The Importance of Fatty Acids as Nutrients during Post-Exercise Recovery
Abstract
1. Introduction
2. Increased Circulating Fatty Acid Availability in Recovery from Exercise
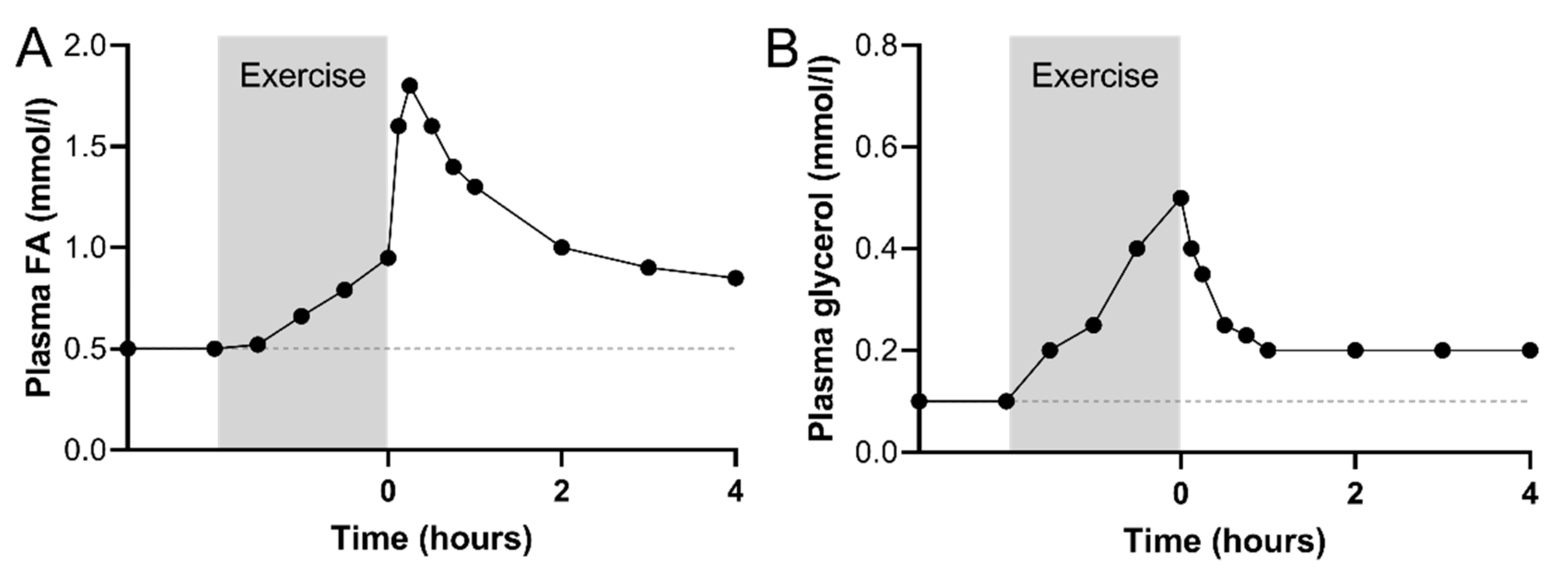
2.1. Immediate Recovery
2.2. Early Recovery
3. Plasma VLDL-TG as an Additional FA Source during Exercise Recovery
4. Potential Mechanisms Regulating Fatty Acid Oxidation in Skeletal Muscle
4.1. Fatty Acid Uptake into the Skeletal Muscle
4.2. The Role of IMTG Metabolism in Skeletal Muscle FA Oxidation in Recovery
4.3. Myocellular FA Handling
5. Muscle Glucose Oxidation and Glycogen Resynthesis during Recovery—A Role of AMPK
6. Molecular Metabolic Adaptations in Skeletal Muscle during Recovery
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borsheim, E.; Bahr, R. Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sports Med. 2003, 33, 1037–1060. [Google Scholar] [CrossRef]
- Andersson-Hall, U.; Pettersson, S.; Edin, F.; Pedersen, A.; Malmodin, D.; Madsen, K. Metabolism and whole-body fat oxidation following postexercise carbohydrate or protein intake. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 37–45. [Google Scholar] [CrossRef]
- Krzentowski, G.; Pirnay, F.; Luyckx, A.S.; Pallikarakis, N.; Lacroix, M.; Mosora, F.; Lefebvre, P.J. Metabolic adaptations in post-exercise recovery. Clin. Physiol. 1982, 2, 277–288. [Google Scholar] [CrossRef]
- Bielinski, R.; Schutz, Y.; Jequier, E. Energy metabolism during the postexercise recovery in man. Am. J. Clin. Nutr. 1985, 42, 69–82. [Google Scholar] [CrossRef]
- Maehlum, S.; Grandmontagne, M.; Newsholme, E.A.; Sejersted, O.M. Magnitude and duration of excess postexercise oxygen consumption in healthy young subjects. Metabolism 1986, 35, 425–429. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Klein, S.; Carraro, F.; Weber, J.M. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am. J. Physiol. 1990, 258, E382–E389. [Google Scholar] [CrossRef]
- Kiens, B.; Richter, E.A. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am. J. Physiol. 1998, 275, E332–E337. [Google Scholar] [CrossRef]
- Horton, T.J.; Pagliassotti, M.J.; Hobbs, K.; Hill, J.O. Fuel metabolism in men and women during and after long-duration exercise. J. Appl. Physiol. (1985) 1998, 85, 1823–1832. [Google Scholar] [CrossRef]
- Kimber, N.E.; Heigenhauser, G.J.; Spriet, L.L.; Dyck, D.J. Skeletal muscle fat and carbohydrate metabolism during recovery from glycogen-depleting exercise in humans. J. Physiol. 2003, 548, 919–927. [Google Scholar] [CrossRef]
- Bergman, B.C.; Perreault, L.; Strauss, A.; Bacon, S.; Kerege, A.; Harrison, K.; Brozinick, J.T.; Hunerdosse, D.M.; Playdon, M.C.; Holmes, W.; et al. Intramuscular triglyceride synthesis: Importance in muscle lipid partitioning in humans. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E152–E164. [Google Scholar] [CrossRef]
