Starch Digestion Enhances Bioaccessibility of Anti-Inflammatory Polyphenols from Borlotti Beans (Phaseolus vulgaris)
Abstract
1. Introduction
2. Materials and Methods
2.1. Bean Samples and Domestic Processing
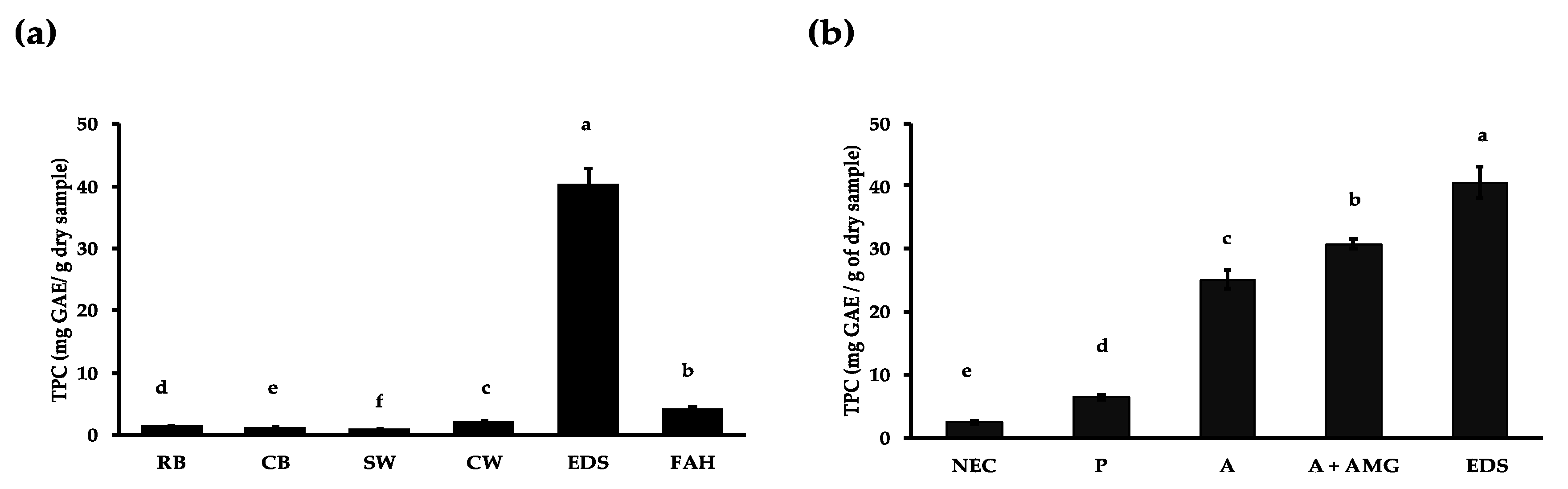
2.2. In Vitro Digestion of Starch and Protein
2.3. Acid Hydrolysis of Fibre
2.4. Determination of Total Polyphenolic Content (TPC) in Bean Extracts
2.5. Separation of Polyphenols Using Liquid Chromatography–Mass Spectrometry (LC–MS)
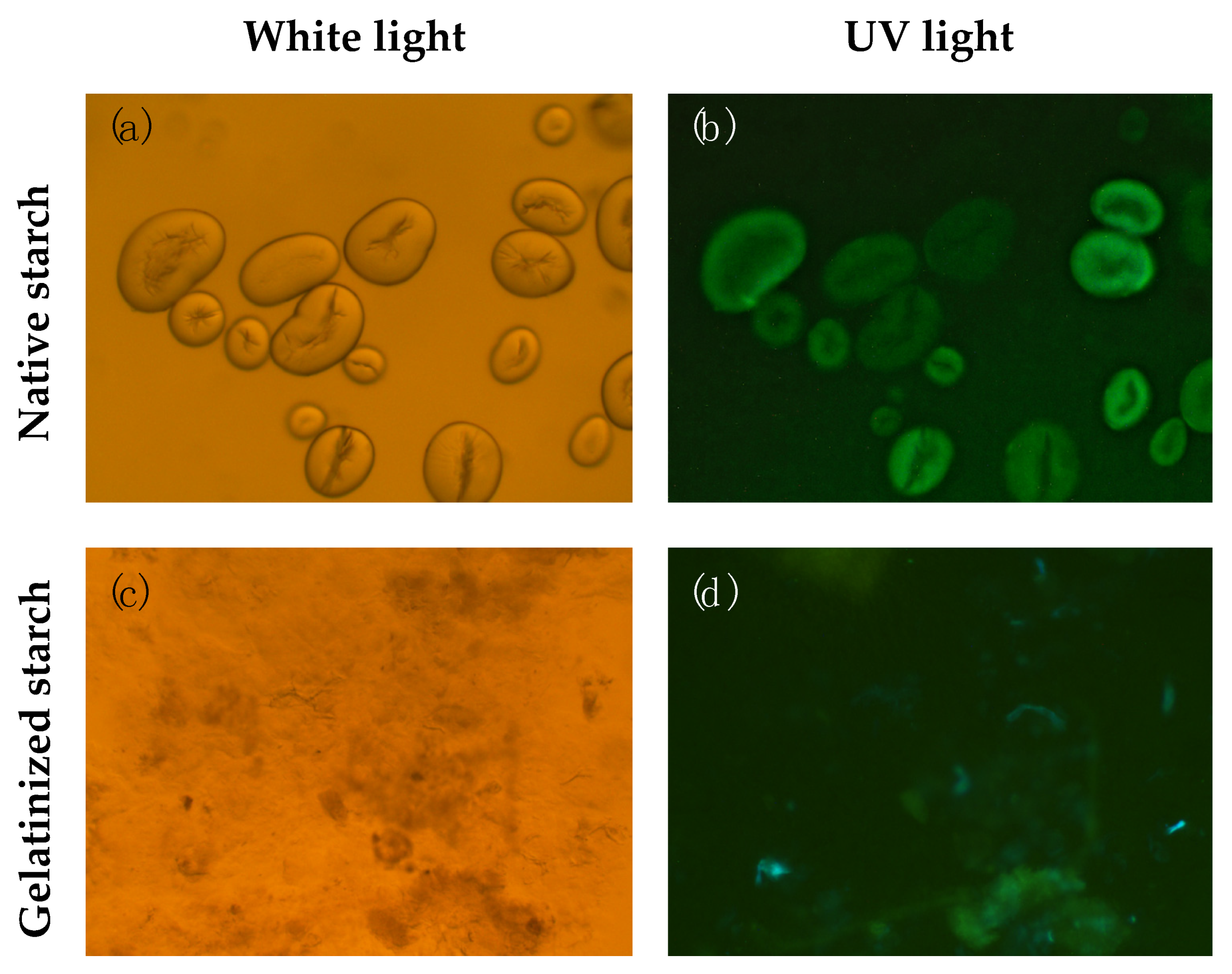
2.6. Starch Isolation and Microscopy
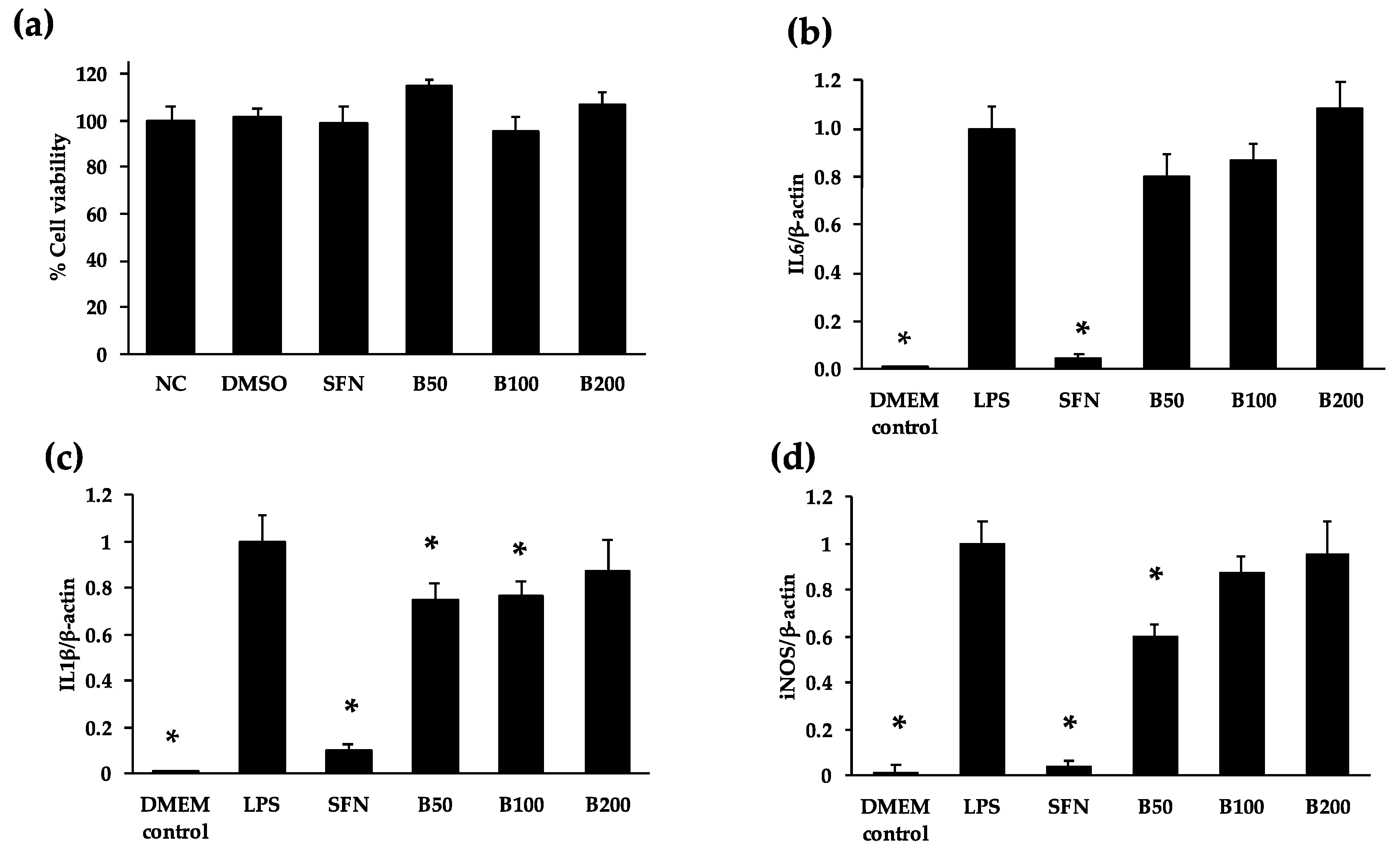
2.7. Determination of Cytotoxicity and Anti-Inflammatory Activity
2.8. Statistical Analysis
3. Results
3.1. Effect of Domestic Processing, Enzymatic Digestion and Fibre Hydrolysis on Polyphenol Release
3.2. Polyphenol Profile of Cooked Bean Extract
3.3. Anti-Inflammatory Properties of Cooked Bean Extracts in RAW 264.7 Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gebrelibanos, M.; Tesfaye, D.; Raghavendra, Y.; Sintayeyu, B. Nutritional and health implications of legumes. Int. J. Pharm. Sci. Res. 2013, 4, 1269–1279. [Google Scholar]
- Foyer, C.H.; Lam, H.M.; Nguyen, H.T.; Siddique, K.H.M.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 1–10. [Google Scholar] [CrossRef]
- Romero-Arenas, O.; Damián-Huato, M.A.; Rivera-Tapia, J.A.; Báez Simón, A.; Huerta Lara, M.; Cabrera Huerta, E. The Nutritional value of Beans (Phaseolus vulgaris L.) and its importance for Feeding of Rural communities in Puebla-Mexico. Int. Res. J. Biol. Sci. 2013, 8, 59–65. [Google Scholar]
- Tonstad, S.; Malik, N.; Haddad, E. A high-fibre bean-rich diet versus a low-carbohydrate diet for obesity. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2014, 27 (Suppl. S2), 109–116. [Google Scholar] [CrossRef]
