 0.15 | 0.92 | 0.34 | 0.05 |
| Isobutyrate | 9.4 | 8.4 | 1.1 | 9.4 | 8.3 | 0.8 | 0.55 | 0.08 | 0.37 | 0.006 | 0.39 |
| Isovalerate | 14.2 | 12.7 | 1.2 | 13.9 | 13.0 | 0.9 | 0.38 | 0.09 | 0.57 | 0.006 | 0.34 |
| Item | Treatment | SEM | DIM | SEM | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | HMB | –10 | –3 | Trt | DIM | Trt×DIM | ST | Trt×ST | |||
| Whole blood | |||||||||||
| Portal flow, L/h | 161 | 170 | 10 | 166 | 165 | 7 | 0.52 | 0.69 | 0.01 | <0.001 | 0.03 |
| O2, mmol/h | −441 | −458 | 13 | −457 | −442 | 12 | 0.42 | 0.30 | 0.21 | 0.001 | 0.04 |
| CO2, mmol/h | 531 | 558 | 22 | 557 | 532 | 22 | 0.40 | 0.36 | 0.10 | 0.09 | 0.29 |
| Plasma | |||||||||||
| HMB, mmol/h | 0.0 | 2.4 | 0.1 | 1.2 | 1.2 | 0.1 | <0.001 | 0.80 | 0.73 | <0.001 | <0.001 |
| Insulin, nmol/h | 19 | 23 | 3.1 | 24 | 18 | 2.5 | 0.37 | 0.009 | 0.02 | <0.001 | <0.001 |
| Glucose, mmol/h | 267 | 309 | 21 | 286 | 290 | 18 | 0.29 | 0.93 | 0.50 | <.0001 | <0.001 |
| Lactate, mmol/h | 40 | 42 | 4.8 | 47 | 36 | 3.8 | 0.85 | <0.001 | 0.21 | <.0001 | 0.19 |
| Urea, mmol/h | −5.6 | −3.0 | 1.7 | −3.5 | −5.1 | 1.3 | 0.30 | 0.25 | 0.40 | 0.28 | 0.64 |
| NEFA, mmol/h | −1.0 | 3.1 | 1.5 | 1.2 | 0.9 | 1.6 | 0.09 | 0.86 | 0.16 | 0.97 | 0.57 |
| TG, mmol/h | −0.1 | −3.5 | 2.7 | −2.8 | −0.8 | 2.8 | 0.44 | 0.64 | 0.85 | 0.51 | 0.60 |
| Essential AA, mmol/h | |||||||||||
| Lysine | 5.2 | 5.9 | 0.3 | 5.5 | 5.5 | 0.3 | 0.20 | 0.82 | 0.12 | <0.001 | 0.004 |
| Methionine | 1.2 | 1.1 | 0.2 | 1.2 | 1.1 | 0.1 | 0.69 | 0.42 | 0.003 | <0.001 | 0.001 |
| Threonine | 3.9 | 4.4 | 0.3 | 4.1 | 4.2 | 0.3 | 0.35 | 0.98 | 0.40 | <0.001 | <0.001 |
| Tryptophan | 0.9 | 1.0 | 0.1 | 1.0 | 1.0 | 0.1 | 0.27 | 0.78 | 0.81 | <0.001 | 0.05 |
| Isoleucine | 5.0 | 5.5 | 0.3 | 5.4 | 5.1 | 0.3 | 0.32 | 0.49 | 0.28 | <0.001 | <0.001 |
| Leucine | 8.8 | 9.6 | 0.4 | 9.3 | 9.0 | 0.4 | 0.30 | 0.44 | 0.20 | <0.001 | <0.001 |
| Valine | 6.6 | 7.2 | 0.5 | 6.8 | 7.0 | 0.4 | 0.49 | 0.76 | 0.35 | <0.001 | 0.001 |
| Histidine | 2.3 | 2.3 | 0.2 | 2.4 | 2.2 | 0.2 | 0.85 | 0.57 | 0.27 | <0.001 | 0.01 |
