Ready-Made Oral Nutritional Supplements Improve Nutritional Outcomes and Reduce Health Care Use—A Randomised Trial in Older Malnourished People in Primary Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Selection of Participants
2.3. Randomisation Procedure
2.4. Interventions
2.5. Measurements and Outcomes
2.6. Ethics
2.7. Statistics and Data Analysis
3. Results
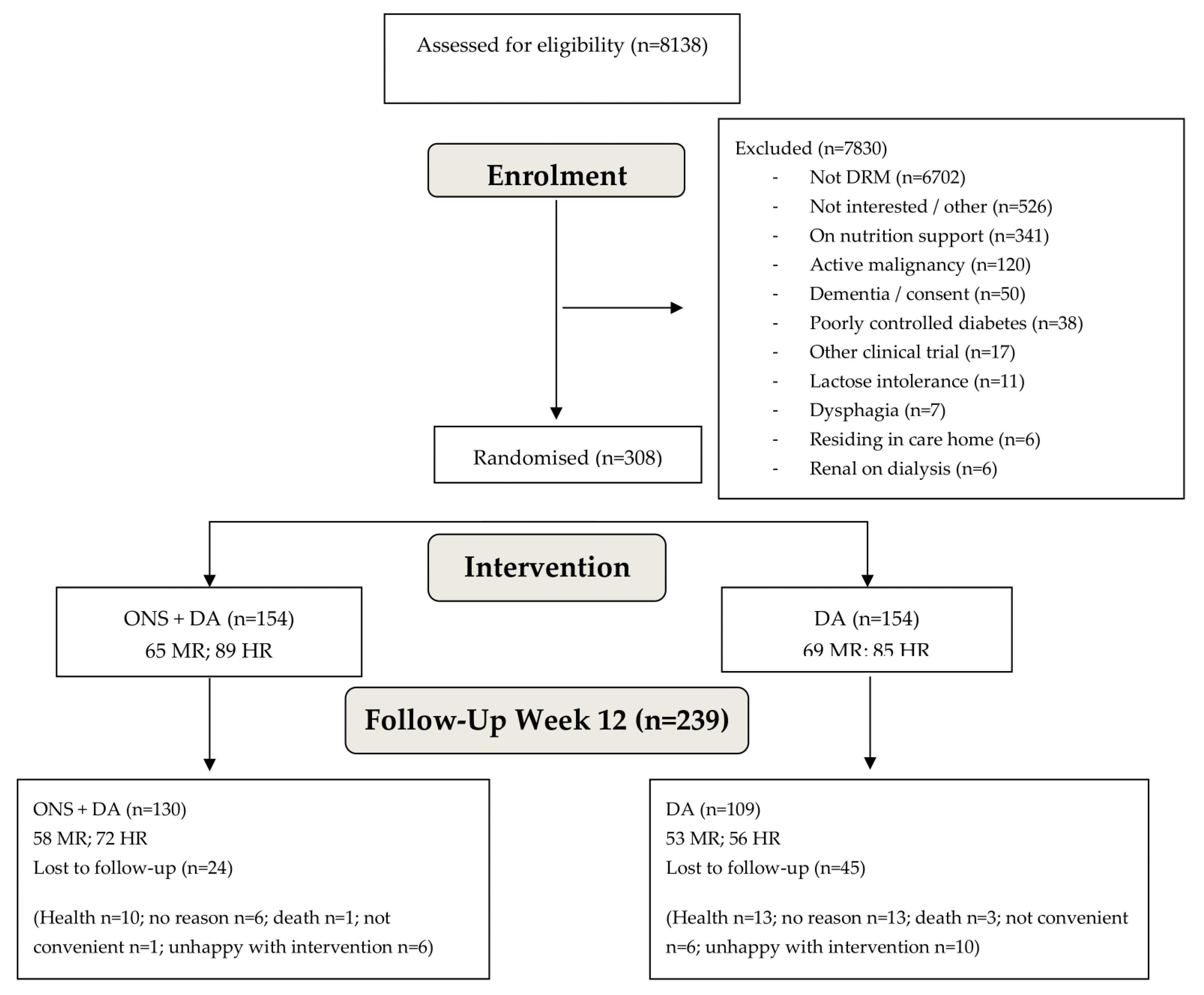
3.1. Recruitment and Baseline Characteristics of Participants
3.2. Dropouts and SAE
3.3. Quality of Life (QoL)
3.3.1. Comparison with Normative Values and Changes within Groups
3.3.2. Differences between Groups
3.4. Healthcare Use and Mortality
3.5. Dietary Intake, Weight and Malnutrition Risk