- Chang, W.C.; Wahlqvist, M.L.; Chang, H.Y.; Hsu, C.C.; Lee, M.S.; Wang, W.S.; Hsiung, C.A. A bean-free diet increases the risk of all-cause mortality among Taiwanese women: The role of the metabolic syndrome. Public Health Nutr. 2012, 15, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ramírez, R.; Galvan-Portillo, M.; Ward, M.; Agudo, A.; González, C.; Oñate-Ocaña, L.; Herrera-Goepfert, R.; Palma-Coca, O. Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City. Int. J. Cancer 2009, 125, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.K.; Beeson, W.L.; Phillips, R.L.; Fraser, G.E. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer 1989, 64, 598–604. [Google Scholar] [CrossRef]
- Aldwairji, M.; Orfila, C.; Burley, V.J. Legume intake and risk of type 2 diabetes in British women. Proc. Nutr. Soc. 2013, 72, E275. [Google Scholar] [CrossRef]
- Villegas, R.; Gao, Y.T.; Yang, G.; Li, H.L.; Elasy, T.A.; Zheng, W.; Shu, X.O. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2008, 87, 162–167. [Google Scholar] [CrossRef]
- Mattei, J.; Sotos-Prieto, M.; Bigornia, S.J.; Noel, S.E.; Tucker, K.L. The mediterranean diet score is more strongly associated with favorable cardiometabolic risk factors over 2 years than other diet quality indexes in puerto rican adults. J. Nutr. 2017, 147, 661–669. [Google Scholar] [CrossRef]
- Viguiliouk, E.; Glenn, A.J.; Nishi, S.K.; Chiavaroli, L.; Seider, M.; Khan, T.; Bonaccio, M.; Iacoviello, L.; Mejia, S.B.; Jenkins, D.J.A.; et al. Associations between Dietary Pulses Alone or with Other Legumes and Cardiometabolic Disease Outcomes: An Umbrella Review and Updated Systematic Review and Meta-analysis of Prospective Cohort Studies. Adv. Nutr. 2019, 10, S308–S319. [Google Scholar] [CrossRef]
