Short-Term Caloric Restriction Attenuates Obesity-Induced Pro-inflammatory Response in Male Rhesus Macaques
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Characteristics and Diets
2.2. WAT Biopsies
2.3. Cell-Based Assays
2.4. RNA-Seq Analysis
2.5. Bioinformatic Analysis
2.6. Flow Cytometry Analysis
3. Results
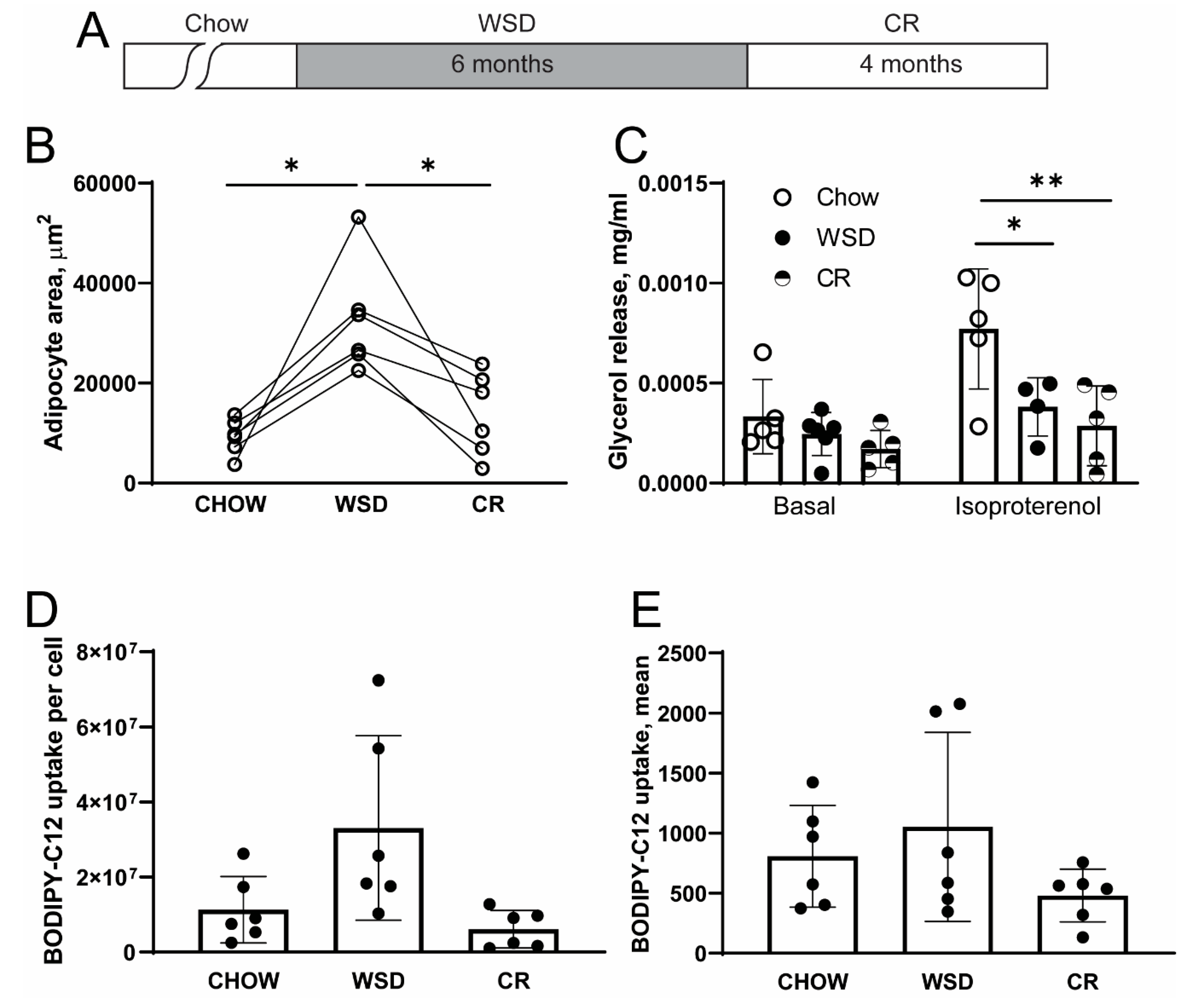
3.1. WSD-Induced Adipocyte Hypertrophy but not β-Adrenergic Resistance is Reversed by CR
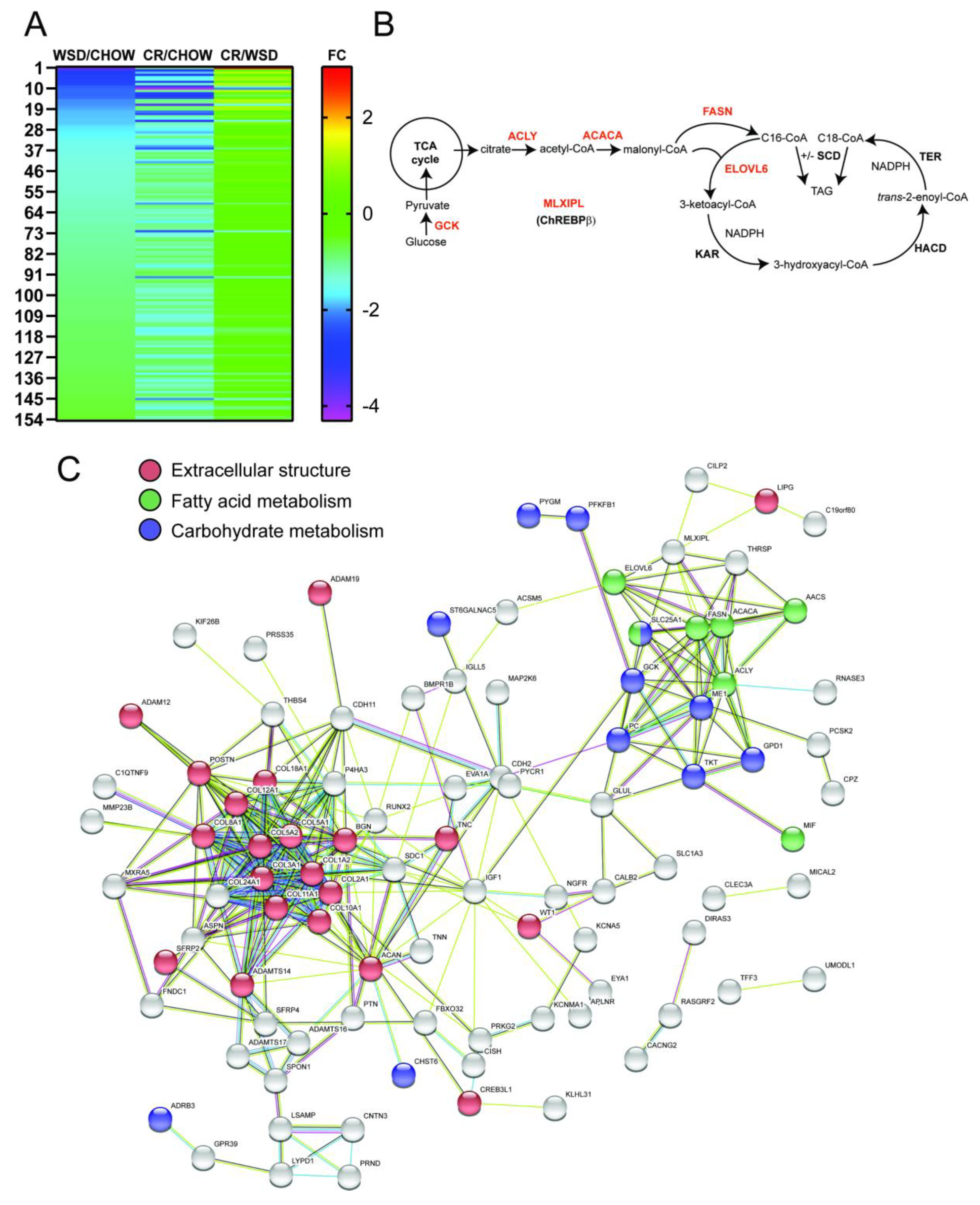
3.2. WSD-Induced Pro-Inflammatory Gene Expression in SC-WAT Is Reversed by CR
3.3. De Novo Lipogenesis and β-Adrenergic Receptor Genes Remain Downregulated after CR
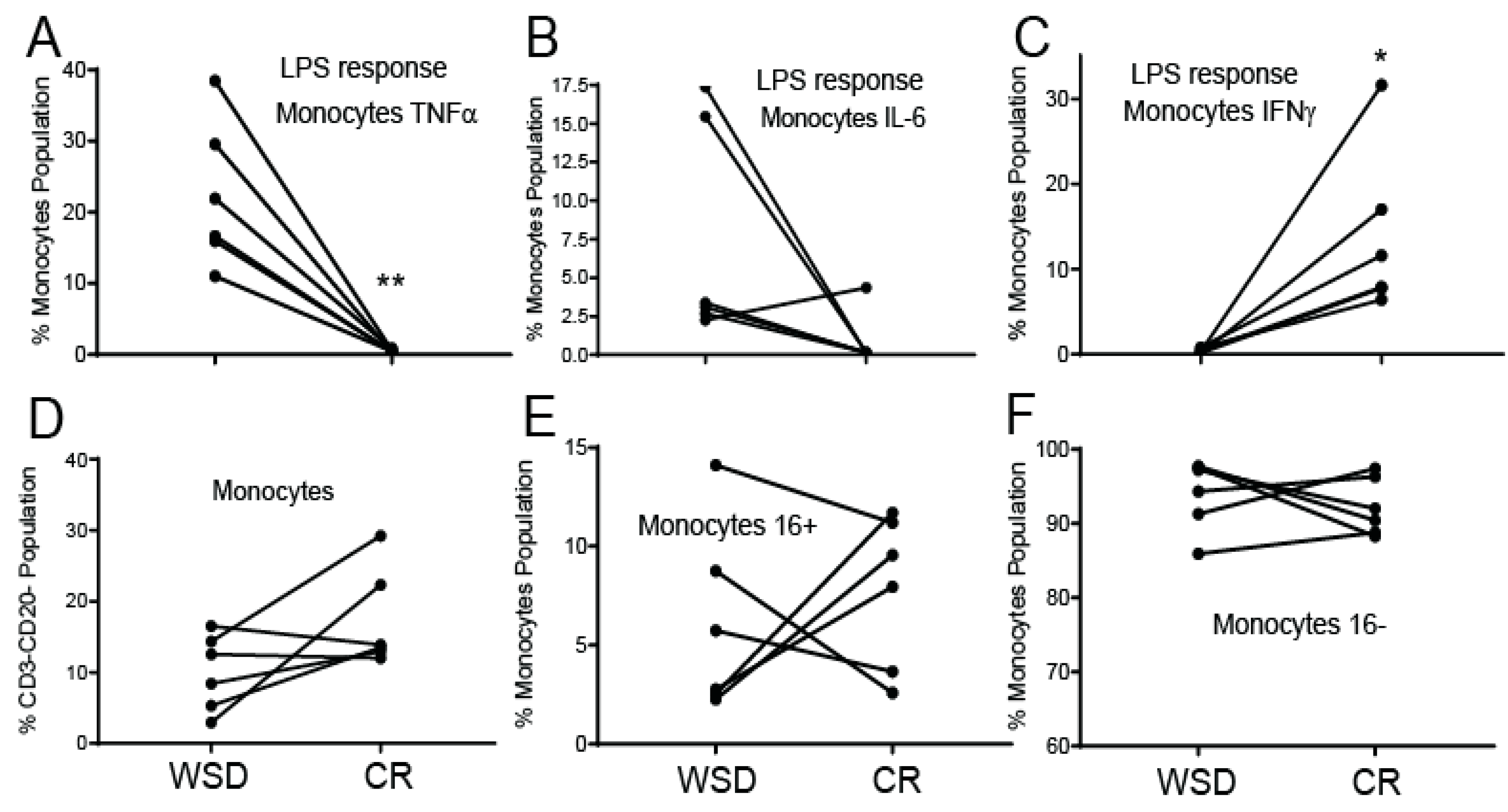
3.4. CR Diminishes a Pro-Inflammatory Response in Circulating Monocytes
4. Discussion
4.1. The Effects of WSD and CR on a Pro-Inflammatory Response
4.2. The Effects of WSD and CR on Lipolysis
4.3. The Effects of WSD and CR on De Novo Lipogenesis Pathway
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Online Data Repository
References
- Kahn, S.E.; Hull, R.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, O. Western-style diet, sex steroids and metabolism. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Suarez, J.A.; Longo, V.D. Protein and amino acid restriction, aging and disease: From yeast to humans. Trends Endocrinol. Metab. 2014, 25, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Longo, V.D. Dietary restriction with and without caloric restriction for healthy aging. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; de Jonge, L.; Frisard, M.I.; DeLany, J.P.; Larson-Meyer, D.E.; Rood, J.; Nguyen, T.; Martin, C.K.; Volaufova, J.; Most, M.M.; et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: A randomized controlled trial. JAMA 2006, 295, 1539–1548. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Heilbronn, L.K.; Redman, L.M.; Newcomer, B.R.; Frisard, M.I.; Anton, S.; Smith, S.R.; Maplstat, A.A.; Ravussin, E. Effect of Calorie Restriction With or Without Exercise on Insulin Sensitivity, β-Cell Function, Fat Cell Size, and Ectopic Lipid in Overweight Subjects. Diabetes Care 2006, 29, 1337–1344. [Google Scholar] [CrossRef]
- Redman, L.M.; Heilbronn, L.K.; Martin, C.K.; Alfonso, A.; Smith, S.R.; Ravussin, E. Effect of Calorie Restriction with or without Exercise on Body Composition and Fat Distribution. J. Clin. Endocrinol. Metab. 2007, 92, 865–872. [Google Scholar] [CrossRef]
