Effects of Extrinsic Wheat Fiber Supplementation on Fecal Weight; A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Product
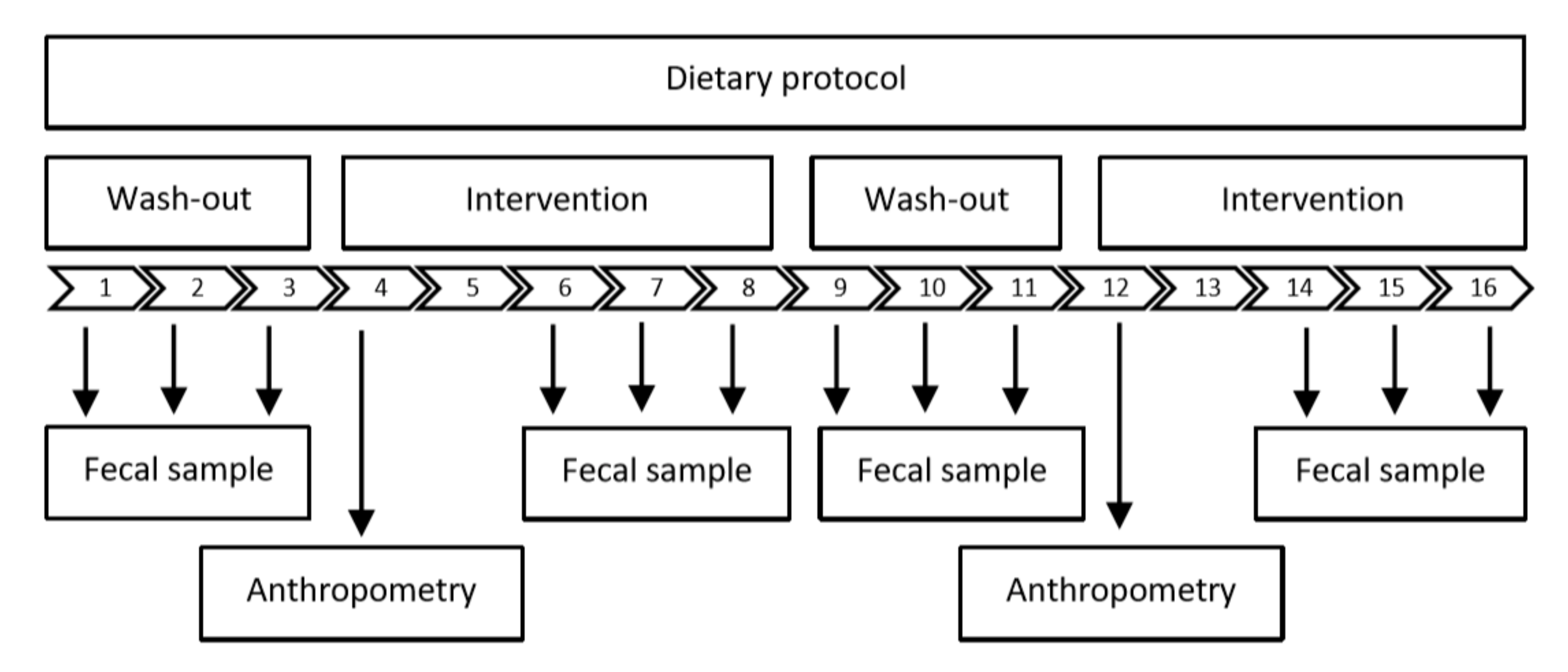
2.3. Study Design
2.4. Anthropometry and Body Composition
2.5. Fecal Samples
2.6. Dietary Protocols
2.7. Quantitation of Short-Chain Fatty Acids (SCFAs)
2.8. High-Throughput 16S rRNA Gene Sequencing
2.9. Data Analysis and Statistics
3. Results
3.1. Subject Characteristics
3.2. Supplementation with Extrinsic Wheat Fiber Increased Fecal Bulk
3.3. Extrinsic Wheat Fiber-Enriched Foods Do Not Alter SFCA Levels vs. Control Diets
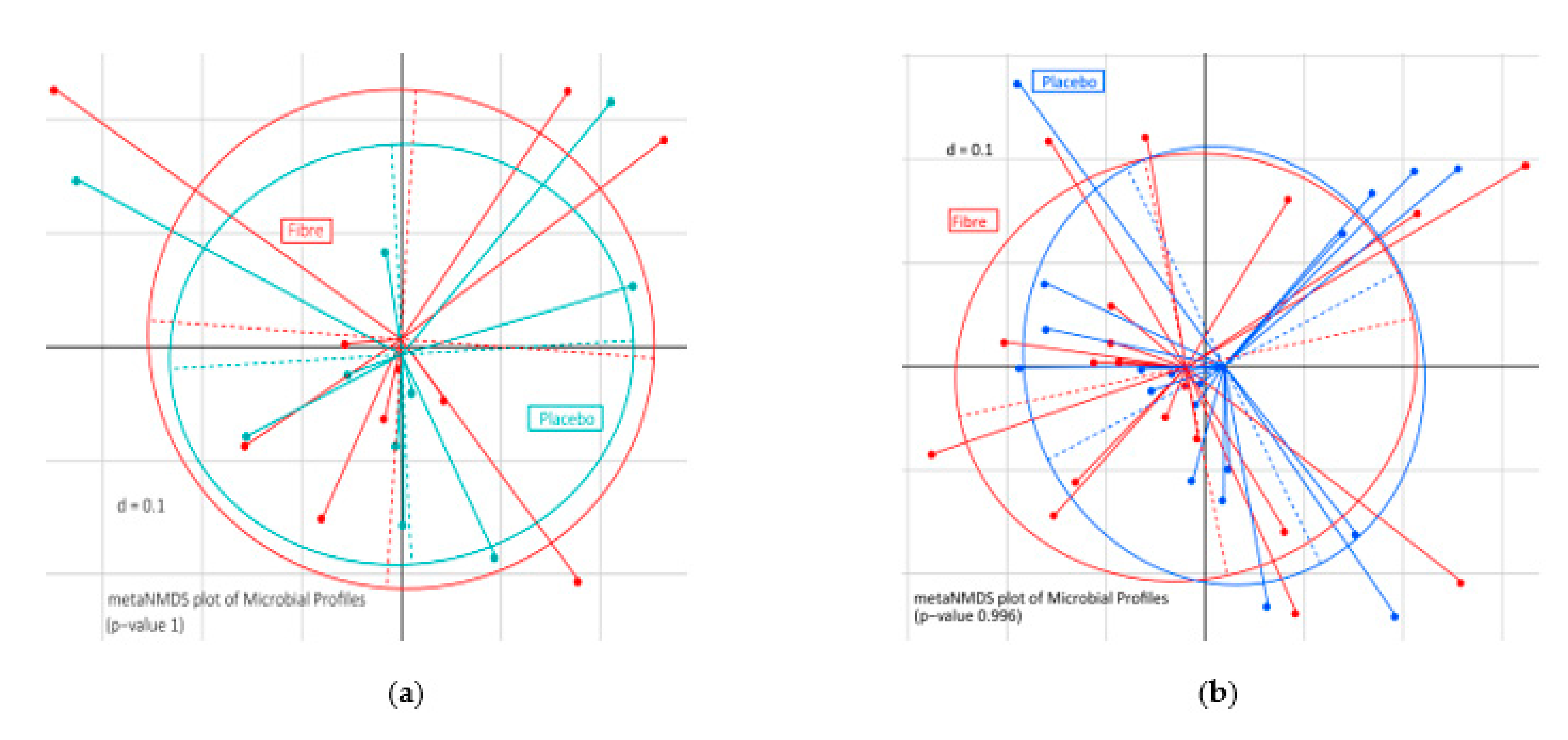
3.4. Extrinsic Wheat Fiber Altered Microbiota Composition
3.5. Analysis of Dietary Protocols
3.6. Evaluation of Product Liking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clark, M.J.; Slavin, J.L. The effect of fiber on satiety and food intake: A systematic review. J. Am. Coll. Nutr. 2013, 32, 200–211. [Google Scholar] [CrossRef]
- Slavin, J.; Green, H. Dietary fibre and satiety. Nutr. Bull. 2007, 32, 32–42. [Google Scholar] [CrossRef]
- Chen, H.L.; Haack, V.S.; Janecky, C.W.; Vollendorf, N.W.; Marlett, J.A. Mechanisms by which wheat bran and oat bran increase stool weight in humans. Am. J. Clin. Nutr. 1998, 68, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muniz, F.J. Dietary fibre and cardiovascular health. Nutr. Hosp. 2012, 27, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Streppel, M.T.; Ocké, M.C.; Boshuizen, H.C.; Kok, F.J.; Kromhout, D. Dietary fiber intake in relation to coronary heart disease and all-cause mortality over 40 y: The Zutphen Study. Am. J. Clin. Nutr. 2008, 88, 1119–1125. [Google Scholar] [CrossRef]
- Jovanovski, E.; Khayyat, R.; Zurbau, A.; Komishon, A.; Mazhar, N.; Sievenpiper, J.L.; Blanco Mejia, S.; Ho, H.V.T.; Li, D.; Jenkins, A.L.; et al. Should Viscous Fiber Supplements Be Considered in Diabetes Control? Results From a Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes Care 2019, 42, 755–766. [Google Scholar] [CrossRef]