- Bennink, M. Consumption of black beans and navy beans (Phaseolus vulgaris) reduced azoxymethane-induced colon cancer in rats. Nutr. Cancer 2002, 44, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.A.; Ross, K.A.; Beta, T.; Fulcher, R.G.; Arntfield, S.D. Effect of pre-dehulling treatments on some nutritional and physical properties of navy and pinto beans (Phaseolus vulgaris L.). LWT Food Sci. Technol. 2008, 41, 771–778. [Google Scholar]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, M.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Messina, V. Nutritional and health benefits of dried beans. Am. J. Clin. Nutr. 2014, 100, 437S–442S. [Google Scholar] [CrossRef]
- Luthria, D.L.; Pastor-Corrales, M.A. Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties. J. Food Compos. Anal. 2006, 19, 205–211. [Google Scholar]
- Lin, L.-Z.; Harnly, J.M.; Pastor-Corrales, M.S.; Luthria, D.L. The polyphenolic profiles of common bean (Phaseolus vulgaris L.). Food Chem. 2008, 107, 399–410. [Google Scholar]
- Xu, B.J.; Chang, S.K. Total phenolic content and antioxidant properties of eclipse black beans (Phaseolus vulgaris L.) as affected by processing methods. J. Food Sci. 2008, 73, H19–H27. [Google Scholar]
- López, A.; El-Naggar, T.; Dueñas, M.; Ortega, T.; Estrella, I.; Hernández, T.; Gómez-Serranillos, M.P.; Palomino, O.M.; Carretero, M.E. Effect of cooking and germination on phenolic composition and biological properties of dark beans (Phaseolus vulgaris L.). Food Chem. 2013, 138, 547–555. [Google Scholar]