| Phenylalanine | 5.2 | 5.6 | 0.3 | 5.5 | 5.3 | 0.2 | 0.41 | 0.33 | 0.13 | <0.001 | <0.001 |
| Nonessential AA, mmol/h | |||||||||||
| Alanine | 21.4 | 23.7 | 1.1 | 23.7 | 21.4 | 1.0 | 0.23 | 0.048 | 0.07 | <0.001 | <0.001 |
| Asparagine | 3.0 | 2.9 | 0.2 | 3.1 | 2.8 | 0.2 | 0.90 | 0.08 | 0.03 | <0.001 | <0.001 |
| Aspartate | 1.9 | 2.0 | 0.2 | 1.8 | 2.0 | 0.2 | 0.94 | 0.39 | 0.31 | <0.001 | 0.48 |
| Cysteine | 2.5 | 3.0 | 0.1 | 2.7 | 2.8 | 0.1 | 0.005 | 0.83 | 0.75 | <0.001 | 0.002 |
| Glutamate | −1.5 | −1.2 | 0.3 | −1.5 | −1.1 | 0.3 | 0.53 | 0.28 | 0.37 | 0.02 | 0.79 |
| Glycine | 18.0 | 19.3 | 1.4 | 19.2 | 18.1 | 1.2 | 0.54 | 0.31 | 0.19 | 0.002 | 0.74 |
| Proline | 11.2 | 12.7 | 0.9 | 12.3 | 11.7 | 0.8 | 0.27 | 0.37 | 0.08 | <0.001 | 0.03 |
| Serine | 6.8 | 8.0 | 0.5 | 7.5 | 7.2 | 0.4 | 0.16 | 0.33 | 0.17 | <0.001 | <0.001 |
| Tyrosine | 3.3 | 3.6 | 0.2 | 3.5 | 3.4 | 0.2 | 0.37 | 0.44 | 0.19 | <0.001 | <0.001 |
| Glutamine | 3.3 | 3.8 | 0.9 | 3.5 | 3.6 | 0.8 | 0.69 | 1.00 | 0.06 | <0.001 | 0.15 |
| VFA, mmol/h | |||||||||||
| Acetate | 122.2 | 104.1 | 6.4 | 108.9 | 117.4 | 5.3 | 0.09 | 0.11 | 0.77 | 0.42 | 0.65 |
| Propionate | 56.6 | 47.6 | 5.9 | 52.4 | 51.8 | 4.5 | 0.33 | 0.76 | 0.10 | 0.67 | 0.38 |
| Butyrate | 21.9 | 17.0 | 2.0 | 18.2 | 20.7 | 1.6 | 0.12 | 0.08 | 0.53 | 0.33 | 0.46 |
| Isobutyrate | 1.6 | 1.6 | 0.1 | 1.7 | 1.5 | 0.1 | 0.74 | 0.26 | 0.52 | 0.86 | 0.19 |
| Isovalerate | 1.2 | 1.1 | 0.1 | 1.2 | 1.0 | 0.1 | 0.40 | 0.29 | 0.15 | 0.72 | 0.13 |
| Item | Treatment | SEM | DIM | SEM | p-Values | ||||
|---|---|---|---|---|---|---|---|---|---|
| CON | HMB | –10 | –3 | Trt | DIM | Trt×DIM | |||
| Essential AA, % | |||||||||
| Lysine | 64.8 | 75.4 | 4.2 | 70.5 | 69.8 | 4.2 | 0.10 | 0.91 | 0.18 |
| Methionine | 49.0 | 45.5 | 6.0 | 48.8 | 45.7 | 6.0 | 0.69 | 0.72 | 0.049 |
| Threonine | 54.3 | 61.5 | 4.6 | 57.4 | 58.4 | 4.6 | 0.28 | 0.88 | 0.47 |
| Tryptophan | 62.9 | 72.0 | 4.1 | 67.8 | 67.1 | 4.1 | 0.14 | 0.90 | 0.67 |
| Isoleucine | 61.9 | 68.5 | 3.5 | 66.8 | 63.6 | 3.5 | 0.21 | 0.53 | 0.29 |
| Leucine | 63.2 | 70.8 | 3.2 | 68.3 | 65.7 | 3.2 | 0.11 | 0.58 | 0.25 |