- Fontana, L.; Villareal, D.T.; Weiss, E.P.; Racette, S.; Steger-May, K.; Klein, S.; Holloszy, J.O. Calorie restriction or exercise: Effects on coronary heart disease risk factors. A randomized, controlled trial. Am. J. Physiol. Metab. 2007, 293, E197–E202. [Google Scholar] [CrossRef]
- Fontana, L. The scientific basis of caloric restriction leading to longer life. Curr. Opin. Gastroenterol. 2009, 25, 144–150. [Google Scholar] [CrossRef]
- Villareal, D.T.; Chode, S.; Parimi, N.; Sinacore, D.R.; Hilton, T.; Armamento-Villareal, R.; Napoli, N.; Qualls, C.; Shah, K. Weight loss, exercise, or both and physical function in obese older adults. New Engl. J. Med. 2011, 364, 1218–1229. [Google Scholar] [CrossRef]
- Katsoulis, K.; Blaudeau, T.E.; Roy, J.P.; Hunter, G.R. Diet-induced Changes in Intra-abdominal Adipose Tissue and CVD Risk in American Women. Obesity (Silver Spring) 2009, 17, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Camhi, S.M.; Stefanick, M.L.; Katzmarzyk, P.T.; Young, D.R. Metabolic syndrome and changes in body fat from a low-fat diet and/or exercise randomized controlled trial. Obesity (Silver Spring) 2010, 18, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Foster-Schubert, K.E.; Alfano, C.M.; Duggan, C.R.; Xiao, L.; Campbell, K.L.; Kong, A.; Bain, C.E.; Wang, C.Y.; Blackburn, G.L.; McTiernan, A. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012, 20, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Carels, R.A.; Darby, L.A.; Cacciapaglia, H.M.; Douglass, O.M. Reducing Cardiovascular Risk Factors in Postmenopausal Women through a Lifestyle Change Intervention. J. Women’s Heal. 2004, 13, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, I.; Warner, J.; Fischer, M.; Park, B.; Hill, B.; Mattison, J.; Lane, M.A.; Roth, G.S.; Ingram, N.K.; Picker, L.J.; et al. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc. Natl. Acad. Sci. USA 2006, 103, 19448–19453. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, I.; Fischer, M.; Warner, J.; Park, B.; Mattison, J.; Ingram, N.K.; Totonchy, T.; Mori, M.; Nikolich-Zugich, J. Optimal window of caloric restriction onset limits its beneficial impact on T-cell senescence in primates. Aging Cell 2008, 7, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.L.; Jain, R.; Rais, M.; White, A.E.; Beer, T.M.; Kievit, P.; Winters-Stone, K.; Messaoudi, I.; Varlamov, O. Perpetuating effects of androgen deficiency on insulin resistance. Int. J. Obes. 2016, 40, 1856–1863. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Messaoudi, I.; Handu, M.; Rais, M.; Sureshchandra, S.; Park, B.S.; Fei, S.; Wright, H.; White, A.E.; Jain, R.; Cameron, J.; et al. Long-lasting effect of obesity on skeletal muscle transcriptome. BMC Genom. 2017, 18, 411. [Google Scholar] [CrossRef]