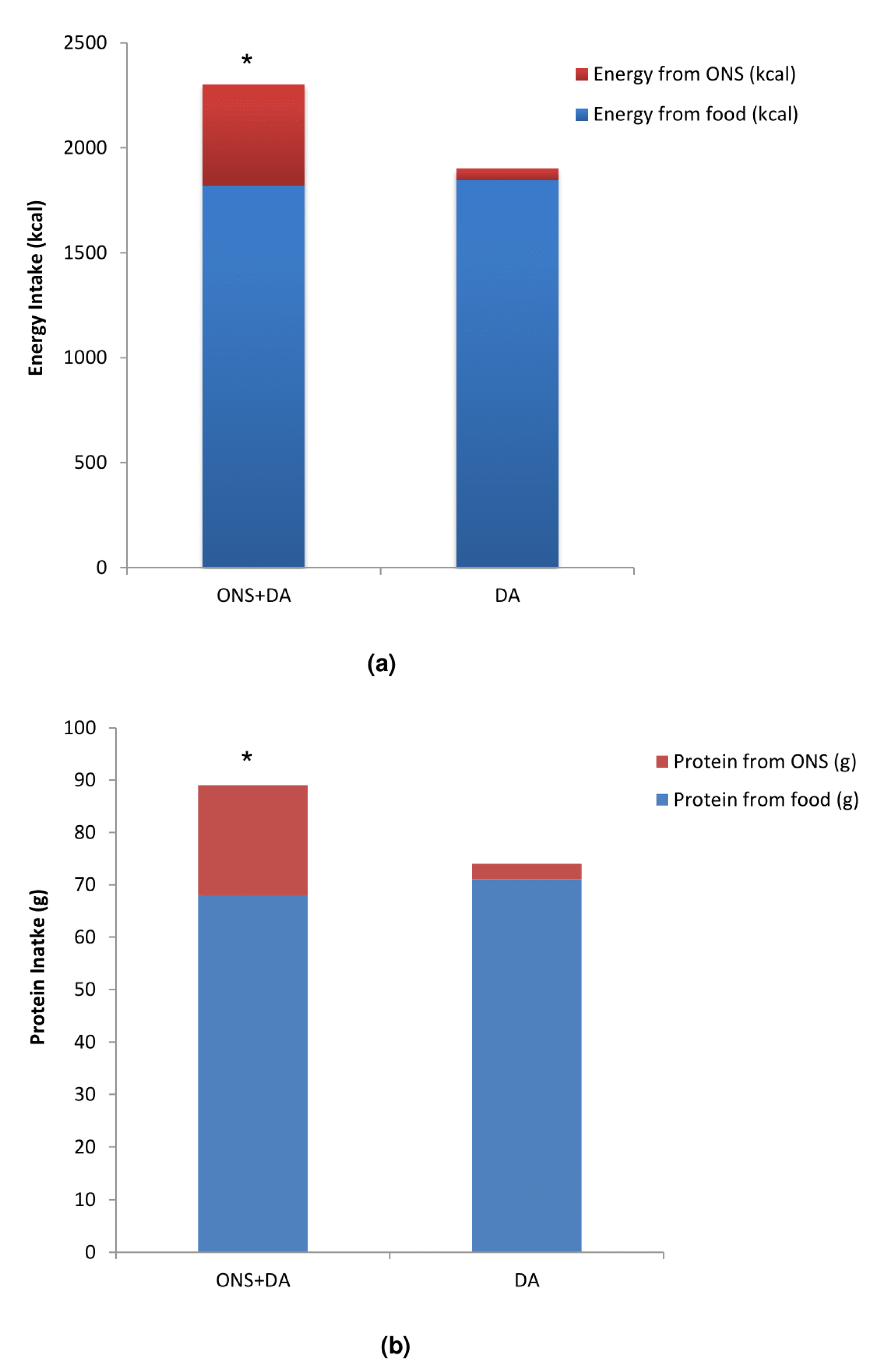
3.5.1. Dietary Intake
3.5.2. Weight and Malnutrition Risk
3.6. Satisfaction and Compliance
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stratton, R.J.; Green, C.J.; Elia, M. Disease-Related Malnutrition: An Evidence Based Approach to Treatment; CABI Publishing: Oxford, UK, 2003. [Google Scholar]
- Elia, M. The cost of malnutrition in England and potential cost savings from nutritional interventions. In A Report from the Malnutrition Action Group of BAPEN and the National Institute for Health Research Southampton Biomedical Research Centre; BAPEN: Redditch, UK, 2015; Available online: http://www.bapen.org.uk/pdfs/economic-report-full.pdf (accessed on 1 October 2018).