| Valine | 61.3 | 68.1 | 4.1 | 64.1 | 65.4 | 4.1 | 0.27 | 0.82 | 0.42 |
| Histidine | 57.9 | 58.7 | 4.7 | 62.0 | 54.6 | 4.7 | 0.92 | 0.28 | 0.26 |
| Phenylalanine | 67.8 | 73.7 | 3.5 | 72.1 | 69.3 | 3.5 | 0.26 | 0.59 | 0.23 |
| Nonessential AA, % | |||||||||
| Alanine | 181.4 | 210.9 | 9.0 | 207.6 | 184.7 | 9.0 | 0.04 | 0.10 | 0.23 |
| Aspartate | 15.5 | 19.8 | 0.8 | 17.7 | 17.6 | 0.8 | 0.003 | 0.97 | 0.96 |
| Cysteine | 51.6 | 53.0 | 5.8 | 49.6 | 55.1 | 5.8 | 0.87 | 0.52 | 0.63 |
| Glutamate | −3.8 | −3.4 | 0.5 | −4.0 | −3.3 | 0.5 | 0.64 | 0.40 | 0.57 |
| Glycine | 126.0 | 140.6 | 8.2 | 137.3 | 129.2 | 8.2 | 0.23 | 0.50 | 0.33 |
| Proline | 65.8 | 77.2 | 5.5 | 73.6 | 69.3 | 5.5 | 0.17 | 0.59 | 0.26 |
| Serine | 58.6 | 69.8 | 4.4 | 65.6 | 62.9 | 4.4 | 0.10 | 0.67 | 0.37 |
| Tyrosine | 86.0 | 95.2 | 4.9 | 92.2 | 89.1 | 4.9 | 0.21 | 0.66 | 0.25 |
| Item | Treatment | SEM | DIM | SEM | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | HMB | –10 | –3 | Trt | DIM | Trt×DIM | ST | Trt×ST | |||
| Whole blood | |||||||||||
| O2, mmol/h | −487 | −617 | 56.6 | −567 | −537 | 53.2 | 0.19 | 0.72 | 0.31 | 0.75 | 0.75 |
| CO2#, mmol/h | 397 | 546 | 84.0 | 514 | 428 | 68.0 | 0.33 | 0.26 | 0.16 | 0.52 | 0.53 |
| RQ | 0.83 | 0.90 | 0.05 | 0.90 | 0.84 | 0.05 | 0.47 | 0.65 | 0.57 | 0.06 | 0.53 |
| Plasma | |||||||||||
| Hepatic vein, L/h | 197 | 260 | 12.0 | 226 | 232 | 12.1 | 0.002 | 0.76 | 0.43 | 0.49 | 0.92 |
| Hepatic artery, L/h | 38 | 90 | 15.7 | 60 | 68 | 14.8 | 0.06 | 0.70 | 0.87 | 1.00 | 1.00 |
| HMB, mmol/h | 0.00 | −0.11 | 0.36 | 0.58 | −0.69 | 0.35 | 0.44 | 0.03 | 0.02 | 0.24 | 0.26 |
| Insulin, nmol/h | −15.3 | −13.4 | 1.9 | −15.3 | −13.4 | 1.8 | 0.26 | 0.37 | 0.36 | <0.001 | <0.001 |
| Glucose, mmol/h | 19.3 | 7.9 | 15.6 | 25.7 | 1.5 | 15.2 | 0.76 | 0.28 | 0.46 | 0.08 | 0.79 |
| Lactate, mmol/h | 49.7 | 79.1 | 12.2 | 59.8 | 68.9 | 12.0 | 0.15 | 0.49 | 0.71 | <0.001 | 0.26 |
| Urea, mmol/h | 45.9 | 38.6 | 3.9 | 45.3 | 39.3 | 3.0 | 0.23 | 0.006 | 0.17 | <0.001 | 0.40 |
| NEFA, mmol/h | −8.9 | −3.1 | 2.4 | −4.7 | −7.3 | 2.2 | 0.11 | 0.41 | 0.86 | 0.59 | 0.23 |