- MacLean, P.; Higgins, J.A.; Giles, E.D.; Sherk, V.D.; Jackman, M.R. The role for adipose tissue in weight regain after weight loss. Obes. Rev. 2015, 16, 45–54. [Google Scholar] [CrossRef]
- Varlamov, O.; Bishop, C.; Handu, M.; Takahashi, D.; Srinivasan, S.; White, A.; Roberts, C.T. Combined androgen excess and Western-style diet accelerates adipose tissue dysfunction in young adult, female nonhuman primates. Hum. Reprod. 2017, 32, 1892–1902. [Google Scholar] [CrossRef]
- Varlamov, O.; Chu, M.; Cornea, A.; Sampath, H.; Roberts, C.T., Jr. Cell-Autonomous Heterogeneity of Nutrient Uptake in White Adipose Tissue of Rhesus Macaques. Endocrinology 2014. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Sampath, H.; Cahana, D.Y.; Kahl, C.A.; Somwar, R.; Cornea, A.; Roberts, C.T.; Varlamov, O. Spatiotemporal dynamics of triglyceride storage in unilocular adipocytes. Mol. Boil. Cell 2014, 25, 4096–4105. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Boil. 2013, 14, R36. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Kolahi, K.; Louey, S.; Varlamov, O.; Thornburg, K. Real-Time Tracking of BODIPY-C12 Long-Chain Fatty Acid in Human Term Placenta Reveals Unique Lipid Dynamics in Cytotrophoblast Cells. PLoS ONE 2016, 11, e0153522. [Google Scholar] [CrossRef]
- Varlamov, O.; Somwar, R.; Cornea, A.; Kievit, P.; Grove, K.L.; Roberts, J.C.T. Single-cell analysis of insulin-regulated fatty acid uptake in adipocytes. Am. J. Physiol. Metab. 2010, 299, E486–E496. [Google Scholar] [CrossRef]
- Somwar, R.; Roberts, J.C.T.; Varlamov, O. Live-cell imaging demonstrates rapid cargo exchange between lipid droplets in adipocytes. FEBS Lett. 2011, 585, 1946–1950. [Google Scholar] [CrossRef]
- Herman, M.; Peroni, O.D.; Villoria, J.; Schön, M.P.; Abumrad, N.A.; Blüher, M.; Klein, S.; Kahn, B.B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012, 484, 333–338. [Google Scholar] [CrossRef]
- Eissing, L.; Scherer, T.; Tödter, K.; Knippschild, U.; Greve, J.W.; Buurman, W.A.; Pinnschmidt, H.O.; Rensen, S.S.; Wolf, A.M.; Bartelt, A.; et al. De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nat. Commun. 2013, 4, 1528. [Google Scholar] [CrossRef]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T., Jr. Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. (Lausanne) 2014, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Catalan, V.; Gómez-Ambrosi, J.; Rotellar, F.; Silva, C.; Gil, M.J.; Rodríguez, A.; Cienfuegos, J.A.; Salvador, J.; Frühbeck, G. The obestatin receptor (GPR39) is expressed in human adipose tissue and is down-regulated in obesity-associated type 2 diabetes mellitus. Clin. Endocrinol. 