Fat and Fat-Free Mass of Preterm and Term Infants from Birth to Six Months: A Review of Current Evidence
Abstract
1. Introduction
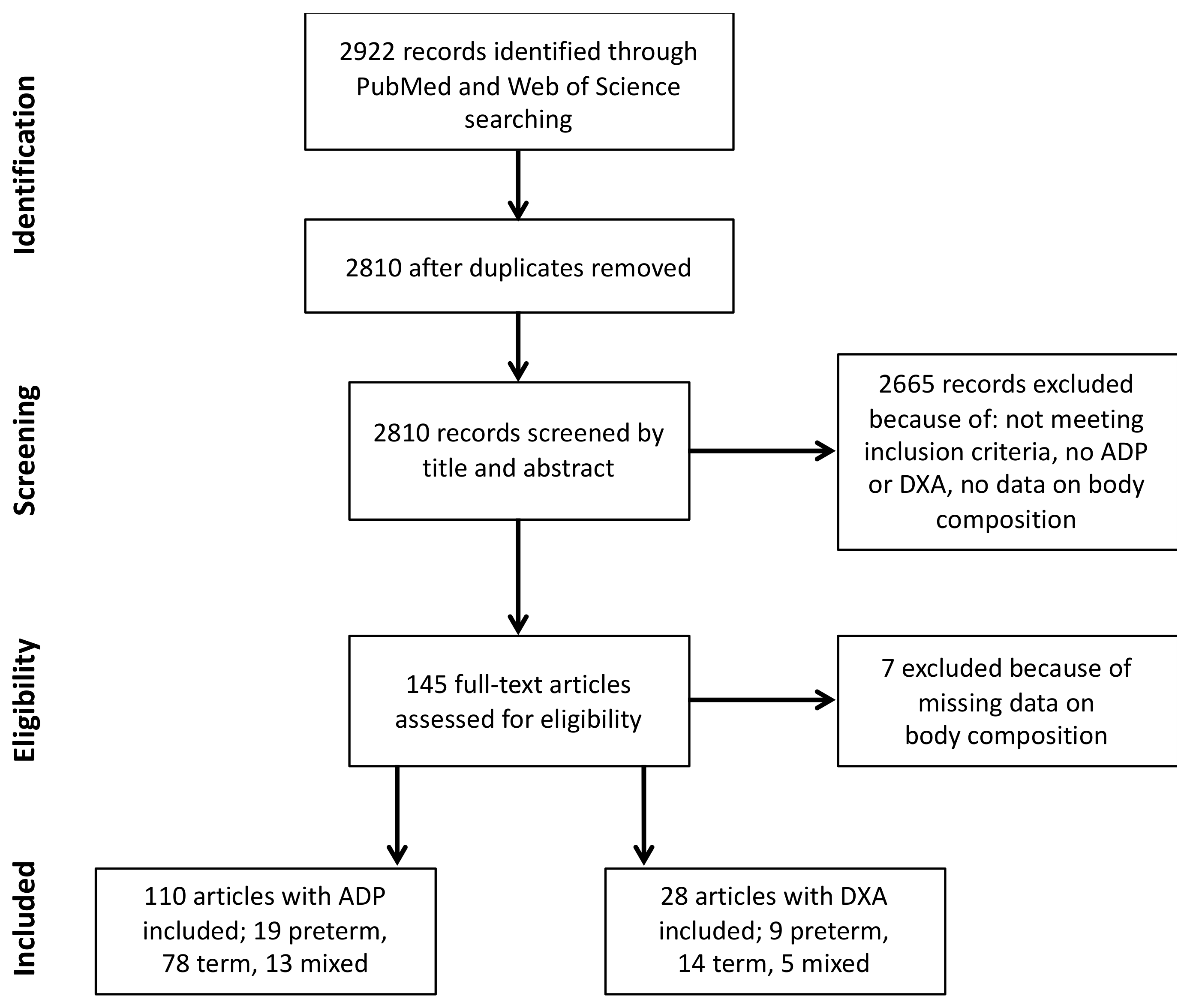
2. Materials and Methods
3. Results
3.1. ADP Study Cohorts
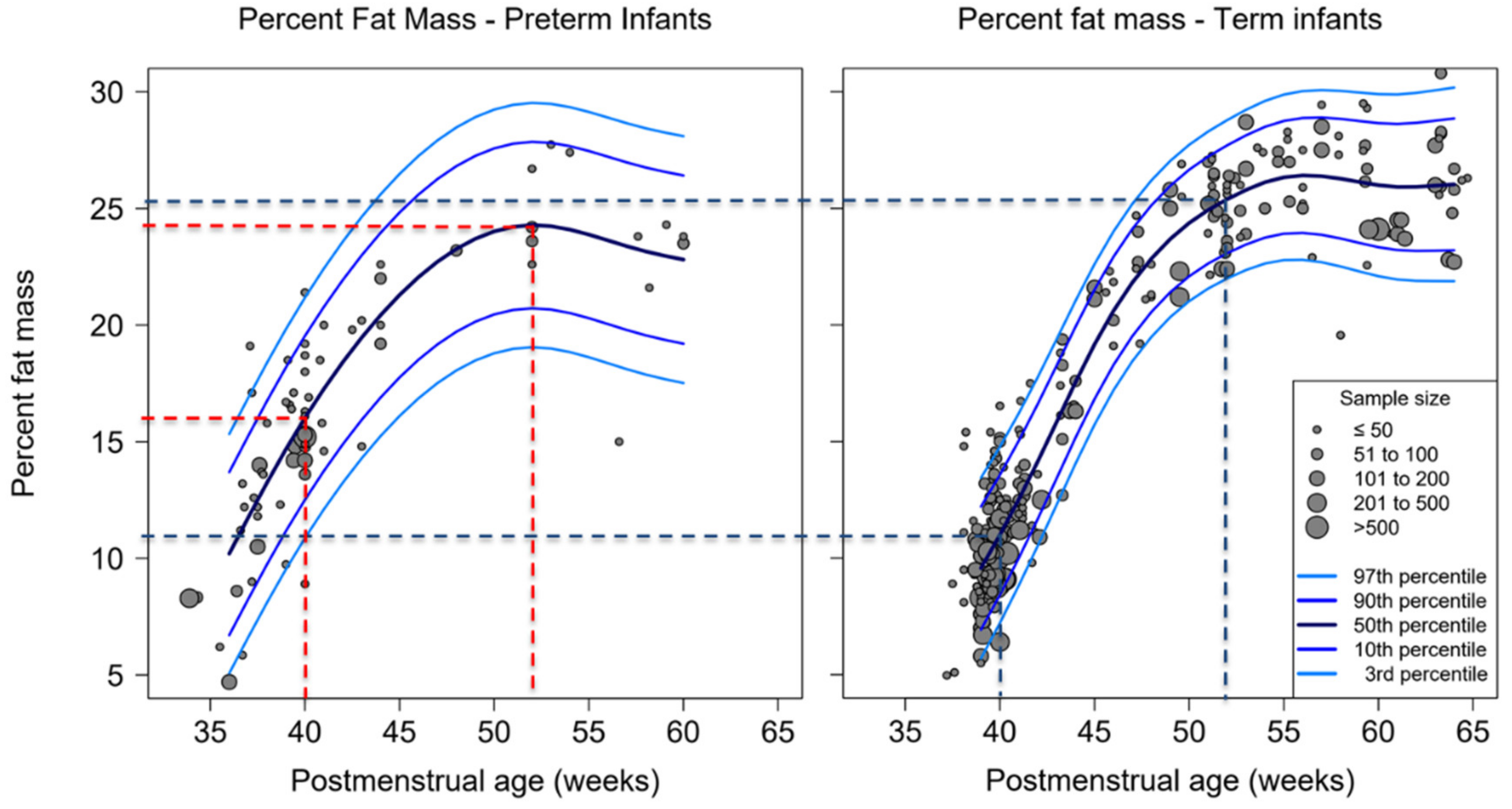
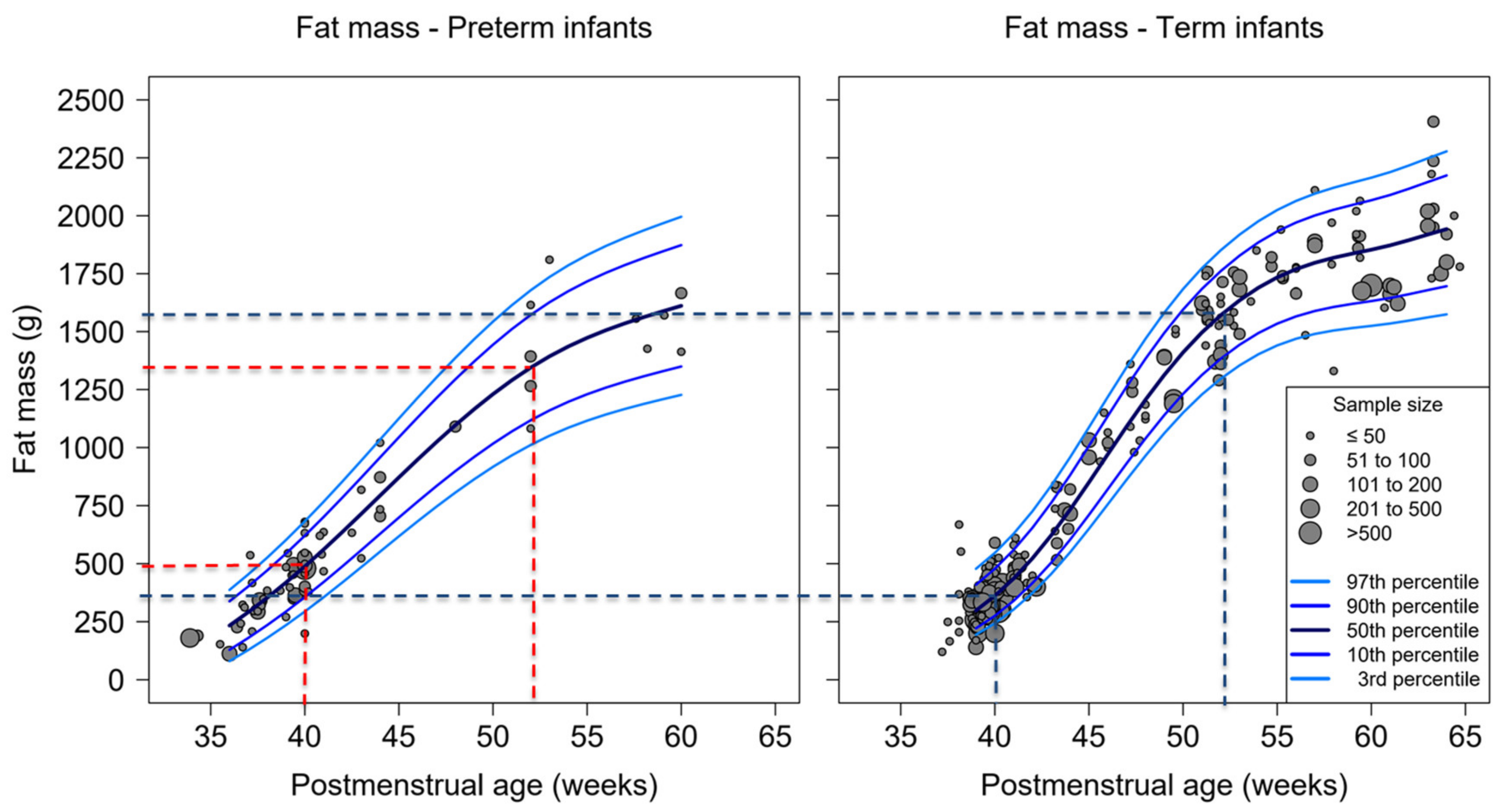
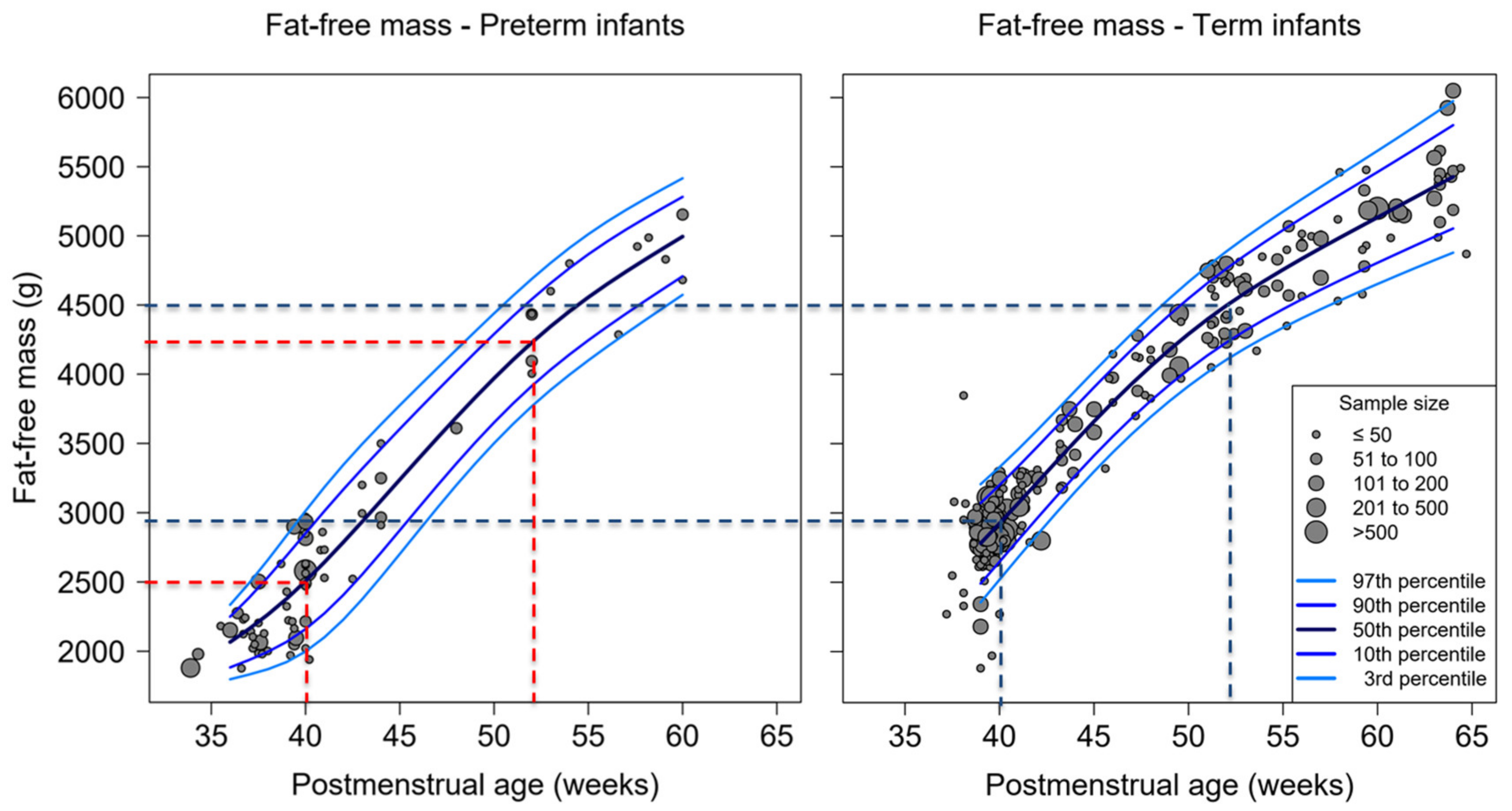
3.2. Percent-Fat Mass, Fat Mass and Fat-Free Mass of Studies Used ADP
3.3. Weight, Length and Head Circumference of Studies Used ADP
3.4. Percent-Fat Mass, Fat Mass and Fat-Free Mass of Studies Used DXA
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rochow, N.; Fusch, G.; Mühlinghaus, A.; Niesytto, C.; Straube, S.; Utzig, N.; Fusch, C. A nutritional program to improve outcome of very low birth weight infants. Clin. Nutr. 2012, 31, 124–131. [Google Scholar] [CrossRef]
- Senterre, T.; Rigo, J. Optimizing early nutritional support based on recent recommendations in VLBW infants allows abolishing postnatal growth restriction. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 536–542. [Google Scholar] [CrossRef]
- Griffin, I.J.; Cooke, R.J. Development of whole body adiposity in preterm infants. Early Hum. Dev. 2012, 88, S19–S24. [Google Scholar] [CrossRef] [PubMed]
