Breast Milk Iodine Concentration Is Associated with Infant Growth, Independent of Maternal Weight
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Methods
2.2. Milk Collection
2.3. Laboratory Analysis
2.4. Infant Anthropometric Measurements
2.5. Statistical Analysis
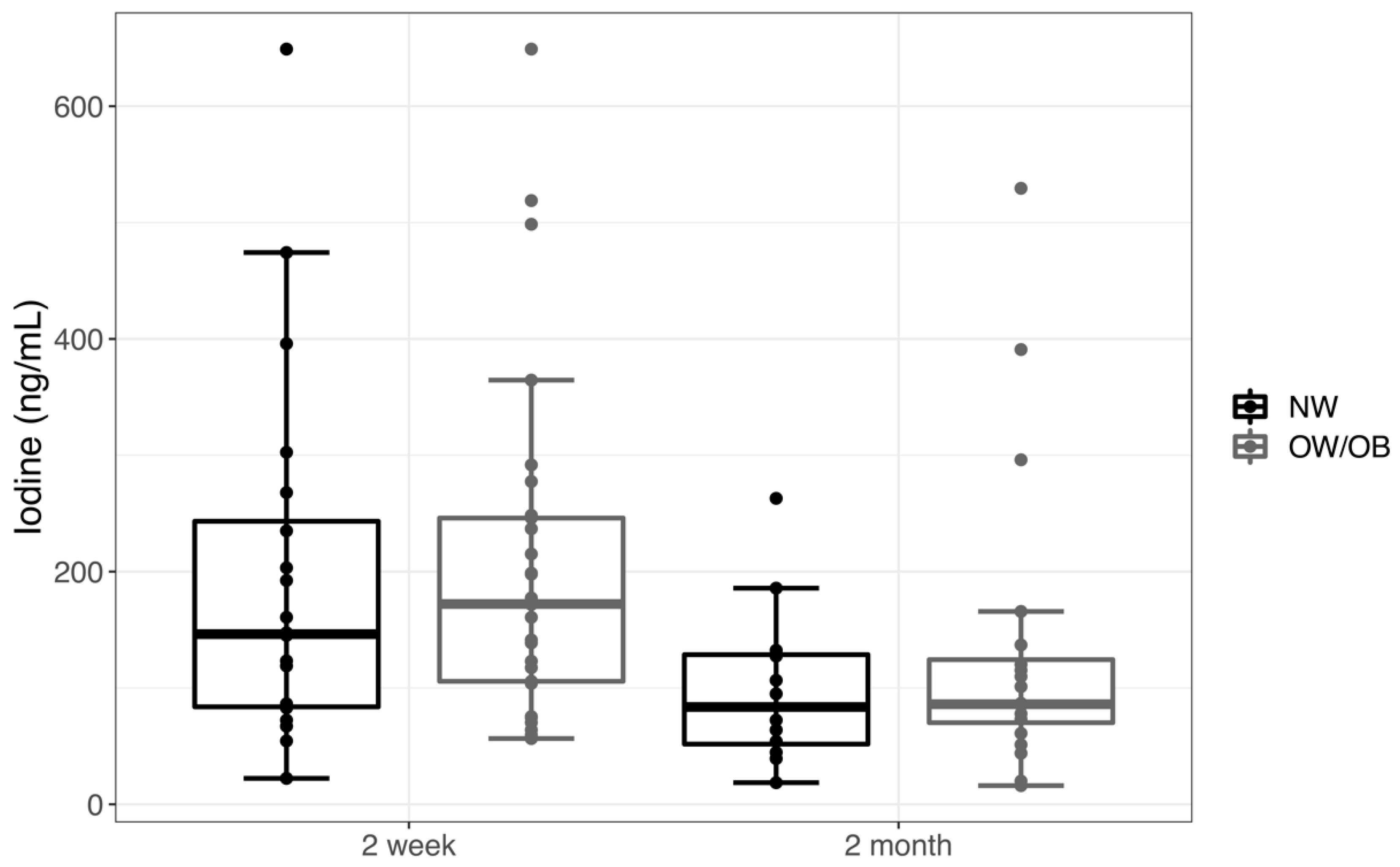
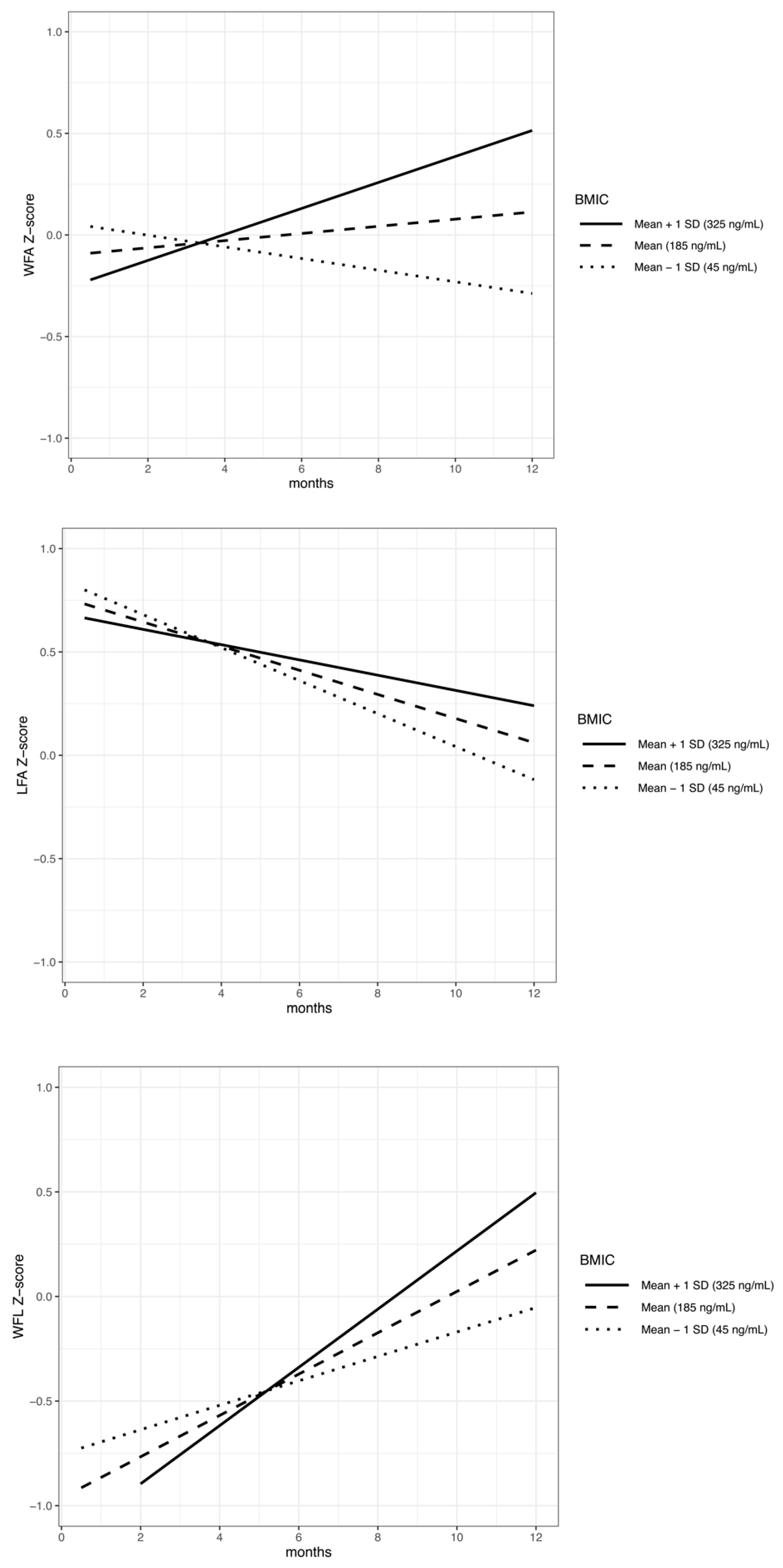
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Zhu, L.; Li, X.; Zheng, H.; Wang, Z.; Hao, Z.; Liu, Y. Maternal iodine status during lactation and infant weight and length in Henan Province, China. BMC Pregnancy Childbirth 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Azizi, F.; Smyth, P. Breastfeeding and maternal and infant iodine nutrition. Clin. Endocrinol. (Oxf) 2009, 70, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Iodine in Human Milk: A Systematic Review. Adv. Nutr. (Bethesda, Md.) 2018, 9, 347S–357S. [Google Scholar] [CrossRef]
- Michaelsen, K.F.; Larsen, P.S.; Thomsen, B.L.; Samuelson, G. The Copenhagen Cohort Study on Infant Nutrition and Growth: Breast-milk intake, human milk macronutrient content, and influencing factors. Am. J. Clin. Nutr. 1994, 59, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. The role of iodine in human growth and development. Semin. Cell Dev. Biol. 2011, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Etling, N.; Padovani, E.; Fouque, F.; Tato, L. First-month variations in total iodine content of human breast milks. Early Hum. Dev. 1986, 13, 81–85. [Google Scholar] [CrossRef]
- Semba, R.D.; Delange, F. Iodine in human milk: Perspectives for infant health. Nutr. Rev. 2001, 59, 269–278. [Google Scholar] [CrossRef]
- van den Hove, M.F.; Beckers, C.; Devlieger, H.; de Zegher, F.; De Nayer, P. Hormone synthesis and storage in the thyroid of human preterm and term newborns: Effect of thyroxine treatment. Biochimie 1999, 81, 563–570. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Are weaning infants at risk of iodine deficiency even in countries with established iodized salt programs? Nestle Nutr. Inst. Workshop Ser. 2012, 70, 137–146. [Google Scholar] [CrossRef]
- Mulrine, H.M.; Skeaff, S.A.; Ferguson, E.L.; Gray, A.R.; Valeix, P. Breast-milk iodine concentration declines over the first 6 mo postpartum in iodine-deficient women. Am. J. Clin. Nutr. 2010, 92, 849–856. [Google Scholar] [CrossRef]
