Human Cytomegalovirus Reactivation During Lactation: Impact of Antibody Kinetics and Neutralization in Blood and Breast Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Breast Milk Preparation
2.3. Viral Monitoring
2.4. HCMV-Specific-IgG Antibodies
2.5. Neutralization Assays
2.6. Statistical Analysis
3. Results
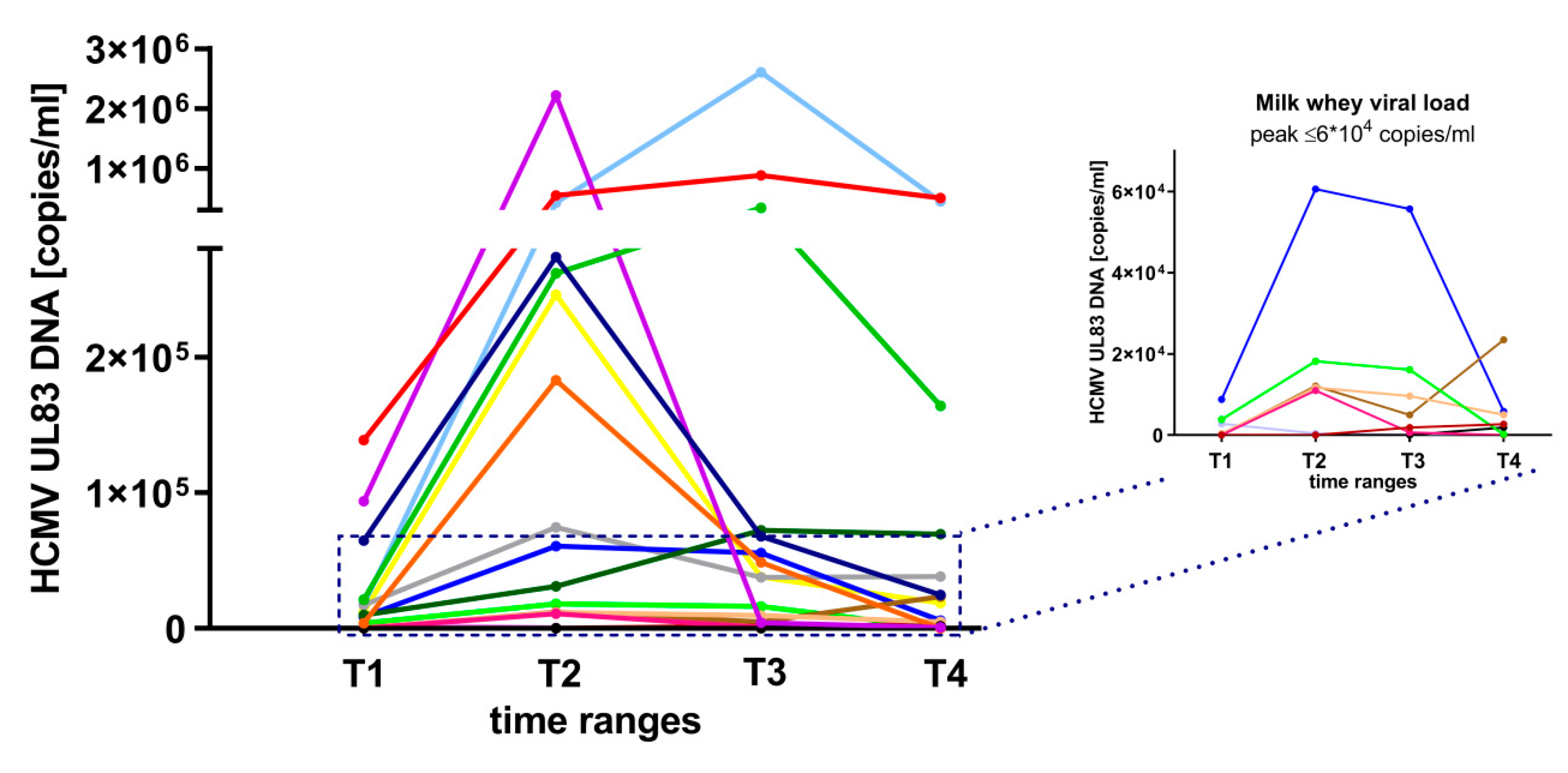
3.1. HCMV-Reactivation in Breast Milk
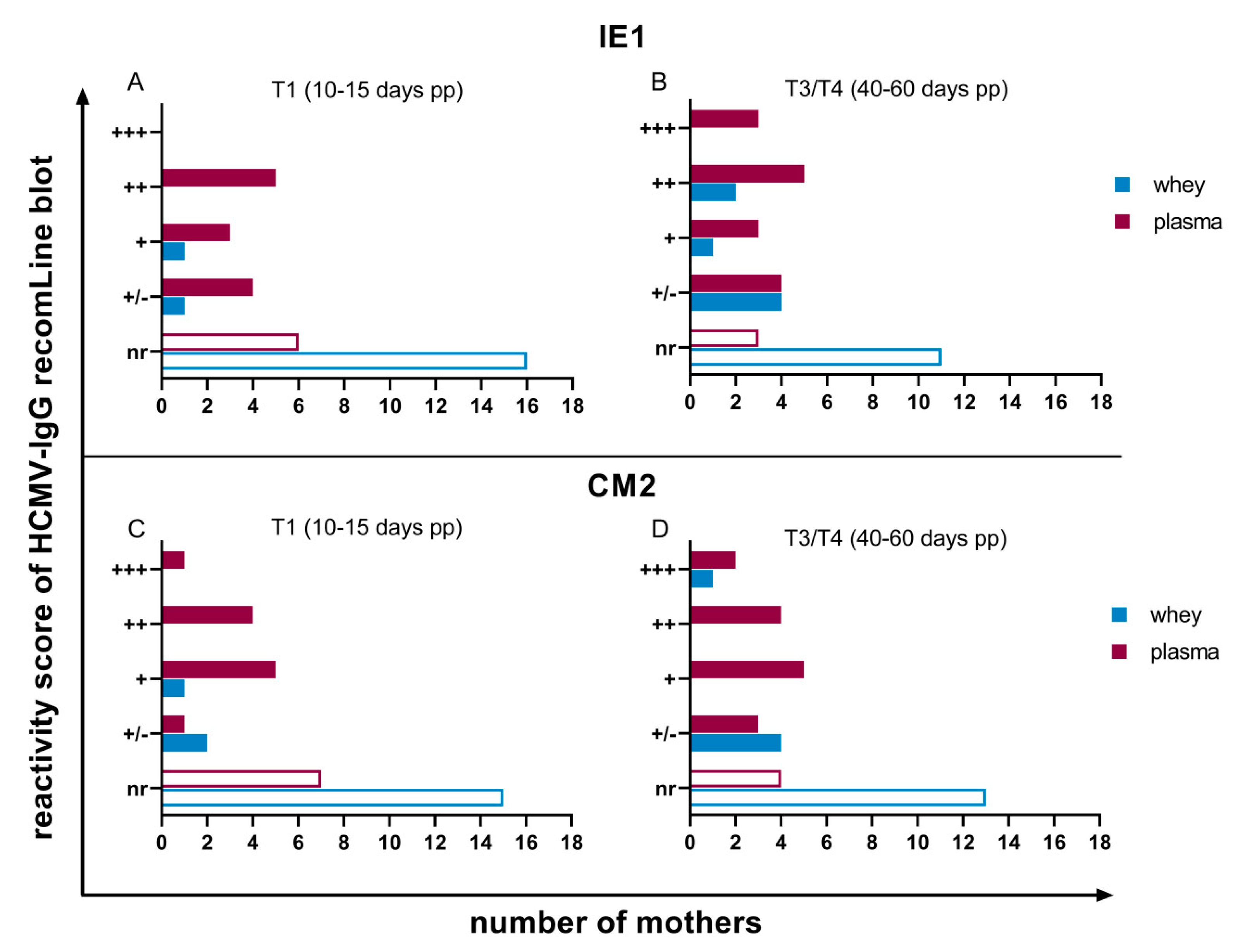
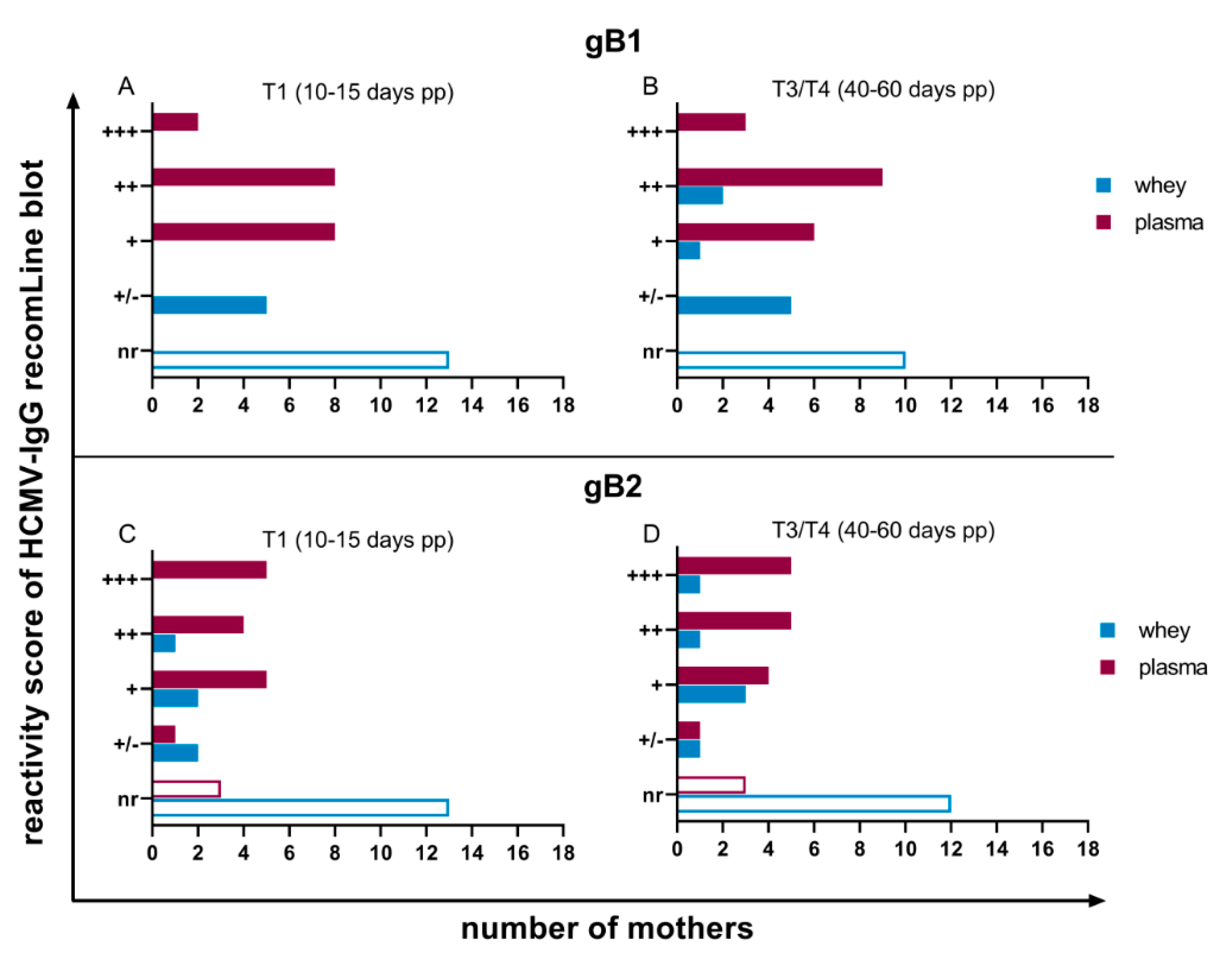
3.2. HCMV Specific-IgG-Antibodies in Plasma and Whey Samples
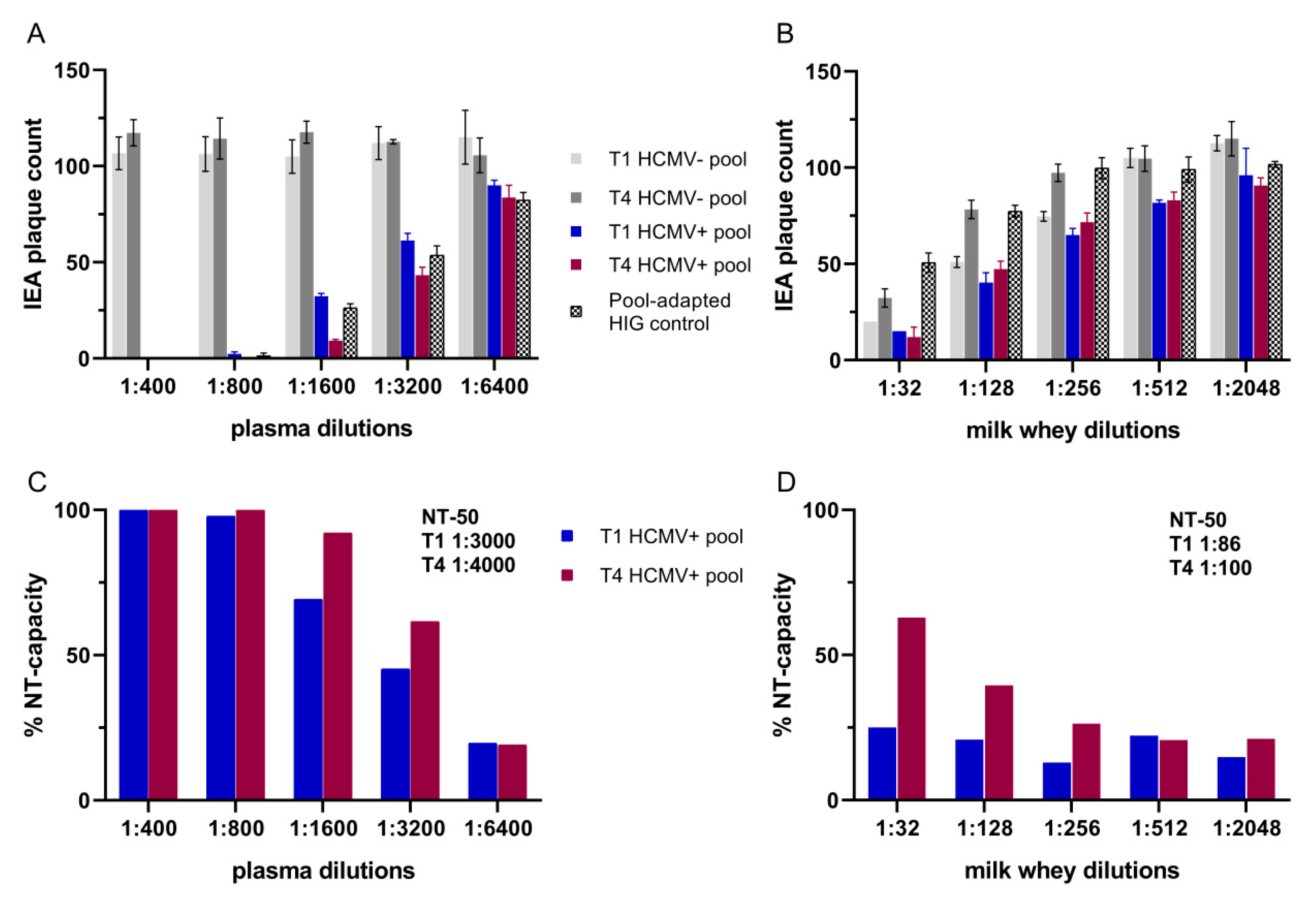
3.3. Neutralization Assays
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Hamprecht, K.; Goelz, R. Postnatal cytomegalovirus infection through human milk in preterm infants: Transmission, clinical presentation, and prevention. Clin. Perinatol. 2017, 44, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hamprecht, K.; Maschmann, J.; Jahn, G.; Poets, C.F.; Goelz, R. Cytomegalovirus transmission to preterm infants during lactation. J. Clin. Virol. 2008, 41, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.J.; Goga, A.E.; Waitt, C.; Gessain, A.; Taylor, G.P.; Rollins, N.; Abrams, E.J.; Lyall, E.H.; Van de Perre, P. Transmission of CMV, HTLV-1, and HIV through breastmilk. Lancet Child. Adolesc. Health 2019, 3, 264–273. [Google Scholar] [CrossRef]
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin. Infect. Dis. 2016, 64, 87–91. [Google Scholar]
- Novakova, V.; Hamprecht, K.; Müller, A.; Arellano-Galindo, J.; Ehlen, M.; Horneff, G. Severe postnatal CMV colitis with an extensive colonic stenosis in a 2-month-old male immunocompetent term infant infected via breast milk. J. Clin. Virol. 2014, 59, 259–263. [Google Scholar] [CrossRef]