- Rochow, N.; Raja, P.; Liu, K.; Fenton, T.; Landau-Crangle, E.; Göttler, S.; Jahn, A.; Lee, S.; Seigel, S.; Campbell, D.; et al. Physiological adjustment to postnatal growth trajectories in healthy preterm infants. Pediatr. Res. 2016, 79, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Landau-Crangle, E.; Rochow, N.; Fenton, T.R.; Liu, K.; Ali, A.; So, H.Y.; Fusch, G.; Marrin, M.L.; Fusch, C. Individualized Postnatal Growth Trajectories for Preterm Infants. J. Parenter. Enter. Nutr. 2018, 42, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Ramel, S.E.; Gray, H.L.; Christiansen, E.; Boys, C.; Georgieff, M.K.; Demerath, E.W. Greater Early Gains in Fat-Free Mass, but Not Fat Mass, Are Associated with Improved Neurodevelopment at 1 Year Corrected Age for Prematurity in Very Low Birth Weight Preterm Infants. J. Pediatr. 2016, 173, 108–115. [Google Scholar] [CrossRef]
- Paviotti, G.; De Cunto, A.; Zennaro, F.; Boz, G.; Travan, L.; Cont, G.; Bua, J.; DeMarini, S. Higher growth, fat and fat-free masses correlate with larger cerebellar volumes in preterm infants at term. Acta Paediatr. 2017, 106, 918–925. [Google Scholar] [CrossRef]
- Embleton, N.D.; Wood, C.L.; Tinnion, R.J. Catch up Growth and the Developmental Origins of Health and Disease (DOHaD) in Preterm Infants. In Nutrition for the Preterm Neonate; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2013; pp. 269–290. [Google Scholar]
- Wells, J.C.K.; Chomtho, S.; Fewtrell, M.S. Programming of body composition by early growth and nutrition. Proc. Nutr. Soc. 2007, 66, 423–434. [Google Scholar] [CrossRef]