- Tuominen, J.A.; Ebeling, P.; Bourey, R.; Koranyi, L.; Lamminen, A.; Rapola, J.; Sane, T.; Vuorinen-Markkola, H.; Koivisto, V.A. Postmarathon paradox: Insulin resistance in the face of glycogen depletion. Am. J. Physiol. 1996, 270, E336–E343. [Google Scholar] [CrossRef]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [CrossRef]
- Bahr, R.; Hostmark, A.T.; Newsholme, E.A.; Gronnerod, O.; Sejersted, O.M. Effect of exercise on recovery changes in plasma levels of FFA, glycerol, glucose and catecholamines. Acta Physiol. Scand. 1991, 143, 105–115. [Google Scholar] [CrossRef]
- Hagenfeldt, L.; Wahren, J. Turnover of free fatty acids during recovery from exercise. J. Appl. Physiol. 1975, 39, 247–250. [Google Scholar] [CrossRef]
- Wee, J.; Charlton, C.; Simpson, H.; Jackson, N.C.; Shojaee-Moradie, F.; Stolinski, M.; Pentecost, C.; Umpleby, A.M. GH secretion in acute exercise may result in post-exercise lipolysis. Growth Horm. IGF Res. 2005, 15, 397–404. [Google Scholar] [CrossRef]
- Henderson, G.C.; Fattor, J.A.; Horning, M.A.; Faghihnia, N.; Johnson, M.L.; Mau, T.L.; Luke-Zeitoun, M.; Brooks, G.A. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J. Physiol. 2007, 584, 963–981. [Google Scholar] [CrossRef]
- Magkos, F.; Mohammed, B.S.; Patterson, B.W.; Mittendorfer, B. Free fatty acid kinetics in the late phase of postexercise recovery: Importance of resting fatty acid metabolism and exercise-induced energy deficit. Metabolism 2009, 58, 1248–1255. [Google Scholar] [CrossRef]
- Hodgetts, V.; Coppack, S.W.; Frayn, K.N.; Hockaday, T.D. Factors controlling fat mobilization from human subcutaneous adipose tissue during exercise. J. Appl. Physiol. (1985) 1991, 71, 445–451. [Google Scholar] [CrossRef]
- Mulla, N.A.; Simonsen, L.; Bulow, J. Post-exercise adipose tissue and skeletal muscle lipid metabolism in humans: The effects of exercise intensity. J. Physiol. 2000, 524, 919–928. [Google Scholar] [CrossRef]
- van, H.G.; Bulow, J.; Sacchetti, M.; Al, M.N.; Lyngso, D.; Simonsen, L. Regional fat metabolism in human splanchnic and adipose tissues; the effect of exercise. J. Physiol. 2002, 543, 1033–1046. [Google Scholar]
- van, H.G.; Sacchetti, M.; Radegran, G.; Saltin, B. Human skeletal muscle fatty acid and glycerol metabolism during rest, exercise and recovery. J. Physiol. 2002, 543, 1047–1058. [Google Scholar]
- Enevoldsen, L.H.; Polak, J.; Simonsen, L.; Hammer, T.; Macdonald, I.; Crampes, F.; De Glisezinski, I.; Stich, V.; Bülow, J. Post-exercise abdominal, subcutaneous adipose tissue lipolysis in fasting subjects is inhibited by infusion of the somatostatin analogue octreotide. Clin. Physiol. Funct. Imaging 2007, 27, 320–326. [Google Scholar] [CrossRef]
- Tsiloulis, T.; Watt, M.J. Exercise and the regulation of adipose tissue metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 175–201. [Google Scholar] [CrossRef]
- Wijnen, J.A.; van Baak, M.A.; de, H.C.; Boudier, H.A.; Tan, F.S.; van Bortel, L.M. Beta-blockade and lipolysis during endurance exercise. Eur. J. Clin. Pharmacol. 1993, 45, 101–105. [Google Scholar] [CrossRef]