- Goelz, R.; Hamprecht, K.; Klingel, K.; Poets, C.F. Intestinal manifestations of postnatal and congenital cytomegalovirus infection in term and preterm infants. J. Clin. Virol. 2016, 83, 29–36. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast milk oligosaccharides: Structure-function relationships in the neonate. Annu. Rev. Nutr. 2014, 34, 143–169. [Google Scholar] [CrossRef]
- Andreas, N.J.; Kampmann, B.; Le-Doare, K.M. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- WHO. World Health Organization Global Strategy for Infant and Young Child Feeding; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Duijts, L.; Jaddoe, V.W.; Hofman, A.; Moll, H.A. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics 2010, 126, e18–e25. [Google Scholar] [CrossRef]
- Wesolowska, A.; Sinkiewicz-Darol, E.; Barbarska, O.; Bernatowicz-Lojko, U.; Borszewska-Kornacka, M.K.; van Goudoever, J.B. Innovative Techniques of Processing Human Milk to Preserve Key Components. Nutrients 2019, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Boix-Amorós, A.; Collado, M.C.; Van’t Land, B.; Calvert, A.; Le Doare, K.; Garssen, J.; Hanna, H.; Khaleva, E.; Peroni, D.G.; Geddes, D.T.; et al. Reviewing the evidence on breast milk composition and immunological outcomes. Nutr. Rev. 2019, 77, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Van de Perre, P. Transfer of antibody via mother’s milk. Vaccine 2003, 21, 3374–3376. [Google Scholar] [CrossRef]
- Hurley, W.L.; Theil, P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef] [PubMed]
- Telemo, E.; Hanson, L.A. Antibodies in milk. J. Mammary Gland Biol. Neoplasia 1996, 1, 243–249. [Google Scholar] [CrossRef]
- Lawrence, R.M. Human breast milk: Current concepts of immunology and infectious diseases. Curr. Probl. Pediatr. Adolesc. Health Care 2007, 37, 7. [Google Scholar] [CrossRef]