- Roggero, P.; Gianni, M.L.; Amato, O.; Piemontese, P.; Morniroli, D.; Wong, W.W.; Mosca, F. Evaluation of air-displacement plethysmography for body composition assessment in preterm infants. Pediatr. Res. 2012, 72, 316–320. [Google Scholar] [CrossRef]
- Fusch, C.; Slotboom, J.; Fuehrer, U.; Schumacher, R.; Keisker, A.; Zimmermann, W.; Moessinger, A.; Boesch, C.; Blum, J. Neonatal Body Composition: Dual-Energy X-Ray Absorptiometry, Magnetic Resonance Imaging, and Three-Dimensional Chemical Shift Imaging versus Chemical Analysis in Piglets. Pediatr. Res. 1999, 46, 465–473. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- De Onis, M.; Garza, C.; Onyango, A.W.; Martorell, R. WHO Child Growth Standards. Acta Paediatr. 2006, 95, 5–101. [Google Scholar] [CrossRef]
- R Development Core Team. A Language and Enviroment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Rigby, R.A.; Stasinopoulos, D.M. Generalized additive models for location, scale and shape (with discussion). J. R. Stat. Soc. Ser. C 2005, 54, 507–554. [Google Scholar] [CrossRef]
- Johnson, M.J.; Wootton, S.A.; Leaf, A.A.; Jackson, A.A. Preterm Birth and Body Composition at Term Equivalent Age: A Systematic Review and Meta-analysis. Pediatrics 2012, 130, e640–e649. [Google Scholar] [CrossRef]
- Al-Theyab, N.A.; Donovan, T.J.; Eiby, Y.A.; Colditz, P.B.; Lingwood, B.E. Fat trajectory after birth in very preterm infants mimics healthy term infants. Pediatr. Obes. 2019, 14, e12472. [Google Scholar] [CrossRef]