- Elia, M.; Russell, C.A. Combating Malnutrition: Recommendations for Action; BAPEN: Redditch, UK, 2009. [Google Scholar]
- Russell, C.A.; Elia, M. Nutrition Screening Surveys in Hospitals in the UK, 2007–2011. In A Report Based on the Amalgamated Data from the Four Nutrition Screening Week Surveys undertaken by BAPEN in 2007, 2008, 2010 and 2011; BAPEN: Redditch, UK, 2014. [Google Scholar]
- Cawood, A.L.; Elia, M.; Stratton, R.J. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res. Rev. 2012, 11, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.; Weekes, C.E. Dietary advice with or without oral nutritional supplements for disease-related malnutrition in adults. Cochrane Database Syst. Rev. 2011, Cd002008. [Google Scholar] [CrossRef] [PubMed]
- Stratton, R.J.; Elia, M. A review of reviews: A new look at the evidence for oral nutritional supplements in clinical practice. Clin. Nutr. Suppl. 2007, 26, 5–23. [Google Scholar] [CrossRef]
- Stratton, R.J.; Smith, T.; Gabe, S. Managing Malnutrition to Improve Lives and Save Money; On behalf of BAPEN (British Society of Enteral and Parenteral Nutrition); BAPEN: Redditch, UK; Available online: www.bapen.org.uk (accessed on 1 October 2018).
- National Institute for Health and Clinical Excellence (NICE). Nutrition Support in Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition (Clinical Guideline 32); National Institute for Health and Clinical Excellence (NICE): London, UK, 2006. [Google Scholar]
- Stratton, R.J.; Hebuterne, X.; Elia, M. A systematic review and meta-analysis of the impact of oral nutritional supplements on hospital readmissions. Ageing Res. Rev. 2013, 12, 884–897. [Google Scholar] [CrossRef]
- Elia, M.; Normand, C.; Laviano, A.; Norman, K. A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in community and care home settings. Clin. Nutr. 2016, 35, 125–137. [Google Scholar] [CrossRef]
- Elia, M.; Normand, C.; Norman, K.; Laviano, A. A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in the hospital setting. Clin. Nutr. 2016, 35, 370–380. [Google Scholar] [CrossRef]
- National Institute for Health and Clinical Excellence (NICE). Quality Standard for Nutrition Support in Adults. NICE Quality Standard 24; National Institute for Health and Clinical Excellence (NICE): London, UK, 2012. [Google Scholar]
- BAPEN. The ‘MUST’ Toolkit. Available online: https://www.bapen.org.uk/screening-and-must/must/must-toolkit (accessed on 17 January 2018).
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Q. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Group, T.E. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar]
- Cook, R.J.; Sackett, D.L. The number needed to treat: A clinically useful measure of treatment effect. BMJ 1995, 310, 452–454. [Google Scholar] [CrossRef]
- Mulhern, B.; Feng, Y.; Shah, K.; Janssen, M.F.; Herdman, M.; van Hout, B.; Devlin, N. Comparing the UK EQ-5D-3L and English EQ-5D-5L Value Sets. Pharmacoeconomics 2018, 36, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G. Missing outcomes in randomized trials: Addressing the dilemma. Open Med 2009, 3, e51–e53. [Google Scholar] [PubMed]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons: Chichester, UK, 1987. [Google Scholar]
- Barnard, J.; Rubin, D.B. Miscellanea. Small-sample degrees of freedom with multiple imputation. Biometrika 1999, 86, 948–955. [Google Scholar] [CrossRef]
- Szende, A.; Janssen, B.; Cabases, J. Self-Reported Population Health: An International Perspective Based on EQ-5D; Springer: Dordrecht, The Netherlands, 2014; p. 196. [Google Scholar]
- NHS Digital.Finalised Patient Reported Outcome Measures (PROMs) in England. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/patient-reported-outcome-measures-proms/provisional-monthly-patient-reported-outcome-measures-proms-in-england-april-2017-to-june-2017 (accessed on 1 October 2018).
