Dietary Saturated Fatty Acids Modulate Pain Behaviour in Trauma-Induced Osteoarthritis in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Rats and Diets
2.2. Surgery
2.3. Metabolic, Cardiovascular, and Liver Parameters
2.4. Pain Behaviour
2.4.1. von Frey Test
2.4.2. Hind Limb Grip Strength
2.4.3. Pressure Application Measurement (PAM)
2.5. Assessment of Articular Cartilage, Subchondral Bone, Lumbar Spinal Cords, and Dorsal Root Ganglia
2.6. RNA Extraction and Real-time PCR
2.7. Quantification of Inflammatory Markers
2.8. Statistical Analysis
3. Results
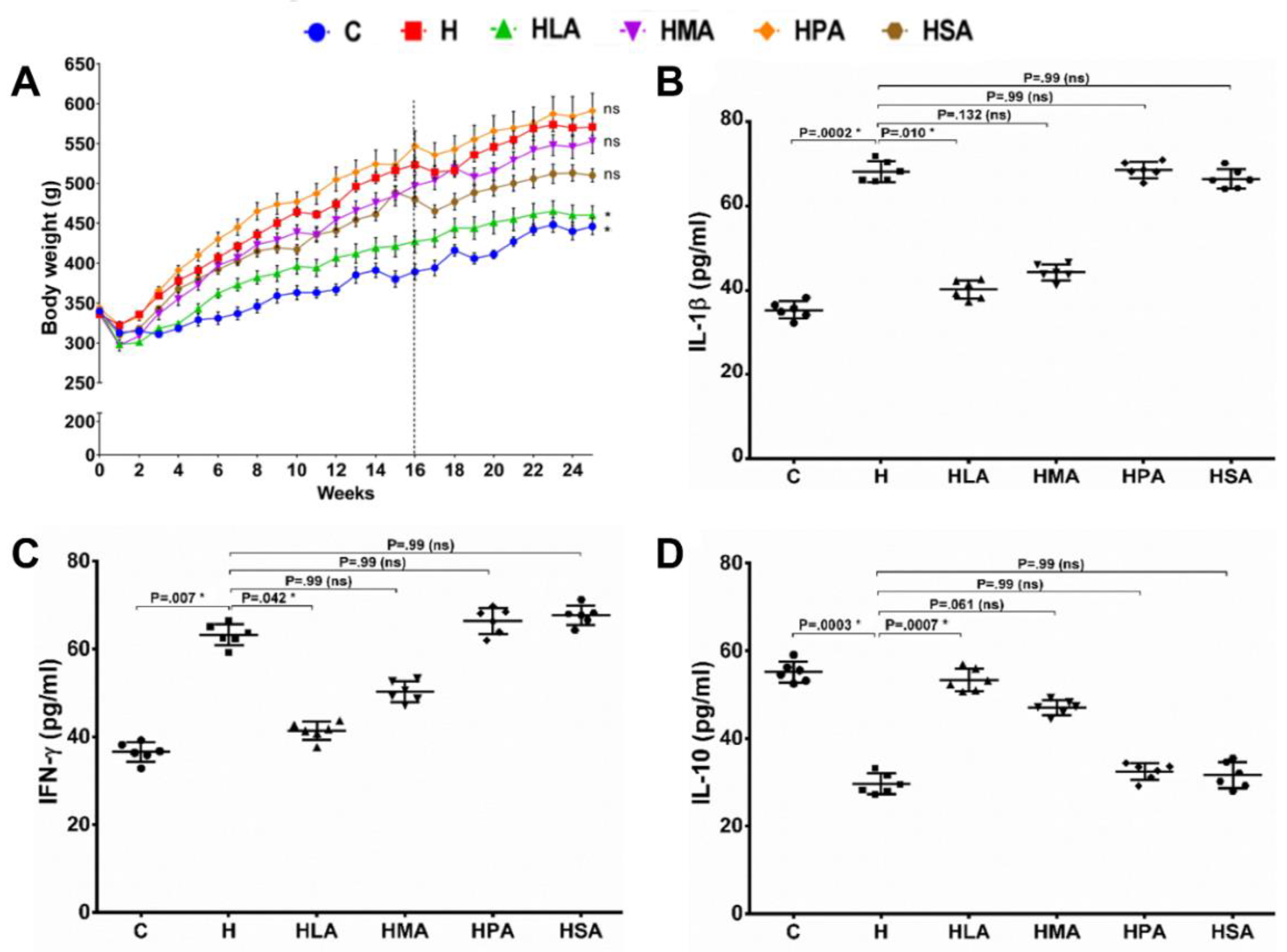
3.1. Metabolic, Cardiovascular and Liver Parameters
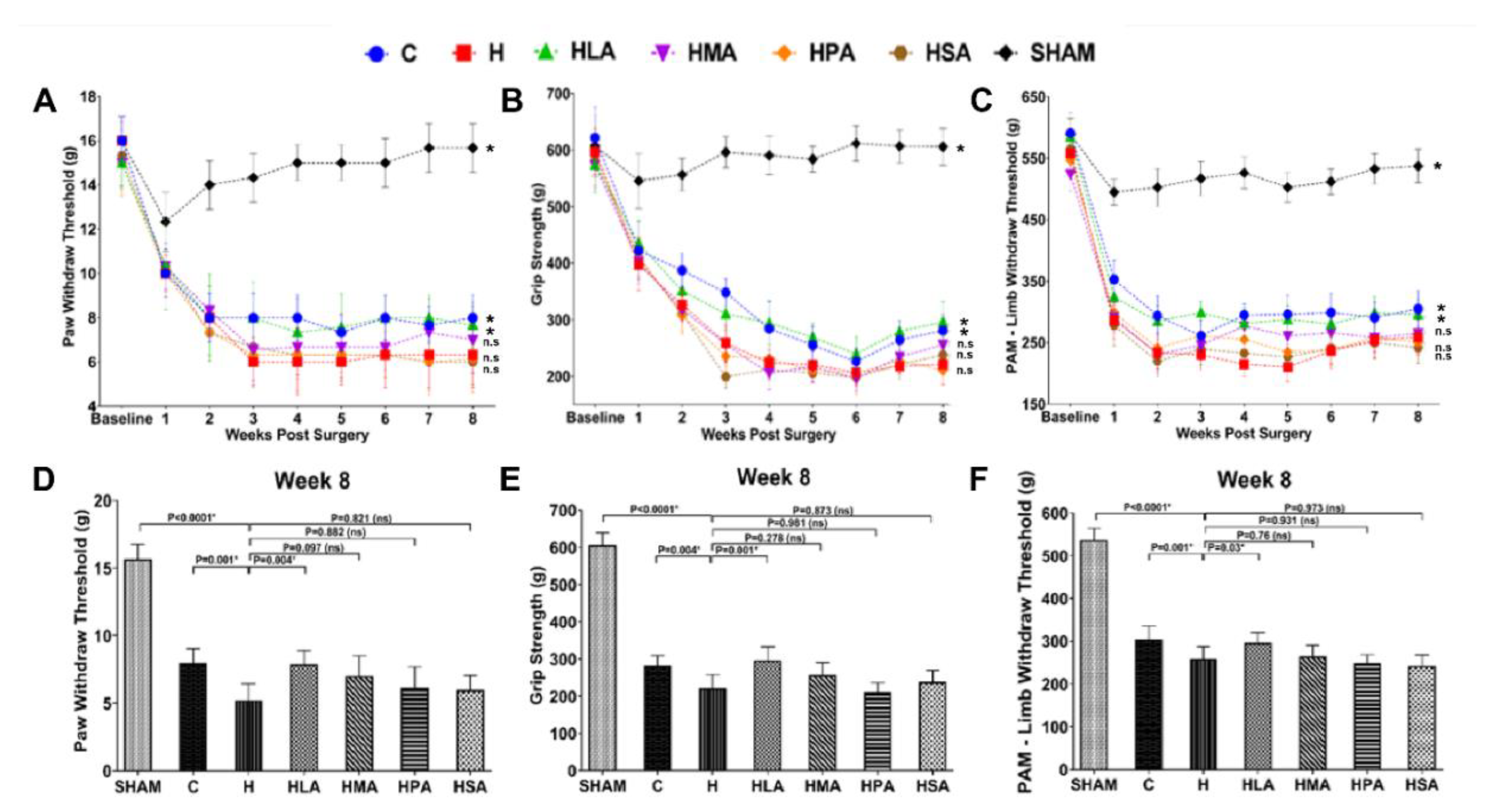
3.2. Pain Behaviour Post-Meniscectomy
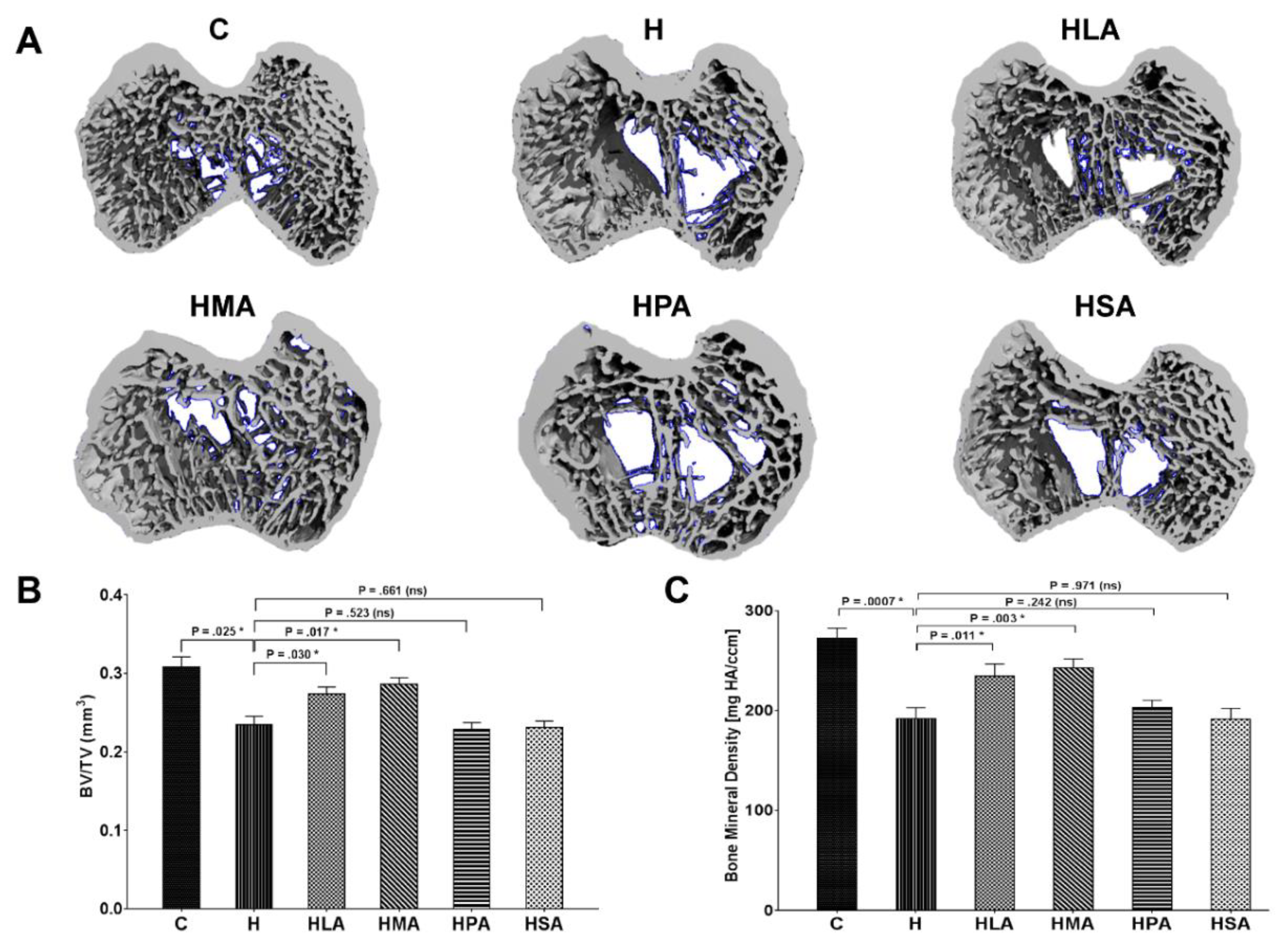
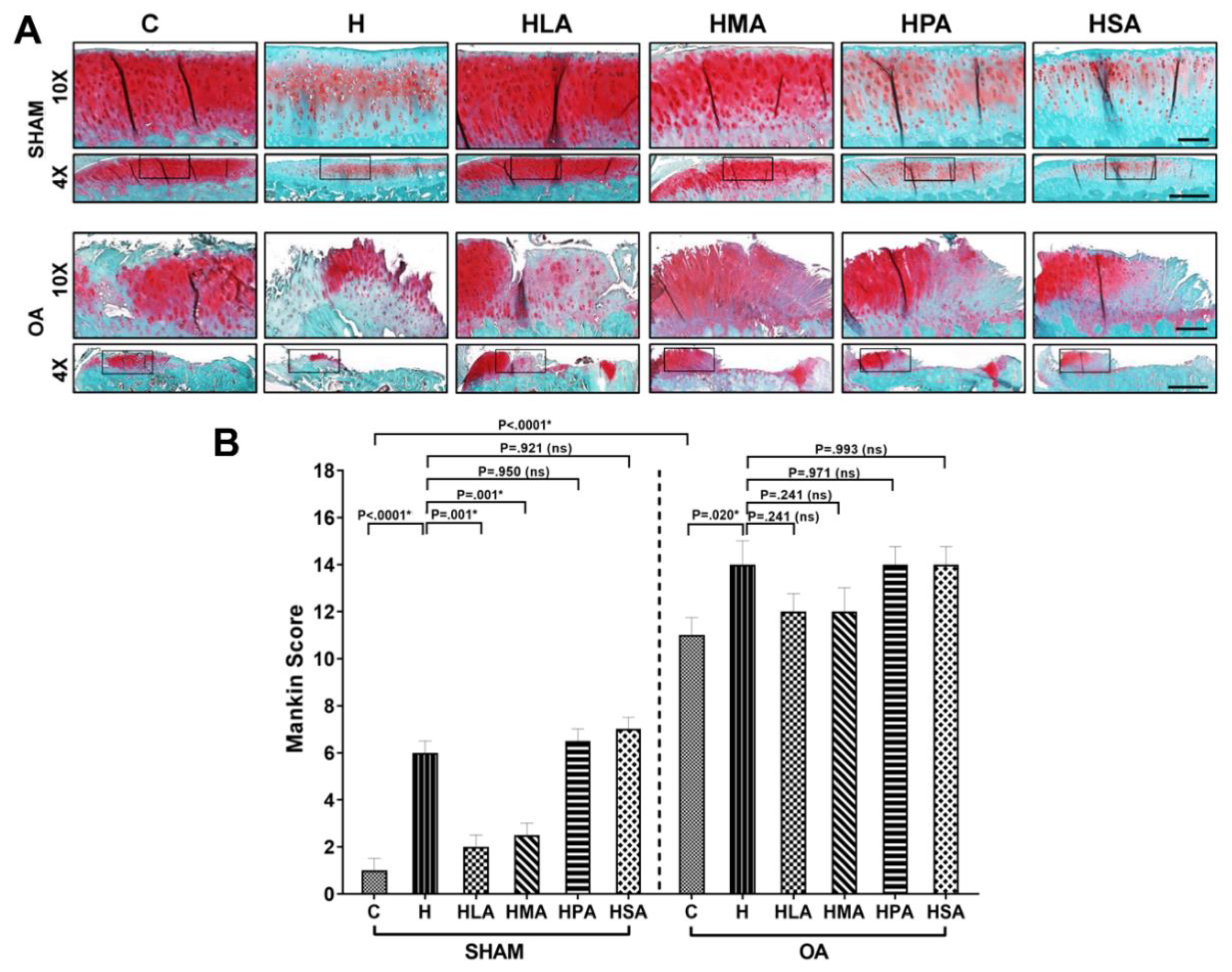
3.3. Structural Changes in Knee Joints
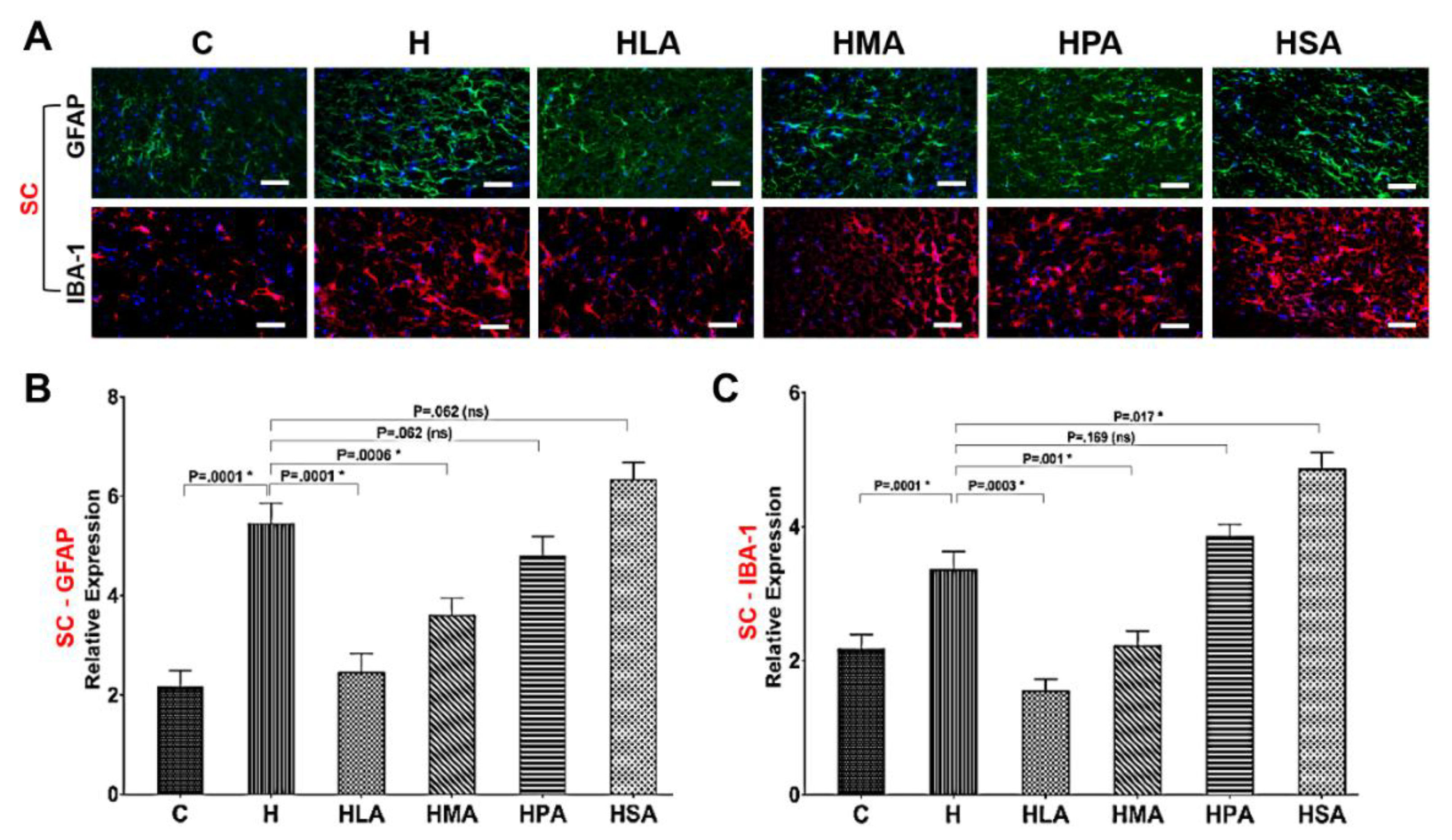
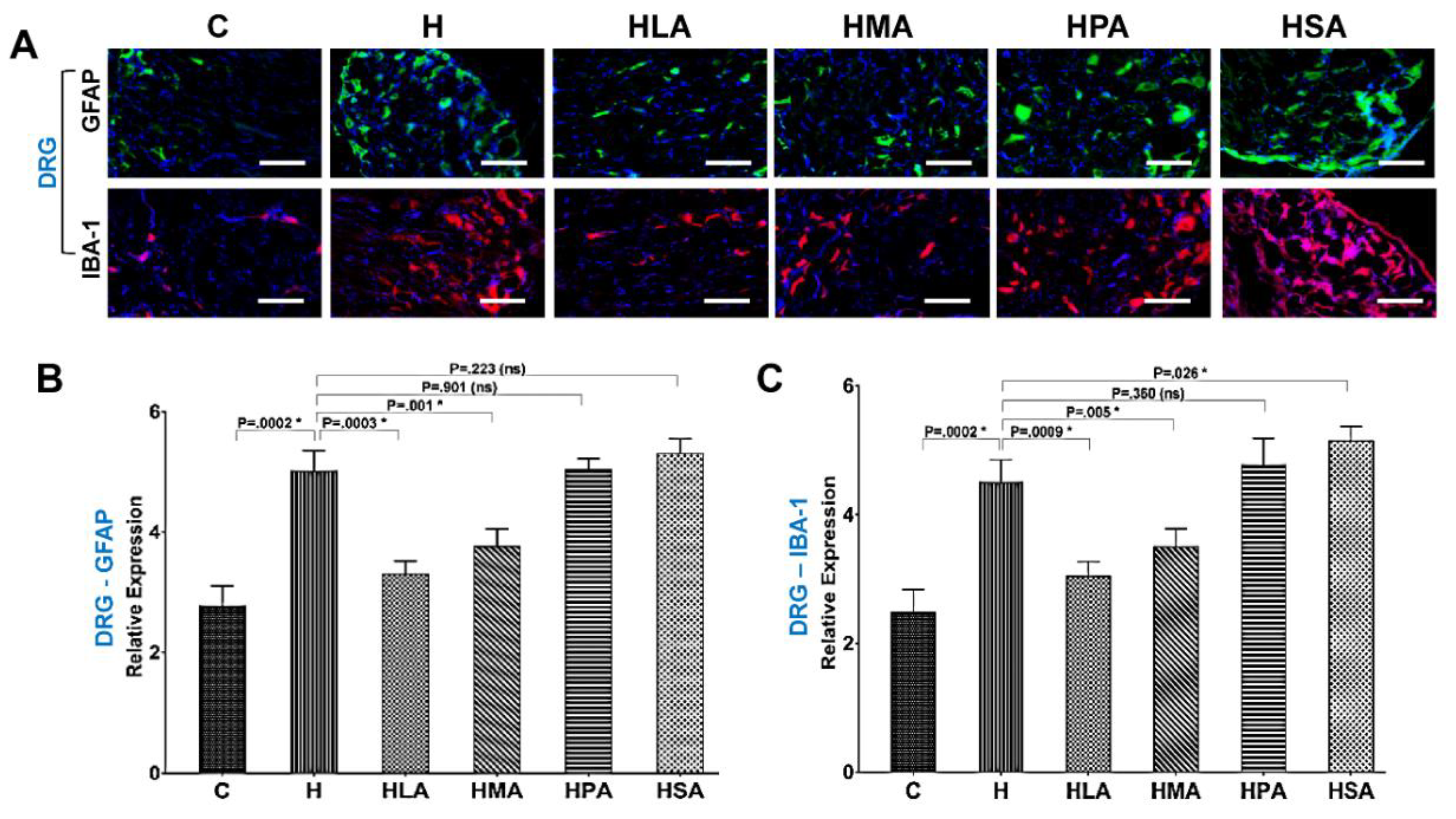
3.4. Spinal Glial Cell Activation Post-Meniscectomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiménez, G.; Cobo-Molinos, J.; Antich, C.; López-Ruiz, E. Osteoarthritis: Trauma vs disease. Adv. Exp. Med. Biol. 2018, 1059, 63–83. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. National Health Survey: First Results, 2017–2018. ABS cat. no. 4364.0.55.001. 2018. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/mf/4364.0.55.001 (accessed on 2 February 2020).
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Rezuș, E.; Cardoneanu, A.; Burlui, A.; Luca, A.; Codreanu, C.; Tamba, B.I.; Stanciu, G.-D.; Dima, N.; Bădescu, C.; Rezuș, C. The link between inflammaging and degenerative joint diseases. Int. J. Mol. Sci. 2019, 20, 614. [Google Scholar] [CrossRef]