- WHO. Reaching Optimal Iodine Nutrition in Pregnant and Lactating Women and Young Children; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Institute of Medicine (US). Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222310/ (accessed on 10 November 2019).
- ACOG Committee opinion No. 549: Obesity in pregnancy. Obstet. Gynecol. 2013, 121, 213–217.
- Dold, S.; Zimmermann, M.B.; Baumgartner, J.; Davaz, T.; Galetti, V.; Braegger, C.; Andersson, M. A dose-response crossover iodine balance study to determine iodine requirements in early infancy. Am. J. Clin. Nutr. 2016, 104, 620–628. [Google Scholar] [CrossRef]
- Chierici, R.; Saccomandi, D.; Vigi, V. Dietary supplements for the lactating mother: Influence on the trace element content of milk. Acta Paediatr. (Oslo, Norway: 1992) Suppl. 1999, 88, 7–13. [Google Scholar] [CrossRef]
- Henjum, S.; Lilleengen, A.M.; Aakre, I.; Dudareva, A.; Gjengedal, E.L.F.; Meltzer, H.M.; Brantsaeter, A.L. Suboptimal Iodine Concentration in Breastmilk and Inadequate Iodine Intake among Lactating Women in Norway. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Osei, J.; Andersson, M.; Reijden, O.V.; Dold, S.; Smuts, C.M.; Baumgartner, J. Breast-Milk Iodine Concentrations, Iodine Status, and Thyroid Function of Breastfed Infants Aged 2–4 Months and Their Mothers Residing in a South African Township. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 381–391. [Google Scholar] [CrossRef]
- Sabatier, M.; Garcia-Rodenas, C.L.; Castro, C.A.; Kastenmayer, P.; Vigo, M.; Dubascoux, S.; Andrey, D.; Nicolas, M.; Payot, J.R.; Bordier, V.; et al. Longitudinal Changes of Mineral Concentrations in Preterm and Term Human Milk from Lactating Swiss Women. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, M.; Bai, Y.; Hao, Y.; Chen, W.; Cui, T.; Guo, W.; Pan, Z.; Lin, L.; Wang, C.; et al. Variation of iodine concentration in breast milk and urine in exclusively breastfeeding women and their infants during the first 24 wk after childbirth. Nutrition (Burbank, Los Angeles County, Calif.) 2019, 71, 110599. [Google Scholar] [CrossRef]
- Tazebay, U.H.; Wapnir, I.L.; Levy, O.; Dohan, O.; Zuckier, L.S.; Zhao, Q.H.; Deng, H.F.; Amenta, P.S.; Fineberg, S.; Pestell, R.G.; et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat. Med. 2000, 6, 871–878. [Google Scholar] [CrossRef]