- Meyer, K.A.; Kushi, L.H.; Jacobs, D.R.; Slavin, J.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000, 71, 921–930. [Google Scholar] [CrossRef]
- Stewart, M.L.; Zimmer, J.P. Postprandial glucose and insulin response to a high-fiber muffin top containing resistant starch type 4 in healthy adults: A double-blind, randomized, controlled trial. Nutrition 2018, 53, 59–63. [Google Scholar] [CrossRef]
- Kay, B.A.; Trigatti, K.; MacNeil, M.B.; Klingel, S.L.; Repin, N.; Douglas Goff, H.; Wright, A.J.; Duncan, A.M. Pudding products enriched with yellow mustard mucilage, fenugreek gum or flaxseed mucilage and matched for simulated intestinal viscosity significantly reduce postprandial peak glucose and insulin in adults at risk for type 2 diabetes. J. Funct. Foods 2017, 37, 603–611. [Google Scholar] [CrossRef]
- Weickert, M.O.; Mohlig, M.; Koebnick, C.; Holst, J.J.; Namsolleck, P.; Ristow, M.; Osterhoff, M.; Rochlitz, H.; Rudovich, N.; Spranger, J.; et al. Impact of cereal fibre on glucose-regulating factors. Diabetologia 2005, 48, 2343–2353. [Google Scholar] [CrossRef]
- Drasar, B.S.; Jenkins, D.J.; Cummings, J.H. The influence of a diet rich in wheat fibre on the human faecal flora. J. Med. Microbiol. 1976, 9, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.M.; Wiggins, H.S.; Englyst, H.N.; Cole, T.J.; Wayman, B.J.; Cummings, J.H. The effect of age, sex and level of intake of dietary fibre from wheat on large-bowel function in thirty healthy subjects. Br. J. Nutr. 1986, 56, 349–361. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Miller, P.E.; Verbeke, K. Effects of cereal fiber on bowel function: A systematic review of intervention trials. World J. Gastroenterol. 2015, 21, 8952–8963. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef]
- Lampe, J.W.; Wetsch, R.F.; Thompson, W.O.; Slavin, J.L. Gastrointestinal effects of sugarbeet fiber and wheat bran in healthy men. Eur. J. Clin. Nutr. 1993, 47, 543–548. [Google Scholar]