- Dimsdale, J.E.; Hartley, L.H.; Guiney, T.; Ruskin, J.N.; Greenblatt, D. Postexercise peril. Plasma catecholamines and exercise. JAMA 1984, 251, 630–632. [Google Scholar] [CrossRef]
- Hagberg, J.M.; Hickson, R.C.; McLane, J.A.; Ehsani, A.A.; Winder, W.W. Disappearance of norepinephrine from the circulation following strenuous exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979, 47, 1311–1314. [Google Scholar] [CrossRef]
- Galbo, H. Hormonal and Metabolic Adaptation to Exercise; Thieme Medical Publishers: New York, NY, USA, 1983. [Google Scholar]
- Gravholt, C.H.; Schmitz, O.; Simonsen, L.; Bulow, J.; Christiansen, J.S.; Moller, N. Effects of a physiological GH pulse on interstitial glycerol in abdominal and femoral adipose tissue. Am. J. Physiol. 1999, 277, E848–E854. [Google Scholar] [CrossRef]
- Hansen, T.K.; Gravholt, C.H.; ORskov, H.; Rasmussen, M.H.; Christiansen, J.S.; Jorgensen, J.O. Dose dependency of the pharmacokinetics and acute lipolytic actions of growth hormone. J. Clin. Endocrinol. Metab. 2002, 87, 4691–4698. [Google Scholar] [CrossRef]
- Moller, N.; Gjedsted, J.; Gormsen, L.; Fuglsang, J.; Djurhuus, C. Effects of growth hormone on lipid metabolism in humans. Growth Horm. IGF Res. 2003, 13, S18–S21. [Google Scholar] [CrossRef]
- Djurhuus, C.B.; Gravholt, C.H.; Nielsen, S.; Pedersen, S.B.; Moller, N.; Schmitz, O. Additive effects of cortisol and growth hormone on regional and systemic lipolysis in humans. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E488–E494. [Google Scholar] [CrossRef]
- Kopchick, J.J.; Berryman, D.E.; Puri, V.; Lee, K.Y.; Jorgensen, J.O.L. The effects of growth hormone on adipose tissue: Old observations, new mechanisms. Nat. Rev. Endocrinol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Arner, P.; Bolinder, J.; Engfeldt, P.; Ostman, J. The antilipolytic effect of insulin in human adipose tissue in obesity, diabetes mellitus, hyperinsulinemia, and starvation. Metabolism 1981, 30, 753–760. [Google Scholar] [CrossRef]
- Wojtaszewski, J.F.; MacDonald, C.; Nielsen, J.N.; Hellsten, Y.; Hardie, D.G.; Kemp, B.E.; Kiens, B.; Richter, E.A. Regulation of 5′AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E813–E822. [Google Scholar] [CrossRef] [PubMed]
- Stallknecht, B.; Lorentsen, J.; Enevoldsen, L.H.; Bülow, J.; Biering-Sørensen, F.; Galbo, H.; Kjær, M. Role of the sympathoadrenergic system in adipose tissue metabolism during exercise in humans. J. Physiol. 2001, 536, 283–294. [Google Scholar] [CrossRef]
- Bergouignan, A.; Kealey, E.H.; Schmidt, S.L.; Jackman, M.R.; Bessesen, D.H. Twenty-four hour total and dietary fat oxidation in lean, obese and reduced-obese adults with and without a bout of exercise. PLoS ONE 2014, 9, e94181. [Google Scholar] [CrossRef]