- Belluzzi, E.; Stocco, E.; Pozzuoli, A.; Granzotto, M.; Porzionato, A.; Vettor, R.; De Caro, R.; Ruggieri, P.; Ramonda, R.; Rossato, M.; et al. Contribution of infrapatellar fat pad and synovial membrane to knee osteoarthritis pain. BioMed Res. Int. 2019, 2019, 6390182. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P.W. Mechanisms that drive bone pain across the lifespan. Br. J. Clin. Pharmacol. 2019, 85, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.D.; Suter, M.R.; Ji, R.R.; Decosterd, I. Glial cells and chronic pain. Neuroscientist 2010, 16, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, X.; Zhang, C.; Zhang, Y.; Yao, W. An update on reactive astrocytes in chronic pain. J. Neuroinflamm. 2019, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.R. Microglia in pain: Detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Courties, A.; Gualillo, O.; Berenbaum, F.; Sellam, J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1955–1965. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Herzog, W.; MacDonald, G.Z.; Reimer, R.A.; Rios, J.L.; Smith, I.C.; Zernicke, R.F.; Hart, D.A. Obesity, metabolic syndrome, and musculoskeletal disease: Common inflammatory pathways suggest a central role for loss of muscle integrity. Front. Physiol. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, E.; van Caam, A.; van der Kraan, P.M. Obesity and osteoarthritis, more than just wear and tear: Pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford) 2015, 54, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.R.; Panchal, S.K.; Friis, T.; Sekar, S.; Crawford, R.; Brown, L.; Xiao, Y.; Prasadam, I. Obesity-associated metabolic syndrome spontaneously induces infiltration of pro-inflammatory macrophage in synovium and promotes osteoarthritis. PLoS ONE 2017, 12, e0183693. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Shafie, S.R.; Prasadam, I.; Crawford, R.; Panchal, S.K.; Brown, L.; Xiao, Y. Saturated fatty acids induce development of both metabolic syndrome and osteoarthritis in rats. Sci. Rep. 2017, 7, 46457. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, M.A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef]