- Chen, P.X.; Tang, Y.; Marcone, M.F.; Pauls, P.K.; Zhang, B.; Liu, R.; Tsao, R. Characterization of free, conjugated and bound phenolics and lipophilic antioxidants in regular- and non-darkening cranberry beans (Phaseolus vulgaris L.). Food Chem. 2015, 185, 298–308. [Google Scholar]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Bordenave, N.; Hamaker, B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014, 5, 18–34. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.X.; Li, X.X.; Li, M. Effect of tea polyphenols on the retrogradation of rice starch. Food Res. Int. 2009, 42, 221–225. [Google Scholar] [CrossRef]
- Barros, F.; Awika, J.M.; Rooney, L.W. Interaction of Tannins and Other Sorghum Phenolic Compounds with Starch and Effects on in vitro Starch Digestibility. J. Agric. Food Chem. 2012, 60, 11609–11617. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, M.; Zhang, G. Interaction between amylose and tea polyphenols modulates the postprandial glycemic response to high-amylose maize starch. J. Agric. Food Chem. 2013, 61, 8608–8615. [Google Scholar] [CrossRef]
- Cohen, R.; Orlova, Y.; Kovalev, M.; Ungar, Y.; Shimoni, E. Structural and functional properties of amylose complexes with genistein. J. Agric. Food Chem. 2008, 56, 4212–4218. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Schwartz, B.; Peri, I.; Shimoni, E. Improving bioavailability and stability of genistein by complexation with high-amylose corn starch. J. Agric. Food Chem. 2011, 59, 7932–7938. [Google Scholar] [CrossRef] [PubMed]
- LaParra, J.; Glahn, R.P.; Miller, D.D. Bioaccessibility of Phenols in Common Beans (Phaseolus vulgaris L.) and Iron (Fe) Availability to Caco-2 Cells. J. Agric. Food Chem. 2008, 56, 10999–11005. [Google Scholar]