- Marshall, G.S.; Rabalais, G.P.; Stout, G.G.; Waldeyer, S.L. Antibodies to recombinant-derived glycoprotein B after natural human cytomegalovirus infection correlate with neutralizing activity. J. Infect. Dis 1992, 165, 381–384. [Google Scholar] [CrossRef]
- Hamprecht, K.; Witzel, S.; Maschmann, J.; Dietz, K.; Baumeister, A.; Mikeler, E.; Goelz, R.; Speer, C.P.; Jahn, G. Rapid detection and quantification of cell free cytomegalovirus by a high-speed centrifugation-based microculture assay: Comparison to longitudinally analyzed viral DNA load and pp67 late transcript during lactation. J. Clin. Virol. 2003, 28, 303–316. [Google Scholar] [CrossRef]
- Hamprecht, K.; Mikeler, E.; Jahn, G. Semi-quantitative detection of cytomegalovirus DNA from native serum and plasma by nested PCR: Influence of DNA extraction procedures. J. Virol. Methods 1997, 69, 125–135. [Google Scholar] [CrossRef]
- Göhring, K.; Dietz, K.; Hartleif, S.; Jahn, G.; Hamprecht, K. Influence of different extraction methods and PCR techniques on the sensitivity of HCMV-DNA detection in dried blood spot (DBS) filter cards. J. Clin. Virol. 2010, 48, 278–281. [Google Scholar] [CrossRef]
- Rothe, M.; Hemprecht, K.; Lang, D. Diagnostic differentiation of primary versus secondary/recurrent infection of human cytomegalovirus by using a recombinant gB ELISA. Biotest Bull. 2000, 6, 147–158. [Google Scholar]
- Vornhagen, R.; Hinderer, W.; Sonneborn, H.-H.; Bein, G.; Matter, L.; Hauw, T.; Enders, G.; Jahn, G.; Plachter, B. IgM-specific serodiagnosis of acute human cytomegalovirus infection using recombinant autologous fusion proteins. J. Virol. Methods 1996, 60, 73–80. [Google Scholar] [CrossRef]
- Bogner, E.; Pecher, G. Pattern of the epitope-specific IgG/IgM response against human cytomegalovirus in patients with multiple myeloma. Clin. Vaccine Immunol. 2013, 20, 1298–1304. [Google Scholar] [CrossRef][Green Version]