- Sondergaard, E.; Rahbek, I.; Sørensen, L.P.; Christiansen, J.S.; Gormsen, L.C.; Jensen, M.D.; Nielsen, S. Effects of exercise on VLDL-triglyceride oxidation and turnover. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E939–E944. [Google Scholar] [CrossRef]
- Magkos, F.; Wright, D.C.; Patterson, B.W.; Mohammed, B.S.; Mittendorfer, B. Lipid metabolism response to a single, prolonged bout of endurance exercise in healthy young men. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E355–E362. [Google Scholar] [CrossRef]
- Thompson, P.D.; Cullinane, E.; Henderson, L.O.; Herbert, P.N. Acute effects of prolonged exercise on serum lipids. Metabolism 1980, 29, 662–665. [Google Scholar] [CrossRef]
- Annuzzi, G.; Jansson, E.; Kaijser, L.; Holmquist, L.; Carlson, L.A. Increased removal rate of exogenous triglycerides after prolonged exercise in man: Time course and effect of exercise duration. Metabolism 1987, 36, 438–443. [Google Scholar] [CrossRef]
- Kiens, B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol. Rev. 2006, 86, 205–243. [Google Scholar] [CrossRef]
- Perreault, L.; Lavely, J.M.; Kittelson, J.M.; Horton, T.J. Gender differences in lipoprotein lipase activity after acute exercise. Obes. Res. 2004, 12, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Kiens, B.; Lithell, H.; Mikines, K.J.; Richter, E.A. Effects of insulin and exercise on muscle lipoprotein lipase activity in man and its relation to insulin action. J. Clin. Investig. 1989, 84, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Seip, R.L.; Angelopoulos, T.J.; Semenkovich, C.F. Exercise induces human lipoprotein lipase gene expression in skeletal muscle but not adipose tissue. Am. J. Physiol. 1995, 268, E229–E236. [Google Scholar] [CrossRef] [PubMed]
- Greiwe, J.S.; Holloszy, J.O.; Semenkovich, C.F. Exercise induces lipoprotein lipase and GLUT-4 protein in muscle independent of adrenergic-receptor signaling. J. Appl. Physiol. (1985) 2000, 89, 176–181. [Google Scholar] [CrossRef]
- Seip, R.L.; Mair, K.; Cole, T.G.; Semenkovich, C.F. Induction of human skeletal muscle lipoprotein lipase gene expression by short-term exercise is transient. Am. J. Physiol. 1997, 272, E255–E261. [Google Scholar] [CrossRef]
- Borsheim, E.; Knardahl, S.; Hostmark, A.T. Short-term effects of exercise on plasma very low density lipoproteins (VLDL) and fatty acids. Med. Sci. Sports Exerc. 1999, 31, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Morio, B.; Holmback, U.; Gore, D.; Wolfe, R.R. Increased VLDL-TAG turnover during and after acute moderate-intensity exercise. Med. Sci. Sports Exerc. 2004, 36, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Malkova, D.; Evans, R.D.; Frayn, K.N.; Humphreys, S.M.; Jones, P.R.; Hardman, A.E. Prior exercise and postprandial substrate extraction across the human leg. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1020–E1028. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, E.P.; Fisher, G. Exercise and dietary-mediated reductions in postprandial lipemia. J. Nutr. Metab. 2014, 2014, 902065. [Google Scholar] [CrossRef] [PubMed]