- Mälkki, Y.; Virtanen, E. Gastrointestinal Effects of Oat Bran and Oat Gum: A Review. LWT Food Sci. Technol. 2001, 34, 337–347. [Google Scholar] [CrossRef]
- Blake, M.R.; Raker, J.M.; Whelan, K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 693–703. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Sequeira, C.; Borchers, C.H. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Lagkouvardos, I.; Joseph, D.; Kapfhammer, M.; Giritli, S.; Horn, M.; Haller, D.; Clavel, T. IMNGS: A comprehensive open resource of processed 16S rRNA microbial profiles for ecology and diversity studies. Sci. Rep. 2016, 6, 33721. [Google Scholar] [CrossRef] [PubMed]
- Lagkouvardos, I.; Fischer, S.; Kumar, N.; Clavel, T. Rhea: A transparent and modular R pipeline for microbial profiling based on 16S rRNA gene amplicons. PeerJ 2017, 5, e2836. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.L.; Correa, D.; Pak, S.C. Probiotics, prebiotics, and low FODMAP diet for irritable bowel syndrome—What is the current evidence? Complement. Ther. Med. 2019, 43, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Parisi, G.C.; Zilli, M.; Miani, M.P.; Carrara, M.; Bottona, E.; Verdianelli, G.; Battaglia, G.; Desideri, S.; Faedo, A.; Marzolino, C.; et al. High-fiber diet supplementation in patients with irritable bowel syndrome (IBS): A multicenter, randomized, open trial comparison between wheat bran diet and partially hydrolyzed guar gum (PHGG). Dig. Dis. Sci. 2002, 47, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- De Wit, N.; Esser, D.; Siebelink, E.; Fischer, A.; Sieg, J.; Mes, J. Extrinsic wheat fibre consumption enhances faecal bulk and stool frequency; a randomized controlled trial. Food Funct. 2019, 10, 646–651. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Peterson, R.D.; Thorne, M.J.; Ferguson, P.W. Wheat fiber and laxation: Dose response and equilibration time. Am. J. Gastroenterol. 1987, 82, 1259–1263. [Google Scholar]