- Fields, D.A.; Gilchrist, J.M.; Catalano, P.M.; Gianni, M.L.; Roggero, P.M.; Mosca, F. Longitudinal Body Composition Data in Exclusively Breast-Fed Infants: A Multicenter Study. Obesity 2011, 19, 1887–1891. [Google Scholar] [CrossRef] [PubMed]
- Andres, A.; Shankar, K.; Badger, T.M. Body Fat Mass of Exclusively Breastfed Infants Born to Overweight Mothers. J. Acad. Nutr. Diet. 2012, 112, 991–995. [Google Scholar] [CrossRef]
- Gianni, M.L.; Roggero, P.; Morlacchi, L.; Garavaglia, E.; Piemontese, P.; Mosca, F. Formula-fed infants have significantly higher fat-free mass content in their bodies than breastfed babies. Acta Paediatr. 2014, 103, e277–e281. [Google Scholar] [CrossRef]
- Liotto, N.; Roggero, P.; Bracco, B.; Menis, C.; Morniroli, D.; Perrone, M.; Giannì, M.L.; Mosca, F.; Nadia, L. Can Basic Characteristics Estimate Body Composition in Early Infancy? J. Pediatr. Gastroenterol. Nutr. 2018, 66, e76–e80. [Google Scholar] [CrossRef]
- Roggero, P.; Gianni, M.L.; Orsi, A.; Piemontese, P.; Amato, O.; Liotto, N.; Morlacchi, L.; Taroni, F.; Fields, D.A.; Catalano, P.M.; et al. Quality of Growth in Exclusively Breast-Fed Infants in the First Six Months of Life: An Italian Study. Pediatr. Res. 2010, 68, 542–544. [Google Scholar] [CrossRef]