- Pekny, M.; Pekna, M. Astrocyte intermediate filaments in CNS pathologies and regeneration. J. Pathol. 2004, 204, 428–437. [Google Scholar] [CrossRef]
- Hathway, G.J.; Vega-Avelaira, D.; Moss, A.; Ingram, R.; Fitzgerald, M. Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain 2009, 144, 110–118. [Google Scholar] [CrossRef]
- Prasadam, I.; Crawford, R.; Xiao, Y. Aggravation of ADAMTS and matrix metalloproteinase production and role of ERK1/2 pathway in the interaction of osteoarthritic subchondral bone osteoblasts and articular cartilage chondrocytes—Possible pathogenic role in osteoarthritis. J. Rheumatol. 2012, 39, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Prasadam, I.; Mao, X.; Wang, Y.; Shi, W.; Crawford, R.; Xiao, Y. Inhibition of p38 pathway leads to OA-like changes in a rat animal model. Rheumatology (Oxford) 2012, 51, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Courties, A.; Berenbaum, F.; Sellam, J. The phenotypic approach to osteoarthritis: A look at metabolic syndrome-associated osteoarthritis. Joint Bone Spine 2019, 86, 725–730. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.M. Animal models of osteoarthritis: Comparisons and key considerations. Vet. Pathol. 2015, 52, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.S.; Prasadam, I.; Xiao, Y.; Momot, K.I. Progression of post-traumatic osteoarthritis in rat meniscectomy models: Comprehensive monitoring using MRI. Sci. Rep. 2018, 8, 6861. [Google Scholar] [CrossRef] [PubMed]
- Angeby Moller, K.; Klein, S.; Seeliger, F.; Finn, A.; Stenfors, C.; Svensson, C.I. Monosodium iodoacetate-induced monoarthritis develops differently in knee versus ankle joint in rats. Neurobiol. Pain 2019, 6, 100036. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef]
- Miller, R.E.; Malfait, A.-M. Osteoarthritis pain: What are we learning from animal models? Best Pract. Res. Clin. Rheumatol. 2017, 31, 676–687. [Google Scholar] [CrossRef]