- Sjøberg, K.A.; Frøsig, C.; Kjøbsted, R.; Sylow, L.; Kleinert, M.; Betik, A.C.; Shaw, C.S.; Kiens, B.; Wojtaszewski, J.F.; Rattigan, S.; et al. Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes 2017, 66, 1501–1510. [Google Scholar] [CrossRef]
- McConell, G.K.; Sjøberg, K.A.; Ceutz, F.; Gliemann, L.; Nyberg, M.P.; Hellsten, Y.; Frøsig, C.; Kiens, B.; Wojtaszewski, J.F.; Richter, E.A. Insulin-induced membrane permeability to glucose in human muscles at rest and following exercise. J. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.A.; Minson, C.T.; Halliwill, J.R. The cardiovascular system after exercise. J. Appl. Physiol. (1985) 2017, 122, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, A.M.; Fritzen, A.M.; Kiens, B. Molecular regulation of fatty acid oxidation in skeletal muscle during aerobic exercise. Trends Endocrinol. Metab. 2018, 29, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Albers, P.H.; Rose, A.J.; Birk, J.B.; Schjerling, P.; Dzamko, N.; Steinberg, G.R.; Kiens, B. Contraction-induced skeletal muscle FAT/CD36 trafficking and FA uptake is AMPK independent. J. Lipid Res. 2011, 52, 699–711. [Google Scholar] [CrossRef]
- Bradley, N.S.; Snook, L.A.; Jain, S.S.; Heigenhauser, G.J.; Bonen, A.; Spriet, L.L. Acute endurance exercise increases plasma membrane fatty acid transport proteins in rat and human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E183–E189. [Google Scholar] [CrossRef]
- Jain, S.S.; Chabowski, A.; Snook, L.A.; Schwenk, R.W.; Glatz, J.F.; Luiken, J.J.; Bonen, A. Additive effects of insulin and muscle contraction on fatty acid transport and fatty acid transporters, FAT/CD36, FABPpm, FATP1, 4 and 6. FEBS Lett. 2009, 583, 2294–2300. [Google Scholar] [CrossRef]
- Clarke, D.C.; Miskovic, D.; Han, X.X.; Calles-Escandon, J.; Glatz, J.F.; Luiken, J.J.; Heikkila, J.J.; Bonen, A. Overexpression of membrane-associated fatty acid binding protein (FABPpm) in vivo increases fatty acid sarcolemmal transport and metabolism. Physiol. Genom. 2004, 17, 31–37. [Google Scholar] [CrossRef]
- Holloway, G.P.; Lally, J.; Nickerson, J.G.; Alkhateeb, H.; Snook, L.A.; Heigenhauser, G.J.; Calles-Escandon, J.; Glatz, J.F.; Luiken, J.J.; Spriet, L.L.; et al. Fatty acid binding protein facilitates sarcolemmal fatty acid transport but not mitochondrial oxidation in rat and human skeletal muscle. J. Physiol. 2007, 582, 393–405. [Google Scholar] [CrossRef]
- Luiken, J.J.; Dyck, D.J.; Han, X.X.; Tandon, N.N.; Arumugam, Y.; Glatz, J.F.; Bonen, A. Insulin induces the translocation of the fatty acid transporter FAT/CD36 to the plasma membrane. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E491–E495. [Google Scholar] [CrossRef]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab. 2018, 27, 1111–1120. [Google Scholar] [CrossRef]