- Grundy, M.M.-L.; Edwards, C.H.; Mackie, A.R.; Gidley, M.J.; Butterworth, P.J.; Ellis, P.R. Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. Br. J. Nutr. 2016, 116, 816–833. [Google Scholar] [CrossRef]
- Wyman, J.B.; Heaton, K.W.; Manning, A.P.; Wicks, A.C. The effect on intestinal transit and the feces of raw and cooked bran in different doses. Am. J. Clin. Nutr. 1976, 29, 1474–1479. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
| Food | Drink | |||||
|---|---|---|---|---|---|---|
| Control Diet | Extrinsic Wheat Fiber-Enriched Diet | p-Value | Control Diet | Extrinsic Wheat Fiber-Enriched Diet | p-Value | |
| n | 10 (5 ♀,5 ♂) | 19 (12 ♀,7 ♂) | ||||
| Fecal Wet Weight (gram per day) | 144.4 ± 38.1 | 209.1 ± 88.0 | 0.02 | 171.2 ± 89.5 | 176.1 ± 64.9 | 0.51 |
| Fecal Dry Weight (gram per day) | 36.3 ± 16.4 | 33.7 ± 17.2 | 0.56 | 40.5 ± 31.3 | 41.8 ± 18.7 | 0.18 |
| Stool Consistency a | 3.5 ± 0.8 | 3.9 ± 1.2 | 0.53 | 3.6 ± 0.7 | 3.5 ± 0.7 | 0.72 |
| Stool Frequency (per day) | 1.2 ± 0.4 | 1.3 ± 0.5 | 0.40 | 1.2 ± 0.32 | 1.2 ± 0.3 | 0.78 |
| Food | Drink | |||||
|---|---|---|---|---|---|---|
| Control Diet | Extrinsic Wheat Fiber-Enriched Diet | p-Value | Control Diet | Extrinsic Wheat Fiber-Enriched Diet | p-Value | |
| n | 10 (5 ♀,5 ♂) | 19 (12 ♀,7 ♂) | ||||
| Acetic Acid (ng/mL) | 2633.3 ± 981.5 | 3131.9 ± 1300.9 a | 0.10 | 2420.2 ± 1298.3 | 2038.7 ± 1208.7 a | 0.26 |
| Butyric Acid (ng/mL) | 1187.3 ± 688.0 | 1102.3 ± 645.8 | 0.72 | 1252.8 ± 650.2 | 1041.8 ± 593.0 | 0.13 |
| Propionic Acid (ng/mL) | 736.4 ± 259.3 b | 771.4 ± 209.0 | 0.65 | 1087.6 ± 469.9 b | 857.5 ± 317.2 | 0.10 |
| 2-Methylbutyric Acid (ng/mL) | 72.6 ± 62.1 | 81.0 ± 41.2 | 0.98 | 77.5 ± 73.8 | 73.0 ± 43.3 | 0.77 |
| Hexanoic Acid (ng/mL) | 151.0 ± 121.0 | 162.8 ± 145.6 | 0.75 | 115.5 ± 137.7 | 112.2 ± 127.1 | 0.47 |
| Isobutyrate (ng/mL) | 347.2 ± 493.9 | 234.1 ± 419.0 | 0.49 | 105.6 ± 76.2 | 101.6 ± 49.1 | 0.80 |