| TG, mmol/h | 5.6 | 3.6 | 2.9 | 8.0 | 1.2 | 2.9 | 0.51 | 0.05 | 0.06 | 0.70 | 0.58 |
| Essential AA, mmol/h | |||||||||||
| Lysine | −1.0 | −1.3 | 0.2 | −1.0 | −1.3 | 0.2 | 0.43 | 0.08 | 0.62 | <0.001 | 0.44 |
| Methionine | −0.1 | 0.6 | 0.1 | 0.3 | 0.2 | 0.1 | <0.001 | 0.39 | 0.02 | 0.002 | 0.40 |
| Threonine | −1.2 | −0.9 | 0.2 | −0.8 | −1.2 | 0.2 | 0.17 | 0.18 | 0.30 | 0.12 | 0.04 |
| Tryptophan | −0.6 | −0.6 | 0.1 | −0.5 | −0.7 | 0.1 | 0.94 | 0.02 | 0.92 | 0.05 | 0.73 |
| Isoleucine | −0.7 | −0.8 | 0.3 | −0.3 | −1.1 | 0.3 | 0.80 | 0.047 | 0.88 | 0.006 | 0.46 |
| Leucine | −1.0 | −1.4 | 0.3 | −1.0 | −1.4 | 0.3 | 0.46 | 0.09 | 0.13 | 0.006 | 0.32 |
| Valine | −0.7 | 0.1 | 0.5 | 0.4 | −1.0 | 0.5 | 0.28 | 0.03 | 0.62 | 0.008 | 0.42 |
| Histidine | −1.6 | −1.5 | 0.3 | −1.5 | −1.5 | 0.3 | 0.60 | 0.93 | 0.28 | 0.84 | 0.84 |
| Phenylalanine | −4.2 | −3.7 | 0.2 | −3.8 | −4.1 | 0.2 | 0.06 | 0.83 | 0.73 | <0.001 | 0.24 |
| Nonessential AA, mmol/h | |||||||||||
| Alanine | −25.4 | −25.4 | 1.7 | −25.7 | −25.0 | 1.7 | 0.70 | 0.76 | 0.37 | <0.001 | 0.03 |
| Asparagine | −1.6 | −1.3 | 0.1 | −1.4 | −1.5 | 0.1 | 0.04 | 0.45 | 0.77 | <0.001 | 0.25 |
| Aspartate | −0.9 | −1.3 | 0.2 | −1.2 | −1.1 | 0.2 | 0.44 | 0.85 | 0.61 | 0.004 | 0.01 |
| Cysteine | −2.0 | −2.3 | 0.4 | −1.9 | −2.4 | 0.3 | 0.60 | 0.30 | 0.55 | 0.01 | 0.08 |
| Glutamate | 35.8 | 40.3 | 2.7 | 39.3 | 36.8 | 2.7 | 0.29 | 0.49 | 0.52 | 0.03 | 0.38 |
| Glycine | −20.9 | −14.7 | 2.0 | −17.0 | −18.5 | 1.7 | 0.07 | 0.33 | 0.84 | 0.85 | 0.54 |
| Proline | −4.4 | −4.2 | 1.1 | −3.3 | −5.3 | 1.0 | 0.92 | 0.11 | 0.38 | 0.19 | 0.68 |
| Serine | −5.2 | −5.6 | 0.7 | −5.7 | −5.1 | 0.6 | 0.99 | 0.34 | 0.25 | <0.001 | 0.002 |
| Tyrosine | −3.3 | −2.8 | 0.3 | −3.1 | −3.1 | 0.2 | 0.21 | 0.92 | 0.57 | <0.001 | 0.32 |
| Glutamine | −1.6 | −5.0 | 0.7 | −3.3 | −3.3 | 0.7 | 0.04 | 0.98 | 0.003 | 0.34 | 0.28 |
| VFA, mmol/h | |||||||||||
| Acetate | −8.3 | −1.8 | 5.5 | −8.9 | −1.2 | 4.9 | 0.45 | 0.16 | 0.79 | 0.34 | 0.71 |
| Propionate | −54.4 | −51.2 | 7.2 | −54.6 | −51.1 | 5.4 | 0.67 | 0.29 | 0.27 | 0.43 | 0.33 |