- Suokas, A.K.; Sagar, D.R.; Mapp, P.I.; Chapman, V.; Walsh, D.A. Design, study quality and evidence of analgesic efficacy in studies of drugs in models of OA pain: A systematic review and a meta-analysis. Osteoarthr. Cartil. 2014, 22, 1207–1223. [Google Scholar] [CrossRef]
- Garcia, M.M.; Goicoechea, C.; Avellanal, M.; Traseira, S.; Martin, M.I.; Sanchez-Robles, E.M. Comparison of the antinociceptive profiles of morphine and oxycodone in two models of inflammatory and osteoarthritic pain in rat. Eur. J. Pharmacol. 2019, 854, 109–118. [Google Scholar] [CrossRef]
- Sudirman, S.; Ong, A.D.; Chang, H.W.; Kong, Z.L. Effect of fucoidan on anterior cruciate ligament transection and medial meniscectomy induced osteoarthritis in high-fat diet-induced obese rats. Nutrients 2018, 10, 686. [Google Scholar] [CrossRef]
- Bhaswant, M.; Fanning, K.; Netzel, M.; Mathai, M.L.; Panchal, S.K.; Brown, L. Cyanidin 3-glucoside improves diet-induced metabolic syndrome in rats. Pharmacol. Res. 2015, 102, 208–217. [Google Scholar] [CrossRef]
- du Preez, R.; Paul, N.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Carrageenans from the red seaweed Sarconema filiforme attenuate symptoms of diet-induced metabolic syndrome in rats. Mar. Drugs 2020, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Leyton, J.; Drury, P.J.; Crawford, M.A. Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br. J. Nutr. 1987, 57, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Perez, T.; Pino, J.; Lopez, V.; Franco, E.; Alonso, A.; Gonzalez-Gay, M.A.; Mera, A.; Lago, F.; Gomez, R.; et al. Biomechanics, obesity, and osteoarthritis. The role of adipokines: When the levee breaks. J. Orthop. Res. 2018, 36, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; He, J.; Wu, B.; Wang, W.; Li, Z. An extensive review regarding the adipokines in the pathogenesis and progression of osteoarthritis. Cytokine 2019, 113, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Chu, I.J.H.; Lim, A.Y.T.; Ng, C.L.W. Effects