- McLeod, G.; Simmer, K.; Sherriff, J.; Nathan, E.; Geddes, D.; Hartmann, P. Feasibility study: Assessing the influence of macronutrient intakes on preterm body composition, using air displacement plethysmography. J. Paediatr. Child Heal. 2015, 51, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Fields, D.A.; Demerath, E.W.; Pietrobelli, A.; Chandler-Laney, P.C. Body Composition at 6 months of Life: Comparison Of Air Displacement Plethysmography and Dual-Energy X-Ray Absorptiometry. Obesity 2012, 20, 2302–2306. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. Body Composition Assessment from Birth to Two Years of Age; International Atomic Energy Agency: Vienna, Austria, 2013. [Google Scholar]
- Barbour, L.A.; Hernandez, T.L.; Reynolds, R.M.; Reece, M.S.; Chartier-Logan, C.; Anderson, M.K.; Kelly, T.; Friedman, J.E.; Van Pelt, R.E. Striking differences in estimates of infant adiposity by new and old DXA software, PEAPOD and skin-folds at 2 weeks and 1 year of life. Pediatr. Obes. 2016, 11, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Lantz, H.; Samuelson, G.; Bratteby, L.; Mallmin, H.; Sjöström, L. Differences in whole body measurements by DXA-scanning using two Lunar DPX-L machines. Int. J. Obes. 1999, 23, 764–770. [Google Scholar] [CrossRef][Green Version]
- Pearson, D.; Horton, B.; Green, D.J. Cross Calibration of Hologic QDR2000 and GE Lunar Prodigy for Whole Body Bone Mineral Density and Body Composition Measurements. J. Clin. Densitom. 2011, 14, 294–301. [Google Scholar] [CrossRef]