- Brown-Grant, K. The iodide concentrating mechanism of the mammary gland. J. Physiol 1957, 135, 644–654. [Google Scholar] [CrossRef]
- Dold, S.; Zimmermann, M.B.; Aboussad, A.; Cherkaoui, M.; Jia, Q.; Jukic, T.; Kusic, Z.; Quirino, A.; Sang, Z.; San Luis, T.O.; et al. Breast Milk Iodine Concentration Is a More Accurate Biomarker of Iodine Status Than Urinary Iodine Concentration in Exclusively Breastfeeding Women. J. Nutr. 2017, 147, 528–537. [Google Scholar] [CrossRef]
- Eskin, B.A.; Bartuska, D.G.; Dunn, M.R.; Jacob, G.; Dratman, M.B. Mammary gland dysplasia in iodine deficiency. Studies in rats. JAMA 1967, 200, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, P.; Dalili, H.; Mehrabi, Y.; Hedayati, M.; Mirmiran, P.; Azizi, F. Breast Milk Iodine Concentration Rather than Maternal Urinary Iodine Is a Reliable Indicator for Monitoring Iodine Status of Breastfed Neonates. Biol. Trace Elem. Res. 2018, 185, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Strum, J.M. Effect of iodide-deficiency on rat mammary gland. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1979, 30, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.M.; Crozier, S.R.; Miles, E.A.; Gale, C.R.; Calder, P.C.; Cooper, C.; Inskip, H.M.; Godfrey, K.M. Preconception Maternal Iodine Status Is Positively Associated with IQ but Not with Measures of Executive Function in Childhood. J. Nutr. 2018, 148, 959–966. [Google Scholar] [CrossRef]
- Soriguer, F.; Valdes, S.; Morcillo, S.; Esteva, I.; Almaraz, M.C.; de Adana, M.S.; Tapia, M.J.; Dominguez, M.; Gutierrez-Repiso, C.; Rubio-Martin, E.; et al. Thyroid hormone levels predict the change in body weight: A prospective study. Eur. J. Clin. Invest. 2011, 41, 1202–1209. [Google Scholar] [CrossRef]
- Torlinska, B.; Bath, S.C.; Janjua, A.; Boelaert, K.; Chan, S.Y. Iodine Status during Pregnancy in a Region of Mild-to-Moderate Iodine Deficiency is not Associated with Adverse Obstetric Outcomes; Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Lecube, A.; Zafon, C.; Gromaz, A.; Fort, J.M.; Caubet, E.; Baena, J.A.; Tortosa, F. Iodine deficiency is higher in morbid obesity in comparison with late after bariatric surgery and non-obese women. Obes. Surg. 2015, 25, 85–89. [Google Scholar] [CrossRef]