| Butyrate | −16.5 | −15.1 | 2.9 | −15.2 | −16.4 | 2.2 | 0.68 | 0.37 | 0.78 | 0.31 | 0.76 |
| Isobutyrate | −1.8 | −1.6 | 0.2 | −1.8 | −1.6 | 0.2 | 0.43 | 0.55 | 0.90 | 0.39 | 0.28 |
| Isovalerate | −1.2 | −1.2 | 0.1 | −1.3 | −1.1 | 0.1 | 0.66 | 0.29 | 0.005 | 0.46 | 0.72 |
| Item | Treatment | SEM | DIM | SEM | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | HMB | –10 | –3 | Trt | DIM | Trt×DIM | ST | Trt×ST | |||
| Plasma, % | |||||||||||
| HMB | 2.9 | 3.0 | 5.1 | 1.8 | 4.1 | 5.4 | 0.93 | 0.72 | 0.50 | 0.16 | 0.63 |
| Insulin | 14.4 | 4.3 | 3.6 | 12.3 | 6.3 | 3.1 | 0.09 | 0.08 | 0.92 | 0.02 | 0.47 |
| Glucose | 9.5 | 8.7 | 0.9 | 10.2 | 8.0 | 0.7 | 0.54 | 0.001 | 0.40 | <0.001 | 0.004 |
| Lactate | 26.5 | 24.5 | 2.6 | 24.5 | 26.6 | 2.3 | 0.48 | 0.52 | 0.33 | 0.03 | 0.006 |
| Urea | −0.8 | −0.6 | 0.3 | −0.6 | −0.9 | 0.3 | 0.59 | 0.44 | 0.85 | 0.82 | 0.48 |
| NEFA | 13.0 | −3.2 | 10.1 | 9.5 | 0.3 | 10.0 | 0.19 | 0.39 | 0.71 | 0.14 | 0.72 |
| TG | 0.0 | 1.6 | 2.5 | 0.8 | 0.9 | 2.3 | 0.57 | 0.82 | 0.27 | 0.83 | 0.38 |
| Essential AA, % | |||||||||||
| Lysine | 13.5 | 5.8 | 3.4 | 12.2 | 7.1 | 2.5 | 0.17 | <0.001 | 0.30 | <0.001 | 0.001 |
| Methionine | 21.1 | 21.7 | 2.3 | 22.0 | 20.9 | 2.3 | 0.72 | 0.82 | 0.22 | 0.09 | 0.90 |
| Threonine | 3.8 | 1.8 | 1.0 | 3.4 | 2.1 | 0.8 | 0.20 | 0.13 | 0.55 | 0.006 | 0.08 |
| Tryptophan | 1.1 | 0.3 | 0.5 | 0.7 | 0.7 | 0.5 | 0.26 | 0.87 | 0.35 | 0.04 | 0.58 |
| Isoleucine | 5.3 | 2.5 | 1.4 | 4.6 | 3.3 | 1.1 | 0.21 | 0.19 | 0.39 | 0.001 | 0.01 |
| Leucine | 5.2 | 2.4 | 1.3 | 4.5 | 3.2 | 1.1 | 0.20 | 0.14 | 0.30 | <0.001 | 0.10 |
| Valine | 3.2 | 2.0 | 0.9 | 3.2 | 2.1 | 0.7 | 0.43 | 0.16 | 0.97 | 0.02 | 0.04 |
| Histidine | 2.8 | 1.1 | 1.5 | 4.3 | −0.4 | 1.5 | 0.51 | 0.04 | 0.41 | 0.53 | 0.68 |
| Phenylalanine | 2.9 | 1.3 | 1.2 | 2.6 | 1.6 | 1.0 | 0.48 | 0.23 | 0.39 | <0.001 | 0.01 |
| Nonessential AA, % | |||||||||||
| Alanine | 5.9 | 3.2 | 0.7 | 4.5 | 4.5 | 0.6 | 0.04 | 0.77 | 0.55 | <0.001 | 0.02 |
| Asparagine | 10.8 | 5.8 | 2.2 | 8.8 | 7.7 | 2.1 | 0.18 | 0.68 | 0.77 | 0.005 | 0.23 |