- Jevons, E.F.P.; Gejl, K.D.; Strauss, J.A.; Ortenblad, N.; Shepherd, S.O. Skeletal muscle lipid droplets are resynthesized before being coated with perilipin proteins following prolonged exercise in elite male triathletes. Am. J. Physiol. Endocrinol. Metab. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- De Bock, K.; Richter, E.A.; Russell, A.P.; Eijnde, B.O.; Derave, W.; Ramaekers, M.; Koninckx, E.; Leger, B.; Verhaeghe, J.; Hespel, P. Exercise in the fasted state facilitates fibre type-specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J. Physiol. 2005, 564, 649–660. [Google Scholar] [CrossRef]
- Starling, R.D.; Trappe, T.A.; Parcell, A.C.; Kerr, C.G.; Fink, W.J.; Costill, D.L. Effects of diet on muscle triglyceride and endurance performance. J. Appl. Physiol. (1985) 1997, 82, 1185–1189. [Google Scholar] [CrossRef]
- Li, L.O.; Grevengoed, T.J.; Paul, D.S.; Ilkayeva, O.; Koves, T.R.; Pascual, F.; Newgard, C.B.; Muoio, D.M.; Coleman, R.A. Compartmentalized acyl-CoA metabolism in skeletal muscle regulates systemic glucose homeostasis. Diabetes 2015, 64, 23–35. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.D.; Mills, S.E.; Long, C.S.; Foster, D.W. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem. J. 1983, 214, 21–28. [Google Scholar] [CrossRef]
- Smith, B.K.; Perry, C.G.; Koves, T.R.; Wright, D.C.; Smith, J.C.; Neufer, P.D.; Muoio, D.M.; Holloway, G.P. Identification of a novel malonyl-CoA IC(50) for CPT-I: Implications for predicting in vivo fatty acid oxidation rates. Biochem. J. 2012, 448, 13–20. [Google Scholar] [CrossRef][Green Version]
- Rasmussen, B.B.; Hancock, C.R.; Winder, W.W. Postexercise recovery of skeletal muscle malonyl-CoA, acetyl-CoA carboxylase, and AMP-activated protein kinase. J. Appl. Physiol. (1985) 1998, 85, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Frøsig, C.; Roepstorff, C.; Brandt, N.; Maarbjerg, S.J.; Birk, J.B.; Wojtaszewski, J.F.; Richter, E.A.; Kiens, B. Reduced malonyl-CoA content in recovery from exercise correlates with improved insulin-stimulated glucose uptake in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E787–E795. [Google Scholar] [CrossRef] [PubMed]
- Penailillo, L.; Blazevich, A.; Nosaka, K. Energy expenditure and substrate oxidation during and after eccentric cycling. Eur. J. Appl. Physiol. 2014, 114, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.K.; Evans, W.J.; Kirwan, J.P. Impaired substrate oxidation in healthy elderly men after eccentric exercise. J. Appl. Physiol. (1985) 2003, 94, 716–723. [Google Scholar] [CrossRef][Green Version]
- Asp, S.; Daugaard, J.R.; Kristiansen, S.; Kiens, B.; Richter, E.A. Eccentric exercise decreases maximal insulin action in humans: Muscle and systemic effects. J. Physiol. 1996, 494, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, A.M.; Sjøberg, K.A.; Høeg, L.D.; Jeppesen, J.; Jordy, A.B.; Serup, A.K.; Fritzen, A.M.; Pilegaard, H.; Myrmel, L.S.; Madsen, L.; et al. Opposite regulation of insulin sensitivity by dietary lipid versus carbohydrate excess. Diabetes 2017, 66, 2583–2595. [Google Scholar] [CrossRef] [PubMed]