- Alvarez-Pedrerol, M.; Guxens, M.; Mendez, M.; Canet, Y.; Martorell, R.; Espada, M.; Plana, E.; Rebagliato, M.; Sunyer, J. Iodine levels and thyroid hormones in healthy pregnant women and birth weight of their offspring. Eur. J. Endocrinol. 2009, 160, 423–429. [Google Scholar] [CrossRef]
- Farebrother, J.; Naude, C.E.; Nicol, L.; Sang, Z.; Yang, Z.; Jooste, P.L.; Andersson, M.; Zimmermann, M.B. Effects of Iodized Salt and Iodine Supplements on Prenatal and Postnatal Growth: A Systematic Review. Adv. Nutr. (Bethesda, Md.) 2018, 9, 219–237. [Google Scholar] [CrossRef]
- Gunnarsdottir, I.; Dahl, L. Iodine intake in human nutrition: A systematic literature review. Food Nutr. Res. 2012, 56. [Google Scholar] [CrossRef]
- Rydbeck, F.; Rahman, A.; Grander, M.; Ekstrom, E.C.; Vahter, M.; Kippler, M. Maternal urinary iodine concentration up to 1.0 mg/L is positively associated with birth weight, length, and head circumference of male offspring. J. Nutr. 2014, 144, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Iodine supplementation for women during the preconception, pregnancy and postpartum period - Harding, KB - 2017. Cochrane Libr. 2019. [CrossRef]
- Aboud, F.E.; Bougma, K.; Lemma, T.; Marquis, G.S. Evaluation of the effects of iodized salt on the mental development of preschool-aged children: A cluster randomized trial in northern Ethiopia. Matern. Child Nutr. 2017, 13. [Google Scholar] [CrossRef]
- Gowachirapant, S.; Jaiswal, N.; Melse-Boonstra, A.; Galetti, V.; Stinca, S.; Mackenzie, I.; Thomas, S.; Thomas, T.; Winichagoon, P.; Srinivasan, K.; et al. Effect of iodine supplementation in pregnant women on child neurodevelopment: A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 853–863. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Connolly, K.; Bozo, M.; Bridson, J.; Rohner, F.; Grimci, L. Iodine supplementation improves cognition in iodine-deficient schoolchildren in Albania: A randomized, controlled, double-blind study. Am. J. Clin. Nutr. 2006, 83, 108–114. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Jooste, P.L.; Mabapa, N.S.; Schoeman, S.; Biebinger, R.; Mushaphi, L.F.; Mbhenyane, X. Vitamin A supplementation in iodine-deficient African children decreases thyrotropin stimulation of the thyroid and reduces the goiter rate. Am. J. Clin. Nutr. 2007, 86, 1040–1044. [Google Scholar] [CrossRef]