of meaningful weight loss beyond symptomatic relief in adults with knee osteoarthritis and obesity: A systematic review and meta-analysis. Obes. Rev. 2018, 19, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Loef, M.; Schoones, J.W.; Kloppenburg, M.; Ioan-Facsinay, A. Fatty acids and osteoarthritis: Different types, different effects. Joint Bone Spine 2019, 86, 451–458. [Google Scholar] [CrossRef]
- Barr, A.J.; Campbell, T.M.; Hopkinson, D.; Kingsbury, S.R.; Bowes, M.A.; Conaghan, P.G. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis Res. Ther. 2015, 17, 228. [Google Scholar] [CrossRef]
- Yu, D.; Xu, J.; Liu, F.; Wang, X.; Mao, Y.; Zhu, Z. Subchondral bone changes and the impacts on joint pain and articular cartilage degeneration in osteoarthritis. Clin. Exp. Rheumatol. 2016, 34, 929–934. [Google Scholar]
- Perrot, S. Osteoarthritis pain. Best Pract. Res. Clin. Rheumatol. 2015, 29, 90–97. [Google Scholar] [CrossRef]
- Orita, S.; Ishikawa, T.; Miyagi, M.; Ochiai, N.; Inoue, G.; Eguchi, Y.; Kamoda, H.; Arai, G.; Toyone, T.; Aoki, Y.; et al. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskelet. Disord. 2011, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Gu, N.; Peng, J.; Murugan, M.; Wang, X.; Eyo, U.B.; Sun, D.; Ren, Y.; DiCicco-Bloom, E.; Young, W.; Dong, H.; et al. Spinal microgliosis due to resident microglial proliferation is required for pain hypersensitivity after peripheral nerve injury. Cell Rep. 2016, 16, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Sagar, D.R.; Burston, J.J.; Hathway, G.J.; Woodhams, S.G.; Pearson, R.G.; Bennett, A.J.; Kendall, D.A.; Scammell, B.E.; Chapman, V. The contribution of spinal glial cells to chronic pain behaviour in the monosodium iodoacetate model of osteoarthritic pain. Mol. Pain 2011, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Balbinot, G.; Schuch, C.P.; Nascimento, P.S.D.; Lanferdini, F.J.; Casanova, M.; Baroni, B.M.; Vaz, M.A. Photobiomodulation therapy partially restores cartilage integrity and reduces chronic pain behavior in a rat model of osteoarthritis: Involvement of spinal glial modulation. Cartilage 2019. [Google Scholar] [CrossRef]