- Rigo, J.; Nyamugabo, K.; Picaud, J.C.; Gerard, P.; Pieltain, C.; De Curtis, M. Reference values of body composition obtained by dual energy X-ray absorptiometry in preterm and term neonates. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 184–190. [Google Scholar] [CrossRef]
- Koo, W.W.K.; Hammami, M.; Hockman, E.M. Use of fan beam dual energy x-ray absorptiometry to measure body composition of piglets. J. Nutr. 2002, 132, 1380–1383. [Google Scholar] [CrossRef]
- Dempster, P.; Aitkens, S. A new air displacement method for the determination of human body composition. Med. Sci. Sports Exerc. 1995, 27, 1692–1697. [Google Scholar] [CrossRef]
- Ma, G.; Yao, M.; Liu, Y.; Lin, A.; Zou, H.; Urlando, A.; Wong, W.W.; Nommsen-Rivers, L.; Dewey, K.G. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. Am. J. Clin. Nutr. 2004, 79, 653–660. [Google Scholar] [CrossRef]
| ADP | DXA | |||
|---|---|---|---|---|
| Preterm | Term | Preterm | Term | |
| # of cohorts | 32 * | 91 * | 14 * | 19 * |
| <28 weeks PMA | 2 | n/a | 2 | n/a |
| 28 to 31 + 6/7 weeks PMA | 19 | n/a | 11 | n/a |
| 32 to 36 + 6/7 weeks PMA | 14 | n/a | 5 | n/a |
| # of longitudinal | 16 | 34 | 10 | 5 |
| # of cross-sectional | 19 | 57 | 4 | 14 |
| Total sample size infants | 2855 | 22,410 | 1147 | 3542 |
| Age at measurement (# of cohorts) | ||||
| <37 weeks PMA | 8 | n/a | 7 | n/a |
| 37 to 41 + 6/7 weeks PMA | 26 | 82 | 8 | 15 |
| 42 to 47 + 6/7 weeks PMA | 5 | 14 | 2 | 3 |
| 48 to 56 + 6/7 weeks PMA | 6 | 25 | 3 | 3 |
| >57 weeks PMA | 3 | 13 | 2 | 3 |