- Fields, D.A.; Demerath, E.W. Relationship of insulin, glucose, leptin, IL-6 and TNF-alpha in human breast milk with infant growth and body composition. Pediatr. Obes. 2012, 7, 304–312. [Google Scholar] [CrossRef]
- WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. (Oslo, Norway: 1992) Suppl. 2006, 450, 76–85.
- Dumrongwongsiri, O.; Chatvutinun, S.; Phoonlabdacha, P.; Sangcakul, A.; Chailurkit, L.O.; Siripinyanond, A.; Suthutvoravut, U.; Chongviriyaphan, N. High Urinary Iodine Concentration Among Breastfed Infants and the Factors Associated with Iodine Content in Breast Milk. Biol. Trace Elem. Res. 2018, 186, 106–113. [Google Scholar] [CrossRef]
- Stoutjesdijk, E.; Schaafsma, A.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. Iodine status during pregnancy and lactation: A pilot study in the Netherlands. Neth J. Med. 2018, 76, 210–217. [Google Scholar]
- Pearce, E.N.; Leung, A.M.; Blount, B.C.; Bazrafshan, H.R.; He, X.; Pino, S.; Valentin-Blasini, L.; Braverman, L.E. Breast milk iodine and perchlorate concentrations in lactating Boston-area women. J. Clin. Endocrinol. Metab. 2007, 92, 1673–1677. [Google Scholar] [CrossRef]
- Hollowell, J.G.; Staehling, N.W.; Hannon, W.H.; Flanders, D.W.; Gunter, E.W.; Maberly, G.F.; Braverman, L.E.; Pino, S.; Miller, D.T.; Garbe, P.L.; et al. Iodine nutrition in the United States. Trends and public health implications: Iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971–1974 and 1988–1994). J. Clin. Endocrinol. Metab. 1998, 83, 3401–3408. [Google Scholar] [CrossRef][Green Version]
- Caldwell, K.L.; Makhmudov, A.; Ely, E.; Jones, R.L.; Wang, R.Y. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid 2011, 21, 419–427. [Google Scholar] [CrossRef]
- Nazeri, P.; Kabir, A.; Dalili, H.; Mirmiran, P.; Azizi, F. Breast-Milk Iodine Concentrations and Iodine Levels of Infants According to the Iodine Status of the Country of Residence: A Systematic Review and Meta-Analysis. Thyroid 2018, 28, 124–138. [Google Scholar] [CrossRef]
- WHO. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination. Available online: https://apps.who.int/iris/bitstream/handle/10665/43781/9789241595827_eng.pdf (accessed on 24 October 2019).