| Isovalerate (ng/mL) | 98.8 ± 73.4 | 91.3 ± 52.7 | 0.79 | 92.6 ± 82.1 | 87.0 ± 46.2 | 0.75 |
| Pentanoic Acid (ng/mL) | 181.2 ± 67.7 | 170.8 ± 71.9 | 0.61 | 194.3 ± 91.2 | 154.3 ± 55.1 | 0.10 |
| Food | Drink | |||||
|---|---|---|---|---|---|---|
| Control Diet | Extrinsic Wheat Fiber-Enriched Diet | p-Value | Control Diet | Extrinsic Wheat Fiber-Enriched Diet | p-Value | |
| n | 10 (5 ♀,5 ♂) | 19 (12 ♀,7 ♂) | ||||
| Energy Intake (kcal per day | 2535 ± 515 | 2637 ± 321 | 0.41 | 2097 ± 474 | 2299 ± 521 | 0.17 |
| Carbohydrates (gram per day) | 289 ± 92 (47 EN%) | 284 ± 48 (44 EN%) | 0.98 | 232 ± 45 (45 EN%) | 257 ± 50 (45 EN%) | 0.13 |
| Protein (gram per day) | 85 ± 15 (13 EN%) | 90 ± 17 (14 EN%) | 0.22 | 83 ± 32 (16 EN%) | 90 ± 36 (16 EN%) | 0.56 |
| Fat (gram per day) | 109 ± 22 (40 EN%) | 117 ± 20 (42 EN%) | 0.15 | 85 ± 32 (37 EN%) | 91 ± 33 (37 EN%) | 0.33 |
| Fiber Total (gram per day) | 22 ± 7 | 35 ± 6 | <0.0001 | 25 ± 10 | 35 ± 10 | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandl, B.; Lee, Y.-M.; Dunkel, A.; Hofmann, T.; Hauner, H.; Skurk, T. Effects of Extrinsic Wheat Fiber Supplementation on Fecal Weight; A Randomized Controlled Trial. Nutrients 2020, 12, 298. https://doi.org/10.3390/nu12020298
Brandl B, Lee Y-M, Dunkel A, Hofmann T, Hauner H, Skurk T. Effects of Extrinsic Wheat Fiber Supplementation on Fecal Weight; A Randomized Controlled Trial. Nutrients. 2020; 12(2):298. https://doi.org/10.3390/nu12020298
Chicago/Turabian StyleBrandl, Beate, Yu-Mi Lee, Andreas Dunkel, Thomas Hofmann, Hans Hauner, and Thomas Skurk. 2020. "Effects of Extrinsic Wheat Fiber Supplementation on Fecal Weight; A Randomized Controlled Trial" Nutrients 12, no. 2: 298. https://doi.org/10.3390/nu12020298
APA StyleBrandl, B., Lee, Y.-M., Dunkel, A., Hofmann, T., Hauner, H., & Skurk, T. (2020). Effects of Extrinsic Wheat Fiber Supplementation on Fecal Weight; A Randomized Controlled Trial. Nutrients, 12(2), 298. https://doi.org/10.3390/nu12020298