| Studies that included maternal smoking history | No information | Yes: 20 | No information | Yes: 5 |
| Breast fed, formula, mix, no information | 3, 2, 12, 23 | 115, 4, 23, 53 | 4, 5, 5, 6 | 2, 0, 5, 13 |
| SGA, AGA, LGA, no information | 2, 28, 0, 5 | 2, 66, 2, 21 | 3, 14, n/a, n/a | 3, 15, 2, 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamatschek, C.; Yousuf, E.I.; Möllers, L.S.; So, H.Y.; Morrison, K.M.; Fusch, C.; Rochow, N. Fat and Fat-Free Mass of Preterm and Term Infants from Birth to Six Months: A Review of Current Evidence. Nutrients 2020, 12, 288. https://doi.org/10.3390/nu12020288
Hamatschek C, Yousuf EI, Möllers LS, So HY, Morrison KM, Fusch C, Rochow N. Fat and Fat-Free Mass of Preterm and Term Infants from Birth to Six Months: A Review of Current Evidence. Nutrients. 2020; 12(2):288. https://doi.org/10.3390/nu12020288
Chicago/Turabian StyleHamatschek, Constanze, Efrah I. Yousuf, Lea Sophie Möllers, Hon Yiu So, Katherine M. Morrison, Christoph Fusch, and Niels Rochow. 2020. "Fat and Fat-Free Mass of Preterm and Term Infants from Birth to Six Months: A Review of Current Evidence" Nutrients 12, no. 2: 288. https://doi.org/10.3390/nu12020288
APA StyleHamatschek, C., Yousuf, E. I., Möllers, L. S., So, H. Y., Morrison, K. M., Fusch, C., & Rochow, N. (2020). Fat and Fat-Free Mass of Preterm and Term Infants from Birth to Six Months: A Review of Current Evidence. Nutrients, 12(2), 288. https://doi.org/10.3390/nu12020288