2007, 66, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.S.; Jin, C.; Madsen, A.N.; Rasmussen, M.; Kuhre, R.E.; Egerod, K.L.; Nielsen, L.B.; Schwartz, T.W.; Holst, B. Deficiency of the GPR39 receptor is associated with obesity and altered adipocyte metabolism. FASEB J. 2011, 25, 3803–3814. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, Inflammation, and Insulin Resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, M.; Stamler, J.S. Hemoglobin induction in mouse macrophages. Proc. Natl. Acad. Sci. USA 1999, 96, 6643–6647. [Google Scholar] [CrossRef]
- Johannsen, D.L.; Knuth, N.D.; Huizenga, R.; Rood, J.C.; Ravussin, E.; Hall, K.D. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J. Clin. Endocrinol. Metab. 2012, 97, 2489–2496. [Google Scholar] [CrossRef]
- Fothergill, E.; Guo, J.; Howard, L.; Kerns, J.C.; Knuth, N.D.; Brychta, R.; Chen, K.Y.; Skarulis, M.C.; Walter, M.; Walter, P.J.; et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity 2016, 24, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L. Physiological adaptations to weight loss and factors favouring weight regain. Int. J. Obes. 2015, 39, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Rossmeislova, L.; Malisova, L.; Kracmerova, J.; Stich, V. Adaptation of human adipose tissue to hypocaloric diet. Int. J. Obes. (Lond) 2013, 37, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Reynisdottir, S.; Ellerfeldt, K.; Wahrenberg, H.; Lithell, H.; Arner, P. Multiple lipolysis defects in the insulin resistance (metabolic) syndrome. J. Clin. Investig. 1994, 93, 2590–2599. [Google Scholar] [CrossRef] [PubMed]
- Stich, V.; Harant, I.; De Glisezinski, I.; Crampes, F.; Berlan, M.; Kunesova, M.; Hainer, V.; Dauzats, M.; Rivière, D.; Garrigues, M.; et al. Adipose Tissue Lipolysis and Hormone-Sensitive Lipase Expression during Very-Low-Calorie Diet in Obese Female Identical Twins1. J. Clin. Endocrinol. Metab. 1997, 82, 739–744. [Google Scholar]
- Sengenes, C.; Stich, V.; Berlan, M.; Hejnova, J.; Lafontan, M.; Pariskova, Z.; Galitzky, J. Increased lipolysis in adipose tissue and lipid mobilization to natriuretic peptides during low-calorie diet in obese women. Int. J. Obes. 2002, 26, 24–32. [Google Scholar] [CrossRef]
- Flechtner-Mors, M.; Ditschuneit, H.H.; Yip, I.; Adler, G. Sympathetic modulation of lipolysis in subcutaneous adipose tissue: Effects of gender and energy restriction. J. Lab. Clin. Med. 1999, 134, 33–41. [Google Scholar] [CrossRef]