- Hahm, S.C.; Song, E.; Jeon, H.; Yoon, Y.W.; Kim, J. Transcutaneous electrical nerve stimulation reduces knee osteoarthritic Pain by inhibiting spinal glial cells in rats. Phys. Ther. 2019, 99, 1211–1223. [Google Scholar] [CrossRef]
- Sagar, D.R.; Ashraf, S.; Xu, L.; Burston, J.J.; Menhinick, M.R.; Poulter, C.L.; Bennett, A.J.; Walsh, D.A.; Chapman, V. Osteoprotegerin reduces the development of pain behaviour and joint pathology in a model of osteoarthritis. Ann. Rheum. Dis. 2014, 73, 1558–1565. [Google Scholar] [CrossRef]
- Mapp, P.I.; Walsh, D.A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 390–398. [Google Scholar] [CrossRef]
- Dowsey, M.M.; Dieppe, P.; Lohmander, S.; Castle, D.; Liew, D.; Choong, P.F. The association between radiographic severity and pre-operative function in patients undergoing primary knee replacement for osteoarthritis. Knee 2012, 19, 860–865. [Google Scholar] [CrossRef]
- Boer, C.G.; Radjabzadeh, D.; Medina-Gomez, C.; Garmaeva, S.; Schiphof, D.; Arp, P.; Koet, T.; Kurilshikov, A.; Fu, J.; Ikram, M.A.; et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 2019, 10, 4881. [Google Scholar] [CrossRef]
| Variables | C | H | HLA | HMA | HPA | HSA |
|---|---|---|---|---|---|---|
| Initial body weight, g | 340 ± 2ab | 336 ± 0b | 340 ± 2ab | 336 ± 1b | 344 ± 3a | 337 ± 1b |
| Final body weight, g | 446 ± 10b | 571 ± 11a | 460 ± 12b | 553 ± 15a | 591 ± 22a | 492 ± 8b |
| Body weight gain, g | 106 ± 10c | 235 ± 11a | 120 ± 11bc | 217 ± 15a | 247 ± 20a | 155 ± 8b |
| Food intake, g/d | 38.1 ± 1.3a | 25.7 ± 0.3cd | 23.8 ± 1.1d | 27.2 ± 1.1bcd | 30.7 ± 1.0b | 29.4 ± 1.5bc |
| Water intake, mL/d | 25.6 ± 2.4b | 23.3 ± 1.3b | 37.9 ± 2.3a | 28.8 ± 1.4b | 34.7 ± 1.0a | 33.8 ± 0.8a |