- Akillioglu, H.G.; Karakaya, S. Changes in Total Phenols, Total Flavonoids, and Antioxidant Activities of Common Beans and Pinto Beans after Soaking, Cooking, and in vitro Digestion Process. Food Sci. Biotechnol. 2010, 19, 633–639. [Google Scholar] [CrossRef]
- Faller, A.L.; Fialho, E.; Liu, R.H. Cellular antioxidant activity of feijoada whole meal coupled with an in vitro digestion. J. Agric. Food Chem. 2012, 60, 4826–4832. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Zafrilla, P.; Tomás-Barberán, F.A. An in vitro method to simulate phenolic compound release from the food matrix in the gastrointestinal tract. Eur. Food Res. Technol. 2002, 214, 155–159. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; Filocamo, A.; Chessa, S.; Saro, M.; Torre, G.; Faulks, R.M.; Dugo, P. Bioaccessibility of pistachio polyphenols, xanthophylls, and tocopherols during simulated human digestion. Nutrition 2013, 29, 338–344. [Google Scholar] [CrossRef]
- Miranda, L.; Deusser, H.; Evers, D. The impact of in vitro digestion on bioaccessibility of polyphenols from potatoes and sweet potatoes and their influence on iron absorption by human intestinal cells. Food Funct. 2013, 4, 1595–1601. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- McCleary, B.V. An integrated procedure for the measurement of total dietary fibre (including resistant starch), non-digestible oligosaccharides and available carbohydrates. Anal. Bioanal. Chem. 2007, 389, 291–308. [Google Scholar] [CrossRef]
- Aldwairji, M.A.; Chu, J.; Burley, V.J.; Orfila, C. Analysis of dietary fibre of boiled and canned legumes commonly consumed in the United Kingdom. J. Food Compos. Anal. 2014, 36, 111–116. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Phenol Explorer Database. Available online: http://phenol-explorer.eu/ (accessed on 26 November 2019).