- Schoppel, K.; Kropff, B.; Schmidt, C.; Vornhagen, R.; Mach, M. The humoral immune response against human cytomegalovirus is characterized by a delayed synthesis of glycoprotein-specific antibodies. J. Infect. Dis. 1997, 175, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Rothe, M.; Pepperl-Klindworth, S.; Lang, D.; Vornhagen, R.; Hinderer, W.; Weise, K.; Sonneborn, H.H.; Plachter, B. An antigen fragment encompassing the AD2 domains of glycoprotein B from two different strains is sufficient for differentiation of primary vs. recurrent human cytomegalovirus infection by ELISA. J. Med. Virol. 2001, 65, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Kagan, K.; Enders, M.; Schampera, M.; Baeumel, E.; Hoopmann, M.; Geipel, A.; Berg, C.; Goelz, R.; De Catte, L.; Wallwiener, D.; et al. Prevention of maternal–fetal transmission of cytomegalovirus after primary maternal infection in the first trimester by biweekly hyperimmunoglobulin administration. Ultrasound Obstetr. Gynecol. 2019, 53, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Maschmann, J.; Müller, D.; Lazar, K.; Goelz, R.; Hamprecht, K. New short-term heat inactivation method of cytomegalovirus (CMV) in breast milk: Impact on CMV inactivation, CMV antibodies and enzyme activities. Arch. Dis. Child. 2019, 104, F604–F608. [Google Scholar] [CrossRef] [PubMed]
- Schampera, M.S.; Schweinzer, K.; Abele, H.; Kagan, K.O.; Klein, R.; Retting, I.; Jahn, G.; Hamprecht, K. Comparison of cytomegalovirus (CMV)-specific neutralization capacity of hyperimmunoglobulin (HIG) versus standard intravenous immunoglobulin (IVIG) preparations: Impact of CMV IgG normalization. J. Clin. Virol. 2017, 90, 40–45. [Google Scholar] [CrossRef]
- Hamprecht, K.; Maschmann, J.; Vochem, M.; Dietz, K.; Speer, C.P.; Jahn, G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet 2001, 357, 513–518. [Google Scholar] [CrossRef]