- Hellström, L.; Reynisdottir, S.; Langin, D.; Rössner, S.; Arner, P. Regulation of lipolysis in fat cells of obese women during long-term hypocaloric diet. Int. J. Obes. Relat. Metab. Disord. : J. Int. Assoc. Study Obes. 1996, 20, 745–752. [Google Scholar]
- Presta, E.; Leibel, R.L.; Hirsch, J. Regional changes in adrenergic receptor status during hypocaloric intake do not predict changes in adipocyte size or body shape. Metabolism 1990, 39, 307–315. [Google Scholar] [CrossRef]
- Berlan, M.; Dang-Tran, L.; Lafontan, M.; Denard, Y. Influence of hypocaloric diet on alpha-adrenergic responsiveness of obese human subcutaneous adipocytes. Int. J. Obes. 1981, 5, 145–153. [Google Scholar]
- Rozen, R.; Banegas, E.; Davilla, M.; Apfelbaum, M. Effects of a very-low-calorie diet on adrenergic responsiveness in human adipose tissue. Int. J. Obes. 1984, 8, 141–149. [Google Scholar] [PubMed]
- Kather, H.; Wieland, E.; Fischer, B.; Wirth, A.; Schlierf, G. Adrenergic regulation of lipolysis in abdominal adipocytes of obese subjects during caloric restriction: Reversal of catecholamine action caused by relief of endogenous inhibition. Eur. J. Clin. Investig. 1985, 15, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, M.; Vidal, H.; Ohisalo, J.J.; Pirinen, E.; Alhava, E.; Uusitupa, M.I.J. Hormone sensitive lipase expression and adipose tissue metabolism show gender difference in obese subjects after weight loss. Int. J. Obes. 2002, 26, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Löfgren, P.; Hoffstedt, J.; Näslund, E.; Wirén, M.; Arner, P. Prospective and controlled studies of the actions of insulin and catecholamine in fat cells of obese women following weight reduction. Diabetologia 2005, 48, 2334–2342. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wallace, M.; Gurmaches, J.S.; Hsiao, W.-Y.; Li, H.; Lee, P.L.; Vernia, S.; Metallo, C.M.; Guertin, D.A. Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism. Nat. Commun. 2016, 7, 11365. [Google Scholar] [CrossRef] [PubMed]
- Minehira, K.; Vega, N.; Vidal, H.; Acheson, K.; Tappy, L. Effect of carbohydrate overfeeding on whole body macronutrient metabolism and expression of lipogenic enzymes in adipose tissue of lean and overweight humans. Int. J. Obes. 2004, 28, 1291–1298. [Google Scholar] [CrossRef]
- Strawford, A.; Antelo, F.; Christiansen, M.; Hellerstein, M.K. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am. J. Physiol. Metab. 2004, 286, E577–E588. [Google Scholar] [CrossRef]