- Liu, L.; Liu, J.; Wang, D.; Shen, H.; Jia, Q. Effect of Urinary Iodine Concentration in Pregnant and Lactating Women, and in Their Infants Residing in Areas with Excessive Iodine in Drinking Water in Shanxi Province, China. Biol. Trace Elem. Res. 2019. [Google Scholar] [CrossRef]
- Iodine. In Drugs and Lactation Database (LactMed) [Internet], [Updated 2019 Feb 28] ed.; National Library of Medicine (US): Bethesda, MD, USA, 2006.
- Mason, J.B.; Deitchler, M.; Gilman, A.; Gillenwater, K.; Shuaib, M.; Hotchkiss, D.; Mason, K.; Mock, N.; Sethuraman, K. Iodine fortification is related to increased weight-for-age and birthweight in children in Asia. Food Nutr. Bull. 2002, 23, 292–308. [Google Scholar] [CrossRef]
- Nazeri, P.; Tahmasebinejad, Z.; Mehrabi, Y.; Hedayati, M.; Mirmiran, P.; Azizi, F. Lactating Mothers and Infants Residing in an Area with an Effective Salt Iodization Program Have No Need for Iodine Supplements: Results from a Double-Blind, Placebo-Controlled, Randomized Controlled Trial. Thyroid 2018, 28, 1547–1558. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Eriksen, K.G.; Christensen, S.H.; Lind, M.V.; Michaelsen, K.F. Human milk composition and infant growth. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 200–206. [Google Scholar] [CrossRef]
| Breast Milk Precision and Recovery | |||||
|---|---|---|---|---|---|
| Target | 0 Standard | 100 µg/L | 500 µg/L | NR UTAK QC | HR UTAK QC |
| Mean | 42.89 | 145.56 µg/L | 551.52 µg/L | 49 ng/mL | 186 ng/mL |
| CV% | 9.32% | 6.65% | 0.43% | 9.6% | 2.5% |
| Recovery | 97.40 µg/L | 450.9 µg/L | 46 µg/L | 179 µg/L | |
| % Recovery | 97.40% | 90.18% | 93.88% | 96.24% | |
| Participant Characteristics | NW | OW/OB | p |
|---|---|---|---|
| (n = 24) | (n = 33) | ||
| Maternal Age: years, mean (SD) | 31.00 (3.66) | 31.61 (3.06) | 0.5 |
| * Maternal Pre-Pregnancy BMI: kg/m2, mean (SD) | 21.25 (1.99) | 30.95 (4.69) | <0.001 |
| * Maternal Race/Ethnicity | 0.002 | ||
| Caucasian, no. (%) | 11 (46) | 28 (85) * | |
| African American, no. (%) | 2 (8) | 1 (3) | |
| Hispanic/Latino, no. (%) | 4 (17) | 1 (3) | |
| Asian/Pacific Islander, no. (%) | 6 (25) | 1 (3) | |
| Indian, no. (%) | 1 (4) | 0 (0) | |
| N/A, no. (%) | 0 (0) | 2 (6) | |
| Gestational Age: weeks, mean (SD) | 39.5 (1.0) | 39.1 (1.3) | 0.16 |
| Mode of Delivery | 0.39 | ||
| Vaginal, no. (%) | 18 (75) | 20 (60) | |
| C-section, no. (%) | 6 (25) | 13 (40) | |
| Country | 0.1 | ||
| Washtenaw, no. (%) | 18 (75) | 17 (52) | |
| Other, no. (%) | 6 (25) | 16 (49) | |