- Bustos, R.; Fahy, B.; Hylton, C.M.; Seale, R.; Nebane, N.M.; Edwards, A.; Martin, C.; Smith, A.M. Starch granule initiation is controlled by a heteromultimeric isoamylase in potato tubers. Proc. Natl. Acad. Sci. USA 2004, 101, 2215–2220. [Google Scholar] [CrossRef] [PubMed]
- Boesch-Saadatmandi, C.; Loboda, A.; Wagner, A.E.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Döring, F.; Wolffram, S.; Rimbach, G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: Role of miR-155. J. Nutr. Biochem. 2011, 22, 293–299. [Google Scholar] [CrossRef]
- Hoover, R.; Zhou, Y. In vitro and in vivo hydrolysis of legume starches by α-amylase and resistant starch formation in legumes—A review. Carbohydr. Polym. 2003, 54, 401–417. [Google Scholar] [CrossRef]
- Karim, Z.; Holmes, M.; Orfila, C. Inhibitory effect of chlorogenic acid on digestion of potato starch. Food Chem. 2017, 217, 498–504. [Google Scholar] [CrossRef]
- Lim, J.; Zhang, X.; Ferruzzi, M.G.; Hamaker, B.R. Starch digested product analysis by HPAEC reveals structural specificity of flavonoids in the inhibition of mammalian α-amylase and α-glucosidases. Food Chem. 2019, 288, 413–421. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2019. [Google Scholar] [CrossRef]
- Phongnarisorn, B.; Orfila, C.; Holmes, M.; Marshall, L.J. Enrichment of Biscuits with Matcha Green Tea Powder: Its Impact on Consumer Acceptability and Acute Metabolic Response. Foods 2018, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Winham, D.M.; Hutchins, A.M.; Thompson, S.V. Glycemic Response to Black Beans and Chickpeas as Part of a Rice Meal: A Randomized Cross-Over Trial. Nutrients 2017, 9, 1095. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Obiro, W.C.; Sinha Ray, S.; Emmambux, M.N. V-amylose Structural Characteristics, Methods of Preparation, Significance, and Potential Applications. Food Rev. Int. 2012, 28, 412–438. [Google Scholar] [CrossRef]
- Angelino, D.; Cossu, M.; Marti, A.; Zanoletti, M.; Chiavaroli, L.; Brighenti, F.; Del Rio, D.; Martini, D. Bioaccessibility and bioavailability of phenolic compounds in bread: A review. Food Funct. 2017, 8, 2368–2393. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef]
- Fereidoon, S.; Han, P. Bioaccessibility and bioavailability of phenolic compounds. J. Food Bioact. 2018, 4. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin−Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Carrasco-Castilla, J.; Hernández-Álvarez, A.J.; Jiménez-Martínez, C.; Jacinto-Hernández, C.; Alaiz, M.; Girón-Calle, J.; Vioque, J.; Dávila-Ortiz, G. Antioxidant and metal chelating activities of Phaseolus vulgaris L. var. Jamapa protein isolates, phaseolin and lectin hydrolysates. Food Chem. 2012, 131, 1157–1164. [Google Scholar]
- Acosta-Estrada, B.A.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-kappaB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar]
- Kim, H.K.; Cheon, B.S.; Kim, Y.H.; Kim, S.Y.; Kim, H.P. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem. Pharmacol. 1999, 58, 759–765. [Google Scholar]