- Solinas, G.; Borén, J.; Dulloo, A. De novo lipogenesis in metabolic homeostasis: More friend than foe? Mol. Metab. 2015, 4, 367–377. [Google Scholar] [CrossRef]
- Yilmaz, M.; Claiborn, K.C.; Hotamisligil, G.S. De Novo Lipogenesis Products and Endogenous Lipokines. Diabetes 2016, 65, 1800–1807. [Google Scholar] [CrossRef]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef]
- Moraes-Vieira, P.M.; Saghatelian, A.; Kahn, B.B. GLUT4 Expression in Adipocytes Regulates De Novo Lipogenesis and Levels of a Novel Class of Lipids with Antidiabetic and Anti-inflammatory Effects. Diabetes 2016, 65, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Pietiläinen, K.H.; Rog, T.; Seppänen-Laakso, T.; Virtue, S.; Gopalacharyulu, P.; Tang, J.; Rodríguez-Cuenca, S.; Maciejewski, A.; Naukkarinen, J.; Ruskeepää, A.-L.; et al. Association of lipidome remodeling in the adipocyte membrane with acquired obesity in humans. PLoS Boil. 2011, 9, e1000623. [Google Scholar] [CrossRef] [PubMed]
- Würtz, P.; Wang, Q.; Kangas, A.; Richmond, R.; Skarp, J.; Tiainen, M.; Tynkkynen, T.; Soininen, P.; Havulinna, A.S.; Kaakinen, M.; et al. Metabolic Signatures of Adiposity in Young Adults: Mendelian Randomization Analysis and Effects of Weight Change. PLoS Med. 2014, 11, e1001765. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Davis, B.A.; Fei, S.S.; White, A.; Nevonen, K.A.; Takahashi, D.; Vinson, A.; True, C.; Roberts, C.T., Jr.; Varlamov, O. Synergistic Effects of Hyperandrogenemia and Obesogenic Western-style Diet on Transcription and DNA Methylation in Visceral Adipose Tissue of Nonhuman Primates. Sci. Rep. 2019, 9, 19232. [Google Scholar] [CrossRef] [PubMed]
- True, C.; Abbott, D.H.; Roberts, J.C.T.; Varlamov, O. Sex Differences in Androgen Regulation of Metabolism in Nonhuman Primates. Adv. Exp. Med. Biol. 2017, 1043, 559–574. [Google Scholar]
- Varlamov, O.; Chu, M.P.; McGee, W.K.; Cameron, J.L.; O’Rourke, R.W.; Meyer, K.A.; Bishop, C.; Stouffer, R.L.; Roberts, J.C.T. Ovarian cycle-specific regulation of adipose tissue lipid storage by testosterone in female nonhuman primates. Endocrinology 2013, 154, 4126–4135. [Google Scholar] [CrossRef]
- Viguerie, N.; Montastier, E.; Maoret, J.-J.; Roussel, B.; Combes, M.; Valle, C.; Villa-Vialaneix, N.; Iacovoni, J.S.; Martínez, J.A.; Holst, C.; et al. Determinants of Human Adipose Tissue Gene Expression: Impact of Diet, Sex, Metabolic Status, and Cis Genetic Regulation. PLoS Genet. 2012, 8, e1002959. [Google Scholar] [CrossRef]