- Hamprecht, K.; Witzel, S.; Maschmann, J.; Speer, C.P.; Jahn, G. Transmission of Cytomegalovirus Infection Through Breast Milk in Term and Pretern Infants. In Short and Long Term Effects of Breast Feeding on Child Health; Koletzko, B.M.K., Hernell, O., Eds.; Springer: Boston, MA, USA, 2002; Volume 478, pp. 231–239. [Google Scholar]
- Simister, N.E.; Story, C.M. Human placental Fc receptors and the transmission of antibodies from mother to fetus. J. Reprod. Immunol. 1997, 37, 1–23. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol 2007, 7, 715. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, L.; Wu, M.; Zhong, T.; Zhou, Y.-H.; Hu, Y. Kinetics of IgG antibody to cytomegalovirus (CMV) after birth and seroprevalence of anti-CMV IgG in Chinese children. Virol. J. 2012, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Maroulis, G.B.; Buckley, R.H.; Younger, J.B. Serum immunoglobulin concentrations during normal pregnancy. Am. J. Obstet. Gynecol. 1971, 109, 971–976. [Google Scholar] [CrossRef]
- Amino, N.; Tanizawa, O.; Miyai, K.; Tanaka, F.; Hayashi, C.; Kawashima, M.; Ichihara, K. Changes of serum immunoglobulins IgG, IgA, IgM, and IgE during pregnancy. Obstet. Gynecol. 1978, 52, 415–420. [Google Scholar] [PubMed]
- Larsson, A.; Palm, M.; Hansson, L.-O.; Basu, S.; Axelsson, O. Reference values for α1-acid glycoprotein, α1-antitrypsin, albumin, haptoglobin, C-reactive protein, IgA, IgG and IgM during pregnancy. Acta Obstet. Gynecol. Scand. 2008, 87, 1084–1088. [Google Scholar] [CrossRef]
- De Haas, S.; Ghossein-Doha, C.; Van Kuijk, S.; Van Drongelen, J.; Spaanderman, M. Physiological adaptation of maternal plasma volume during pregnancy: A systematic review and meta-analysis. Ultrasound Obstetr. Gynecol. 2017, 49, 177–187. [Google Scholar] [CrossRef]