- Dawson, J.; Fitzpatrick, R.; Carr, A.; Murray, D. Questionnaire on the perceptions of patients about total hip replacement. J. Bone Joint Surg. Br. 1996, 78, 185–190. [Google Scholar] [CrossRef]
- Dawson, J.; Fitzpatrick, R.; Murray, D.; Carr, A. Questionnaire on the perceptions of patients about total knee replacement. J. Bone Joint Surg. Br. 1998, 80, 63–69. [Google Scholar] [CrossRef]
- Klem, T.M.; Sybrandy, J.E.; Wittens, C.H. Measurement of health-related quality of life with the Dutch translated Aberdeen Varicose Vein Questionnaire before and after treatment. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 470–476. [Google Scholar] [CrossRef]
- Parsons, E.L.; Stratton, R.J.; Cawood, A.L.; Smith, T.R.; Elia, M. Oral nutritional supplements in a randomised trial are more effective than dietary advice at improving quality of life in malnourished care home residents. Clin. Nutr. 2017, 36, 134–142. [Google Scholar] [CrossRef]
- Krondl, M.; Coleman, P.H.; Bradley, C.L.; Lau, D.; Ryan, N. Subjectively healthy elderly consuming a liquid nutrition supplement maintained body mass index and improved some nutritional parameters and perceived well being. J. Am. Diet. Assoc. 1999, 99, 1542–1548. [Google Scholar] [CrossRef]
- Gariballa, S.; Forster, S. Dietary supplementation and quality of life of older patients: A randomized, double-blind, placebo-controlled trial. J. Am. Geriatr. Soc. 2007, 55, 2030–2034. [Google Scholar] [CrossRef]
- Norman, K.; Kirchner, H.; Freudenreich, M.; Ockenga, J.; Lochs, H.; Pirlich, M. Three month intervention with protein and energy rich supplements improve muscle function and quality of life in malnourished patients with non-neoplastic gastrointestinal disease—A randomized controlled trial. Clin. Nutr. 2008, 27, 48–56. [Google Scholar] [CrossRef]
- Tidermark, J.; Zethraeus, N.; Svensson, O.; Tornkvist, H.; Ponzer, S. Quality of life related to fracture displacement among elderly patients with femoral neck fractures treated with internal fixation. J. Orthop. Trauma 2002, 16, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Edington, J.; Barnes, R.; Bryan, F.; Dupress, E.; Frost, G.; Hickson, M.; Lancaster, J.; Mongia, S.; Smith, J.; Torrance, A.; et al. A prospective randomised controlled trial of nutritional supplementation in malnourished elderly in the community: Clinical and health economic outcomes. Clin. Nutr. 2004, 23, 195–204. [Google Scholar] [CrossRef]
- Payette, H. Nutrition as a determinant of functional autonomy and quality of life in aging: A research program. Can. J. Physiol. Pharmacol. 2005, 83, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, T.; Iyoki, M.; Sugimoto, T.; Kobayashi, M.; Hanazaki, K. Oral supplementation with carbohydrate- and branched-chain amino acid-enriched nutrients improves postoperative quality of life in patients undergoing hepatic resection. Amino Acids 2011, 40, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; McKenzie, J.; de Mutsert, R.; Azar, R.; Teta, D.; Plauth, M.; Cano, N. Use of a renal-specific oral supplement by haemodialysis patients with low protein intake does not increase the need for phosphate binders and may prevent a decline in nutritional status and quality of life. Nephrol. Dial. Trans. 2008, 23, 2902–2910. [Google Scholar] [CrossRef]