- Comalada, M.; Ballester, I.; Bailon, E.; Sierra, S.; Xaus, J.; Galvez, J.; de Medina, F.S.; Zarzuelo, A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure-activity relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lafuente, A.; Moro, C.; Manchon, N.; Gonzalo-Ruiz, A.; Villares, A.; Guillamon, E.; Rostagno, M.; Mateo-Vivaracho, L. In vitro anti-inflammatory activity of phenolic rich extracts from white and red common beans. Food Chem. 2014, 161, 216–223. [Google Scholar] [CrossRef]
- Chen, P.X.; Zhang, H.; Marcone, M.F.; Pauls, K.P.; Liu, R.; Tang, Y.; Zhang, B.; Renaud, J.B.; Tsao, R. Anti-inflammatory effects of phenolic-rich cranberry bean (Phaseolus vulgaris L.) extracts and enhanced cellular antioxidant enzyme activities in Caco-2 cells. J. Funct. Foods 2017, 38, 675–685. [Google Scholar]
- Zhang, C.; Monk, J.M.; Lu, J.T.; Zarepoor, L.; Wu, W.; Liu, R.; Pauls, K.P.; Wood, G.A.; Robinson, L.; Tsao, R. Cooked navy and black bean diets improve biomarkers of colon health and reduce inflammation during colitis. Br. J. Nutr. 2014, 111, 1549–1563. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
| Target | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| β-actin | CCTCTATGCCAACACAGTGC | CCTGCTTGCTGATCCACATC |
| IL-6 | AGTTGCCTTCTTGGGACTGA | CAGAATTGCCATTGCACAAC |
| IL-1b | CAGGCAGGCAGTATCACTCA | AGCTCATATGGGTCCGACAG |
| iNOS | GCAGCCTGTGAGACCTTTG | GCATTGGAAGTGAAGCGTTTC |
| Peak | Retention Time (min) | Main Ions Detected | Possible Parent Compound |
|---|---|---|---|
| 1 | 0.4 | +291.31 −289.27 +579.42 | Procyanidin B-type |
| 2 | 0.5 | −648.99 | apigenin-7-O-(6″-malonyl-apiosyl-glycoside) |
| 3 | 0.5 | +355.82 | chlorogenic acid derivative |
| 4 | 0.5 | +959.33 −958.37 | not identified |
| 5 | 0.6 | +355.82 −515.20 | chlorogenic acid glycoside |
| 6 | 0.6 | +379.41 −355.45 | not identified |
| 7 | 0.7 | +355.41 −227.2 | not identified |
| 8 | 1.0 | +338.62 −521.12 | petunidin-3-O-(6′′-acetyl glycoside) |
| 9 | 1.1 | +338.58 −521.17 | petunidin-3-O-(6′′-acetyl-glycoside) |
| 10 | 1.1 | +338.63 −521.18 | petunidin-3-O-(6′′-acetyl-glycoside) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Hernandez, L.M.; Nugraheni, K.; Benohoud, M.; Sun, W.; Hernández-Álvarez, A.J.; Morgan, M.R.A.; Boesch, C.; Orfila, C. Starch Digestion Enhances Bioaccessibility of Anti-Inflammatory Polyphenols from Borlotti Beans (Phaseolus vulgaris). Nutrients 2020, 12, 295. https://doi.org/10.3390/nu12020295
Perez-Hernandez LM, Nugraheni K, Benohoud M, Sun W, Hernández-Álvarez AJ, Morgan MRA, Boesch C, Orfila C. Starch Digestion Enhances Bioaccessibility of Anti-Inflammatory Polyphenols from Borlotti Beans (Phaseolus vulgaris). Nutrients. 2020; 12(2):295. https://doi.org/10.3390/nu12020295
Chicago/Turabian StylePerez-Hernandez, Lucia Margarita, Kartika Nugraheni, Meryem Benohoud, Wen Sun, Alan Javier Hernández-Álvarez, Michael R. A. Morgan, Christine Boesch, and Caroline Orfila. 2020. "Starch Digestion Enhances Bioaccessibility of Anti-Inflammatory Polyphenols from Borlotti Beans (Phaseolus vulgaris)" Nutrients 12, no. 2: 295. https://doi.org/10.3390/nu12020295
APA StylePerez-Hernandez, L. M., Nugraheni, K., Benohoud, M., Sun, W., Hernández-Álvarez, A. J., Morgan, M. R. A., Boesch, C., & Orfila, C. (2020). Starch Digestion Enhances Bioaccessibility of Anti-Inflammatory Polyphenols from Borlotti Beans (Phaseolus vulgaris). Nutrients, 12(2), 295. https://doi.org/10.3390/nu12020295