- Meier, J.; Lienicke, U.; Tschirch, E.; Krüger, D.H.; Wauer, R.R.; Prösch, S. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J. Clin. Microbiol. 2005, 43, 1318–1324. [Google Scholar] [CrossRef]
- Hamprecht, K.; Maschmann, J.; Vochem, M.; Speer, C.P.; Jahn, G. Transmission of cytomegalovirus to preterm infants by breast-feeding. Monogr. Virol. 2003, 24, 43–52. [Google Scholar]
- Azenkot, T.; Zaniello, B.; Green, M.L.; Selke, S.; Huang, M.L.; Magaret, A.; Wald, A.; Johnston, C. Cytomegalovirus shedding from breastmilk and mucosal sites in healthy postpartum women: A pilot study. J. Med. Virol. 2019, 91, 894–898. [Google Scholar] [CrossRef]
- Kassim, O.O.; Afolabi, O.; Ako-Nai, K.A.; Torimiro SE, A.; Littleton, G.K.; Oke, O.O.; Grissom, F.E. Cytomegalovirus antibodies in breast milk and sera of mother-infant pairs. J. Trop. Pediatr. 1987, 33, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Triantis, V.; Bode, L.; Van Neerven, R. Immunological effects of human milk oligosaccharides. Front. Pediatrics 2018, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Beljaars, L.; van der Strate, B.W.; Bakker, H.I.; Reker-Smit, C.; Wiegmans, F.C.; Harmsen, M.C.; Molema, G.; Meijer, D.K. Inhibition of cytomegalovirus infection by lactoferrin in vitro and in vivo. Antivir. Res. 2004, 63, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.H.; Osbakk, S.A.; Vorland, L.H.; Traavik, T.; Gutteberg, T.J. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antivir. Res. 2001, 51, 141–149. [Google Scholar] [CrossRef]
- Van der Strate, B.; Beljaars, L.; Molema, G.; Harmsen, M.; Meijer, D. Antiviral activities of lactoferrin. Antivir. Res. 2001, 52, 225–239. [Google Scholar] [CrossRef]