- Beattie, A.H.; Prach, A.T.; Baxter, J.P.; Pennington, C.R. A randomised controlled trial evaluating the use of enteral nutritional supplements postoperatively in malnourished surgical patients. Gut 2000, 46, 813–818. [Google Scholar] [CrossRef]
- Brown, F.; Fry, G.; Cawood, A.L.; Stratton, R.J. Economic impact of implementing malnutrition screening and nutritional management in older adults in general practice. J. Nutr. Health Aging 2019. [Google Scholar] [CrossRef]
- Baggaley, E.; Whincup, L.; Ashman, K.; Cawood, A.L.; Davies, D.; Burns, E.; Stratton, R.J. Effectiveness of implementing a nurse led policy for the management of malnutrition. Clin. Nutr. 2013, 32, S231. [Google Scholar] [CrossRef]
- Kominek, O.; Cawood, A.L.; Janik, L.; Evill, R.; Fitzsimmons, B.; Webb, L.; Stratton, R.J. Local implementation of a pathway to manage malnourished COPD patients in the community. Eur. Respir. J. 2017, 50, PA1609. [Google Scholar]
- Cawood, A.L.; Smith, A.; Pickles, S.; Church, S.; Dalrymple-Smith, J.; Elia, M.; Stratton, R.J. Effectiveness of implementing MUST into care homes within Peterborough Primary Care Trust England. Clin. Nutr. 2009, 4, 81. [Google Scholar] [CrossRef]
- Hubbard, G.P.; Elia, M.; Holdoway, A.; Stratton, R.J. A systematic review of compliance to oral nutritional supplements. Clin. Nutr. 2012, 31, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.; Weekes, C.E. Dietary advice for illness-related malnutrition in adults. Cochrane Database Syst. Rev. 2008, CD002008. [Google Scholar] [CrossRef]
- Reinders, I.; Volkert, D.; de Groot, L.; Beck, A.M.; Feldblum, I.; Jobse, I.; Neelemaat, F.; de van der Schueren, M.A.E.; Shahar, D.R.; Smeets, E.; et al. Effectiveness of nutritional interventions in older adults at risk of malnutrition across different health care settings: Pooled analyses of individual participant data from nine randomized controlled trials. Clin. Nutr. 2019, 38, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | ONS + DA (n154) | DA (n154) | ALL (n308) | p Value * | |||
|---|---|---|---|---|---|---|---|
| Gender | 0.716 a | ||||||
| Male: n | 52 | 49 | 101 | ||||
| Female: n | 102 | 105 | 207 | ||||
| MUST Categories | 0.646 a | ||||||
| MUST—Medium risk: n | 65 | 69 | 134 | ||||
| MUST—High risk: n | 89 | 85 | 174 | ||||
| Age (y): mean ± SD | 71.31 ± 11.18 | 71.63 ± 10.31 | 71.47 ± 10.74 | 0.795 b | |||
| Height (m): mean ± SD | 1.64 ± 0.09 | 1.64 ± 0.09 | 1.64 ± 0.09 | 0.704 b | |||
| Weight (kg): mean ± SD | 51.94 ± 8.72 | 52.29 ± 10.28 | 52.12 ± 9.52 | 0.749 b | |||
| BMI (kg/m2): mean ± SD | 19.32 ± 2.31 | 19.49 ± 2.66 | 19.40 ± 2.49 | 0.561 b | |||
| CCI: mean ± SD | 0.94 ± 0.92 | 1.09 ± 0.94 | 1.02 ± 0.93 | 0.159 b | |||
| Number of systems † mean ± SD | 2.62 ± 1.22 | 2.45 ± 1.11 | 2.54 ± 1.17 | 0.188 b | |||
| Energy intake (kcal): mean ± SD | 1759 ± 565 | 1712 ± 564 | 1735 ± 564 | 0.465 b | |||
| Protein intake (g): mean ± SD | 65.4 ± 21.6 | 64.0 ± 22.1 | 64.7 ± 21.8 | 0.579 b | |||
| EQ-5D-5L index: mean ± SD | 0.75 ± 0.22 | 0.72 ± 0.24 | 0.74 ± 0.23 | 0.155 b | |||
| EQ-5D-5L VAS: mean ± SD | 66.54 ± 19.18 | 63.53 ± 19.03 | 65.03 ± 19.28 | 0.172 b | |||
| Health problems | 0.608 a | ||||||
| Respiratory; n (%) | 54 | (35.1%) | 52 | (33.8%) | 106 | (34.4%) | |
| Gastrointestinal; n (%) | 40 | (26.0%) | 50 | (32.5%) | 90 | (29.2%) | |
| Musculoskeletal; n (%) | 24 | (15.6%) | 17 | (11%) | 41 | (13.3%) | |
| Cardiovascular System; n (%) | 10 | (6.5%) | 12 | (7.8%) | 22 | (7.1%) | |