| Maternal income: dollars, mean (SD) | 0.04 | ||
| <60,000, no. (%) | 21 (88) | 19 (68) | |
| >60,000, no. (%) | 3 (12) | 9 (32) | |
| Unknown, no. (%) | 0 (0) | 5 (15) | |
| Smoker | >0.99 | ||
| Yes, no. (%) | 1 (4) | 1 (3) | |
| No, no. (%) | 19 (80) | 27 (82) | |
| N/A, no. (%) | 4 (17) | 5 (15) | |
| Infant birth weight: kg mean (SD) | 3.42 (0.36) | 3.52 (0.42) | 0.35 |
| Infant birth weight: Z-score mean (SD) | 0.33 (0.78) | 0.42 (0.81) | 0.68 |
| Infant sex | >0.99 | ||
| Male, no. (%) | 11 (54) | 16 (49) | |
| Female, no. (%) | 13 (54) | 17 (51) | |
| Infant age at 2 week time point: days, mean (SD) | 15.9 (3) | 16.9 (3) | 0.27 |
| Infant age at 2 month time point: days, mean (SD) | 62.0 (7.2) | 62.5 (6.7) | 0.8 |
| Variables | WFAZ | LFAZ | WFLZ | |
|---|---|---|---|---|
| Model n | Difference (β (p-value)) in change in infant anthropometry Z-score | |||
| BMIC at 2 weeks (ng/mL) | 35 | −0.1883 (0.706) | 1.23581 (0.028) * | −1.1945 (0.053) |
| Time (months) | 35 | −0.0011 (0.170) | −0.00056 (0.527) | −0.0015 (0.136) |
| Birth WFAZ | 35 | 0.53027 (0.0006) * | −0.18117 (0.002) * | 0.16434 (0.016) * |
| Maternal BMI (kg/m2) | 35 | −0.00532 (0.769) | 0.42163 (0.0004) * | 0.24395 (0.022) * |
| Infant sex | 35 | 0.23087 (0.247) | −0.03543 (0.080) | 0.01866 (0.411) |
| Maternal BMI interaction with time | 35 | −0.00126 (0.536) | 0.00359 (0.118) | −0.00452 (0.089) |
| BMIC at 2 weeks interaction with time | 35 | 0.00033 (0.0007) * | 0.00015 (0.154) | 0.00029 (0.021) * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellsworth, L.; McCaffery, H.; Harman, E.; Abbott, J.; Gregg, B. Breast Milk Iodine Concentration Is Associated with Infant Growth, Independent of Maternal Weight. Nutrients 2020, 12, 358. https://doi.org/10.3390/nu12020358
Ellsworth L, McCaffery H, Harman E, Abbott J, Gregg B. Breast Milk Iodine Concentration Is Associated with Infant Growth, Independent of Maternal Weight. Nutrients. 2020; 12(2):358. https://doi.org/10.3390/nu12020358
Chicago/Turabian StyleEllsworth, Lindsay, Harlan McCaffery, Emma Harman, Jillian Abbott, and Brigid Gregg. 2020. "Breast Milk Iodine Concentration Is Associated with Infant Growth, Independent of Maternal Weight" Nutrients 12, no. 2: 358. https://doi.org/10.3390/nu12020358
APA StyleEllsworth, L., McCaffery, H., Harman, E., Abbott, J., & Gregg, B. (2020). Breast Milk Iodine Concentration Is Associated with Infant Growth, Independent of Maternal Weight. Nutrients, 12(2), 358. https://doi.org/10.3390/nu12020358