| Aspartate | 10.4 | 6.5 | 2.9 | 8.6 | 8.3 | 2.3 | 0.38 | 0.79 | 0.65 | 0.04 | 0.33 |
| Cysteine | −1.4 | −0.8 | 0.4 | −0.6 | −1.6 | 0.4 | 0.23 | 0.10 | 0.83 | 0.07 | 0.02 |
| Glutamate | 27.1 | 17.3 | 3.4 | 23.8 | 20.5 | 2.5 | 0.09 | 0.03 | 0.89 | 0.92 | 0.51 |
| Glycine | −0.0 | −0.3 | 0.4 | −0.2 | −0.1 | 0.4 | 0.73 | 0.63 | 0.36 | 0.26 | 0.73 |
| Proline | 1.5 | 1.5 | 0.6 | 1.9 | 1.1 | 0.5 | 0.91 | 0.21 | 0.65 | 0.003 | 0.13 |
| Serine | 12.1 | 8.1 | 1.2 | 10.2 | 9.9 | 1.0 | 0.049 | 0.59 | 0.11 | 0.002 | 0.45 |
| Tyrosine | 1.9 | 1.0 | 1.1 | 1.9 | 1.0 | 0.9 | 0.57 | 0.19 | 0.18 | 0.01 | 0.26 |
| Glutamine | −11.7 | −12.4 | 1.7 | −9.6 | −14.5 | 1.7 | 0.78 | 0.04 | 0.87 | 0.35 | 0.37 |
| VFA, % | |||||||||||
| Acetate | 59.9 | 53.6 | 4.7 | 54.0 | 59.4 | 3.7 | 0.36 | 0.07 | 0.96 | 0.63 | 0.47 |
| Propionate | −830 | 242 | 410 | 281 | −869 | 350 | 0.28 | 0.11 | 0.004 | 0.03 | <0.001 |
| Butyrate | 9.0 | 9.4 | 12.1 | 8.7 | 9.7 | 12.1 | 0.85 | 0.81 | 0.33 | 0.09 | 0.57 |
| Isobutyrate | 72.4 | 60.4 | 4.9 | 68.4 | 64.3 | 4.2 | 0.11 | 0.27 | 0.26 | 0.15 | 0.64 |
| Isovalerate | 6.6 | 8.2 | 4.2 | 6.0 | 8.8 | 4.1 | 0.64 | 0.45 | 0.30 | 0.95 | 0.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Kristensen, N.B.; Krogh, U.; Theil, P.K. Net Absorption and Metabolism of β-Hydroxy- β-Methyl Butyrate during Late Gestation in a Pig Model. Nutrients 2020, 12, 561. https://doi.org/10.3390/nu12020561
Hu L, Kristensen NB, Krogh U, Theil PK. Net Absorption and Metabolism of β-Hydroxy- β-Methyl Butyrate during Late Gestation in a Pig Model. Nutrients. 2020; 12(2):561. https://doi.org/10.3390/nu12020561
Chicago/Turabian StyleHu, Liang, Niels Bastian Kristensen, Uffe Krogh, and Peter Kappel Theil. 2020. "Net Absorption and Metabolism of β-Hydroxy- β-Methyl Butyrate during Late Gestation in a Pig Model" Nutrients 12, no. 2: 561. https://doi.org/10.3390/nu12020561
APA StyleHu, L., Kristensen, N. B., Krogh, U., & Theil, P. K. (2020). Net Absorption and Metabolism of β-Hydroxy- β-Methyl Butyrate during Late Gestation in a Pig Model. Nutrients, 12(2), 561. https://doi.org/10.3390/nu12020561