| Central nervous system; n (%) | 7 | (4.5%) | 4 | (2.6%) | 11 | (3.6%) | |
| Other; n (%) | 19 | (12.3%) | 19 | (12.3%) | 38 | (12.3%) | |
| Timepoint | ONS + DA (n154) | DA (n154) | ALL (n308) | p Value a | p Value b |
|---|---|---|---|---|---|
| Week 4 | |||||
| % dropouts | 12/154 (7.8%) | 27/154 (17.5%) | 39/308 (12.7%) | 0.010 | 0.016 |
| Dropouts v no dropouts | 12 v 142 | 27 v 127 | 39 v 269 | ||
| Week 8 | |||||
| % dropouts | 19/154 (12.3%) | 37/154 (24.0%) | 56/308 (18.1%) | 0.008 | 0.006 |
| Dropouts v no dropouts | 19 v 135 | 37 v 117 | 56 v 252 | ||
| Week 12 | |||||
| % dropouts | 24/154 (15.6%) | 45/154 (29.2%) | 69/308 (22.4%) | 0.004 | 0.006 |
| Dropouts v no dropouts | 24 v 130 | 45 v 109 | 69 v 239 |
| Quality of Life | Baseline | Average Over Intervention | p Value a | |||
|---|---|---|---|---|---|---|
| ONS + DA | DA | |||||
| Intention to treat analysis | ||||||
| n= 154 | n= 154 | |||||
| EQ-5D-5L index | 0.735 | 0.753 | 0.011 | 0.743 | 0.010 | 0.425 |
| ED-5D-5L VAS | 65.0 | 67.6 | 1.1 | 66.2 | 1.3 | 0.344 |
| Per protocol analysis | ||||||
| n= 126 | n= 104 | |||||
| EQ-5D-5L index | 0.735 | 0.783 | 0.009 | 0.780 | 0.010 | 0.815 |
| ED-5D-5L VAS | 66.8 | 69.8 | 1.0 | 70.6 | 1.1 | 0.582 |
| Healthcare Use | ONS + DA | DA | p Value a | ||
|---|---|---|---|---|---|
| Intention to treat (n154:n154) | |||||
| Total HCP * visits | 5.099 | 0.726 | 7.676 | 1.046 | 0.010 |
| Total hospital admissions | 0.070 | 0.032 | 0.118 | 0.033 | 0.297 |
| Total emergency admissions | 0.046 | 0.028 | 0.100 | 0.031 | 0.202 |
| Total elective admissions | 0.025 | 0.012 | 0.021 | 0.013 | 0.808 |
| Total length of stay (days) | 0.670 | 0.490 | 1.782 | 0.807 | 0.161 |
| Per protocol †(nONS + DA:nDA) | |||||
| Total HCP * visits n118:n103 | 4.139 | 0.458 | 4.926 | 0.525 | 0.261 |
| Total hospital admissions n128:n108 | 0.054 | 0.030 | 0.140 | 0.034 | 0.060 |
| Total emergency admissions n125:n106 | 0.028 | 0.025 | 0.114 | 0.029 | 0.026 |
| Total elective admissions n125:n107 | 0.026 | 0.013 | 0.029 | 0.015 | 0.877 |
| Total length of stay (days) n126:n107 | 0.152 | 0.127 | 0.572 | 0.146 | 0.031 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, T.R.; Cawood, A.L.; Walters, E.R.; Guildford, N.; Stratton, R.J. Ready-Made Oral Nutritional Supplements Improve Nutritional Outcomes and Reduce Health Care Use—A Randomised Trial in Older Malnourished People in Primary Care. Nutrients 2020, 12, 517. https://doi.org/10.3390/nu12020517
Smith TR, Cawood AL, Walters ER, Guildford N, Stratton RJ. Ready-Made Oral Nutritional Supplements Improve Nutritional Outcomes and Reduce Health Care Use—A Randomised Trial in Older Malnourished People in Primary Care. Nutrients. 2020; 12(2):517. https://doi.org/10.3390/nu12020517
Chicago/Turabian StyleSmith, Trevor R., Abbie L. Cawood, Emily R. Walters, Natasha Guildford, and Rebecca J. Stratton. 2020. "Ready-Made Oral Nutritional Supplements Improve Nutritional Outcomes and Reduce Health Care Use—A Randomised Trial in Older Malnourished People in Primary Care" Nutrients 12, no. 2: 517. https://doi.org/10.3390/nu12020517
APA StyleSmith, T. R., Cawood, A. L., Walters, E. R., Guildford, N., & Stratton, R. J. (2020). Ready-Made Oral Nutritional Supplements Improve Nutritional Outcomes and Reduce Health Care Use—A Randomised Trial in Older Malnourished People in Primary Care. Nutrients, 12(2), 517. https://doi.org/10.3390/nu12020517