- Saccoccio, F.M.; Jenks, J.A.; Itell, H.L.; Li, S.H.; Berry, M.; Pollara, J.; Casper, C.; Gantt, S.; Permar, S.R. Humoral Immune Correlates for Prevention of Postnatal Cytomegalovirus Acquisition. J. Infect. Dis. 2019, 220, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Mathur, N.; Dwarkadas, A.; Sharma, V.; Saha, K.; Jain, N. Anti-infective factors in preterm human colostrum. Acta Paediatr. 1990, 79, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Ehlinger, E.P.; Webster, E.M.; Kang, H.H.; Cangialose, A.; Simmons, A.C.; Barbas, K.H.; Burchett, S.K.; Gregory, M.L.; Puopolo, K.P.; Permar, S.R. Maternal cytomegalovirus-specific immune responses and symptomatic postnatal cytomegalovirus transmission in very low-birth-weight preterm infants. J. Infect. Dis. 2011, 204, 1672–1682. [Google Scholar] [CrossRef]
- Asanuma, H.; Numazaki, K.; Nagata, N.; Hotsubo, T.; Horino, K.; Chiba, S. Role of milk whey in the transmission of human cytomegalovirus infection by breast milk. Microbiol. Immunol. 1996, 40, 201–204. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazar, K.; Rabe, T.; Goelz, R.; Hamprecht, K. Human Cytomegalovirus Reactivation During Lactation: Impact of Antibody Kinetics and Neutralization in Blood and Breast Milk. Nutrients 2020, 12, 338. https://doi.org/10.3390/nu12020338
Lazar K, Rabe T, Goelz R, Hamprecht K. Human Cytomegalovirus Reactivation During Lactation: Impact of Antibody Kinetics and Neutralization in Blood and Breast Milk. Nutrients. 2020; 12(2):338. https://doi.org/10.3390/nu12020338
Chicago/Turabian StyleLazar, Katrin, Tabea Rabe, Rangmar Goelz, and Klaus Hamprecht. 2020. "Human Cytomegalovirus Reactivation During Lactation: Impact of Antibody Kinetics and Neutralization in Blood and Breast Milk" Nutrients 12, no. 2: 338. https://doi.org/10.3390/nu12020338
APA StyleLazar, K., Rabe, T., Goelz, R., & Hamprecht, K. (2020). Human Cytomegalovirus Reactivation During Lactation: Impact of Antibody Kinetics and Neutralization in Blood and Breast Milk. Nutrients, 12(2), 338. https://doi.org/10.3390/nu12020338