| Energy intake, kJ/d | 428 ± 14c | 548 ± 9b | 571 ± 19b | 596 ± 23b | 681 ± 21a | 654 ± 28a |
| Feed efficiency, g/kJ | 0.25 ± 0.02c | 0.43 ± 0.02a | 0.21 ± 0.02c | 0.36 ± 0.02b | 0.36 ± 0.02b | 0.24 ± 0.01c |
| Variables | C | H | HLA | HMA | HPA | HSA |
|---|---|---|---|---|---|---|
| Abdominal circumference, cm | 19.8 ± 0.2d | 23.6 ± 0.1ab | 21.1 ± 0.5c | 22.9 ± 0.4ab | 23.8 ± 0.6a | 22.3 ± 0.2b |
| Retroperitoneal fat, mg/mm* | 190 ± 25b | 489 ± 34a | 201 ± 11b | 389 ± 42a | 379 ± 37a | 272 ± 34b |
| Epididymal fat, mg/mm* | 97 ± 8.9c | 231 ± 15a | 110 ± 13c | 221 ± 11a | 208 ± 17a | 163 ± 24b |
| Omental fat, mg/mm* | 144 ± 18c | 261 ± 33a | 133 ± 11c | 229 ± 19ab | 207 ± 19abc | 163 ± 16bc |
| Abdominal fat pads, mg/mm* | 431 ± 50b | 981 ± 76a | 444 ± 25b | 840 ± 60a | 794 ± 60a | 598 ± 67b |
| Bone mineral density, g/cm2 | 0.185 ± 0.004ab | 0.193 ± 0.005a | 0.186 ± 0.003ab | 0.186 ± 0.004ab | 0.189 ± 0.003ab | 0.176 ± 0.004b |
| Bone mineral content, g | 12.9 ± 0.5c | 16.7 ± 0.6ab | 13.2 ± 0.6c | 15.3 ± 0.6b | 17.5 ± 0.70a | 13.0 ± 0.4c |
| Total fat mass, g | 98 ± 13c | 209 ± 20a | 105 ± 15c | 205 ± 20a | 231 ± 22a | 129 ± 15c |
| Total lean mass, g | 329 ± 5 | 350 ± 17 | 348 ± 9 | 333 ± 19 | 339 ± 12 | 359 ± 20 |
| Blood glucose AUC, mmol/L×min | 720 ± 39ab | 773 ± 26a | 644 ± 24b | 653 ± 18b | 720 ± 20ab | 638 ± 23b |
| Fasting blood glucose, mmol/L | 3.5 ± 0.5 | 4.6 ± 0.2 | 3.6 ± 0.3 | 4.0 ± 0.3 | 4.6 ± 0.3 | 4.2 ± 0.3 |
| Blood glucose at 120 min, mmol/L | 5.2 ± 0.2ab | 6.3 ± 0.3a | 4.9 ± 0.3b | 5.0 ± 0.3b | 5.3 ± 0.3ab | 5.2 ± 0.2ab |
| Plasma NEFA, mmol/L | 1.46 ± 0.19b | 3.58 ± 0.56a | 2.04 ± 0.44ab | 3.25 ± 0.53a | 3.47 ± 0.28a | 3.40 ± 0.56a |
| Plasma triglycerides, mmol/L | 0.51 ± 0.04b | 1.33 ± 0.17ab | 0.56 ± 0.12b | 1.71 ± 0.40a | 1.55 ± 0.32ab | 1.11 ± 0.23ab |
| Plasma total cholesterol, mmol/L | 1.41 ± 0.05 | 1.61 ± 0.11 | 1.46 ± 0.05 | 1.64 ± 0.08 | 1.63 ± 0.03 | 1.34 ± 0.08 |