- Capel, F.; Klimčáková, E.; Viguerie, N.; Roussel, B.; Vítková, M.; Kováčiková, M.; Polak, J.; Kováčová, Z.; Galitzky, J.; Maoret, J.-J.; et al. Macrophages and Adipocytes in Human Obesity: Adipose Tissue Gene Expression and Insulin Sensitivity During Calorie Restriction and Weight Stabilization. Diabetes 2009, 58, 1558–1567. [Google Scholar] [CrossRef]
- Giles, E.D.; Steig, A.J.; Jackman, M.R.; Higgins, J.A.; Johnson, G.C.; Lindstrom, R.C.; MacLean, P. Exercise Decreases Lipogenic Gene Expression in Adipose Tissue and Alters Adipocyte Cellularity during Weight Regain After Weight Loss. Front. Physiol. 2016, 7, 188. [Google Scholar] [CrossRef]
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef]

| WSD/CHOW | CR/CHOW | CR/WSD | |||||
|---|---|---|---|---|---|---|---|
| Ensembl_ID | Entrez Gene Name | LogFC | FDR | LogFC | FDR | LogFC | FDR |
| ALAS2 | 5’-aminolevulinate synthase 2 | 3.56 | 3 × 10−52 | 1.24 | 2 × 10−4 | −2.33 | 1 × 10−19 |
| HBZ | hemoglobin subunit zeta | 3.35 | 7 × 10−49 | 0.79 | 0.066 | −2.56 | 5 × 10−23 |
| HBD | hypophosphatemic bone disease | 3.14 | 2 × 10−47 | 1.28 | 4 × 10−5 | −1.86 | 5.3 × 10−13 |
| HBB | hemoglobin subunit beta | 3.11 | 1 × 10−46 | 1.26 | 5 × 10−5 | −1.85 | 5.7 × 10−13 |
| HBA1 | hemoglobin subunit alpha 1 | 3.09 | 1 × 10−46 | 0.87 | 0.013 | −2.21 | 1.7 × 10−18 |
| HBA2 | hemoglobin subunit alpha 2 | 3.09 | 1 × 10−46 | 0.87 | 0.013 | −2.21 | 1.8 × 10−18 |
| HBE1 | hemoglobin subunit epsilon 1 | 3.08 | 1.7 × 10−45 | 1.23 | 9 × 10−5 | −1.85 | 5.6 × 10−13 |
| S100A9 | S100 calcium binding protein A9 | 3.06 | 4 × 10−35 | 0.54 | 0.516 | −2.53 | 1.4 × 10−18 |
| PF4 | platelet factor 4 | 2.98 | 4.8 × 10−22 | 1.70 | 1 × 10−4 | −1.28 | 0.00054 |
| GYPE | glycophorin E | 2.87 | 4 × 10−32 | 1.00 | 0.02 | −1.87 | 2 × 10−10 |
| GYPA | glycophorin A | 2.80 | 5.7 × 10−35 | 1.11 | 0.002 | −1.69 | 1.4 × 10−9 |
| HBBP1 | hemoglobin subunit beta pseudogene 1 | 2.75 | 3.3 × 10−7 | −0.28 | 1 | −3.03 | 1.1 × 10−6 |
| HBG1 | hemoglobin subunit gamma 1 | 2.63 | 3.3 × 10−8 | 1.05 | 0.021 | −1.58 | 2.8 × 10−7 |
| HBG2 | hemoglobin subunit gamma 2 | 2.63 | 3.6 × 10−23 | 1.05 | 0.021 | −1.58 | 2.8 × 10−7 |
| GYPB | glycophorin B (MNS blood group) | 2.58 | 1 × 10−19 | 0.46 | 0.84 | −2.12 | 6.3 × 10−10 |
| CXCL1 | C-X-C motif chemokine ligand 1 | 2.38 | 1.2 × 10−13 | 1.13 | 0.095 | −1.25 | 0.00614 |
| S100A8 | S100 calcium binding protein A8 | 2.28 | 1 × 10−20 | 0.07 | 1 | −2.21 | 3.7 × 10−13 |
| S100A7 | S100 calcium binding protein A7 | 1.69 | 0.028 | −0.03 | 1 | −1.72 | 0.0441 |
| MSR1 | macrophage scavenger receptor 1 | 1.50 | 7.5 × 10−13 | 0.51 | 0.231 | −1.00 | 0.000509 |