| Variables | C | H | HLA | HMA | HPA | HSA |
|---|---|---|---|---|---|---|
| Systolic blood pressure, mmHg | 128 ± 3b | 153 ± 4a | 119 ± 4b | 149 ± 9a | 152 ± 5a | 154 ± 4a |
| LV diastolic stiffness constant (ĸ) | 20.2 ± 1.2 | 23.2 ± 1.3 | 20.6 ± 1.6 | 23.8 ± 0.5 | 22.3 ± 1.6 | 24.2 ± 0.5 |
| LV + Septum wet weight, mg/mm* | 21.2 ± 1.0b | 26.0 ± 1.3a | 20.9 ± 1.0b | 25.3 ± 0.9ab | 23.8 ± 1.4ab | 23.4 ± 1.1ab |
| RV wet weight, mg/mm* | 5.28 ± 0.88 | 5.31 ± 0.22 | 5.04 ± 0.43 | 4.67 ± 0.35 | 4.78 ± 0.48 | 5.10 ± 0.24 |
| Liver wet weight, mg/mm* | 236 ± 10b | 345 ± 13a | 267 ± 17b | 350 ± 11a | 325 ± 16a | 263 ± 3.0b |
| Plasma ALT, U/L | 34.1 ± 7.7 | 28.5 ± 2.1 | 28.4 ± 7.1 | 32.6 ± 3.9 | 21.4 ± 1.5 | 35.1 ± 6.9 |
| Plasma AST, U/L | 78.2 ± 4.8 | 74.6 ± 8.2 | 71.9 ± 7.5 | 80.4 ± 8.7 | 62.8 ± 1.7 | 70.5 ± 4.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekar, S.; Panchal, S.K.; Ghattamaneni, N.K.; Brown, L.; Crawford, R.; Xiao, Y.; Prasadam, I. Dietary Saturated Fatty Acids Modulate Pain Behaviour in Trauma-Induced Osteoarthritis in Rats. Nutrients 2020, 12, 509. https://doi.org/10.3390/nu12020509
Sekar S, Panchal SK, Ghattamaneni NK, Brown L, Crawford R, Xiao Y, Prasadam I. Dietary Saturated Fatty Acids Modulate Pain Behaviour in Trauma-Induced Osteoarthritis in Rats. Nutrients. 2020; 12(2):509. https://doi.org/10.3390/nu12020509
Chicago/Turabian StyleSekar, Sunderajhan, Sunil K Panchal, Naga KR Ghattamaneni, Lindsay Brown, Ross Crawford, Yin Xiao, and Indira Prasadam. 2020. "Dietary Saturated Fatty Acids Modulate Pain Behaviour in Trauma-Induced Osteoarthritis in Rats" Nutrients 12, no. 2: 509. https://doi.org/10.3390/nu12020509
APA StyleSekar, S., Panchal, S. K., Ghattamaneni, N. K., Brown, L., Crawford, R., Xiao, Y., & Prasadam, I. (2020). Dietary Saturated Fatty Acids Modulate Pain Behaviour in Trauma-Induced Osteoarthritis in Rats. Nutrients, 12(2), 509. https://doi.org/10.3390/nu12020509