| CCL5 | C-C motif chemokine ligand 5 | 1.44 | 3.3 × 10−7 | 0.40 | 0.819 | −1.03 | 0.010258 |
| CCR1 | C-C motif chemokine receptor 1 | 1.30 | 7 × 10−9 | 0.25 | 0.901 | −1.04 | 0.000282 |
| CXCR2 | C-X-C motif chemokine receptor 2 | 1.25 | 7.2 × 10−6 | 0.23 | 0.995 | −1.02 | 0.006017 |
| CXCL10 | C-X-C motif chemokine ligand 10 | 1.24 | 1.5 × 10−5 | 0.10 | 1 | −1.14 | 0.004538 |
| CCL3 | C-C motif chemokine ligand 3 | 1.14 | 4 × 10−6 | 0.38 | 0.712 | −0.77 | 0.040866 |
| MMD | monocyte to macrophage differentiation associated | 1.13 | 6.7 × 10−8 | −0.27 | 0.86 | −1.41 | 4.3 × 10−8 |
| WSD/CHOW | CR/CHOW | CR/WSD | |||||
|---|---|---|---|---|---|---|---|
| Ensembl_ID | Entrez Gene Name | LogFC | FDR | LogFC | FDR | LogFC | FDR |
| ELOVL6 | ELOVL fatty acid elongase 6 | −2.203 | 0.000 | −2.959 | 0.000 | −0.756 | 0.023 |
| FASN | fatty acid synthase | −1.880 | 0.000 | −2.139 | 0.000 | −0.259 | 0.720 |
| ACLY | ATP citrate lyase | −1.469 | 0.000 | −2.310 | 0.000 | −0.841 | 0.003 |
| ACACA | acetyl-CoA carboxylase alpha | −1.384 | 0.000 | −1.936 | 0.000 | −0.552 | 0.133 |
| ADCY10 | adenylate cyclase 10 (soluble) | −1.251 | 0.000 | −1.970 | 0.000 | −0.719 | 0.044 |
| GPR39 | G protein-coupled receptor 39 | −1.200 | 0.000 | −1.208 | 0.000 | −0.008 | 1.000 |
| RUNX2 | runt related transcription factor 2 | −1.100 | 0.000 | −0.771 | 0.021 | 0.329 | 0.621 |
| PLIN2 | perilipin 2 | 1.546 | 0.000 | 0.350 | 0.623 | −1.196 | 0.000 |
| LDLR | low density lipoprotein receptor | 1.555 | 0.000 | −0.491 | 0.284 | −2.046 | 0.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wright, H.; Handu, M.; Jankeel, A.; Messaoudi, I.; Varlamov, O. Short-Term Caloric Restriction Attenuates Obesity-Induced Pro-inflammatory Response in Male Rhesus Macaques. Nutrients 2020, 12, 511. https://doi.org/10.3390/nu12020511
Wright H, Handu M, Jankeel A, Messaoudi I, Varlamov O. Short-Term Caloric Restriction Attenuates Obesity-Induced Pro-inflammatory Response in Male Rhesus Macaques. Nutrients. 2020; 12(2):511. https://doi.org/10.3390/nu12020511
Chicago/Turabian StyleWright, Hollis, Mithila Handu, Allen Jankeel, Ilhem Messaoudi, and Oleg Varlamov. 2020. "Short-Term Caloric Restriction Attenuates Obesity-Induced Pro-inflammatory Response in Male Rhesus Macaques" Nutrients 12, no. 2: 511. https://doi.org/10.3390/nu12020511
APA StyleWright, H., Handu, M., Jankeel, A., Messaoudi, I., & Varlamov, O. (2020). Short-Term Caloric Restriction Attenuates Obesity-Induced Pro-inflammatory Response in Male Rhesus Macaques. Nutrients, 12(2), 511. https://doi.org/10.3390/nu12020511