- Pilegaard, H.; Ordway, G.A.; Saltin, B.; Neufer, P.D. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E806–E814. [Google Scholar] [CrossRef] [PubMed]
- Fritzen, A.M.; Lundsgaard, A.M.; Jeppesen, J.; Christiansen, M.L.; Biensø, R.; Dyck, J.R.; Pilegaard, H.; Kiens, B. 5′-AMP activated protein kinase alpha controls substrate metabolism during post-exercise recovery via regulation of pyruvate dehydrogenase kinase. J. Physiol. 2015, 593, 4765–4780. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Chegary, M.; Te Brinke, H.; Wijnen, W.J.; Glatz, J.F.; Luiken, J.J.; Wijburg, F.A.; Wanders, R.J. Pyruvate dehydrogenase kinase 4 expression is synergistically induced by AMP-activated protein kinase and fatty acids. Cell Mol. Life Sci. 2009, 66, 1283–1294. [Google Scholar] [CrossRef]
- Maehlum, S.; Felig, P.; Wahren, J. Splanchnic glucose and muscle glycogen metabolism after glucose feeding during postexercise recovery. Am. J. Physiol. 1978, 235, E255–E260. [Google Scholar] [CrossRef]
- Rose, A.J.; Howlett, K.; King, D.S.; Hargreaves, M. Effect of prior exercise on glucose metabolism in trained men. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E766–E771. [Google Scholar] [CrossRef]
- Mu, J.; Barton, E.R.; Birnbaum, M.J. Selective suppression of AMP-activated protein kinase in skeletal muscle: Update on ‘lazy mice’. Biochem. Soc. Trans. 2003, 31, 236–241. [Google Scholar] [CrossRef]
- Jørgensen, S.B.; Wojtaszewski, J.F.; Viollet, B.; Andreelli, F.; Birk, J.B.; Hellsten, Y.; Schjerling, P.; Vaulont, S.; Neufer, P.D.; Richter, E.A.; et al. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005, 19, 1146–1148. [Google Scholar] [CrossRef]
- Barnes, B.R.; Marklund, S.; Steiler, T.L.; Walter, M.; Hjälm, G.; Amarger, V.; Mahlapuu, M.; Leng, Y.; Johansson, C.; Galuska, D.; et al. The 5′-AMP-activated protein kinase gamma3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J. Biol. Chem. 2004, 279, 38441–38447. [Google Scholar] [CrossRef]
- Hingst, J.R.; Bruhn, L.; Hansen, M.B.; Rosschou, M.F.; Birk, J.B.; Fentz, J.; Foretz, M.; Viollet, B.; Sakamoto, K.; Færgeman, N.J.; et al. Exercise-induced molecular mechanisms promoting glycogen supercompensation in human skeletal muscle. Mol. Metab. 2018, 16, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Kjøbsted, R.; Roll, J.L.; Jørgensen, N.O.; Birk, J.B.; Foretz, M.; Viollet, B.; Chadt, A.; Al-Hasani, H.; Wojtaszewski, J.F. AMPK and TBC1D1 regulate muscle glucose uptake after, but not during, exercise and contraction. Diabetes 2019, 68, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Kjøbsted, R.; Munk-Hansen, N.; Birk, J.B.; Foretz, M.; Viollet, B.; Björnholm, M.; Zierath, J.R.; Treebak, J.T.; Wojtaszewski, J.F. Enhanced muscle insulin sensitivity after contraction/exercise is mediated by AMPK. Diabetes 2017, 66, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Kjøbsted, R.; Chadt, A.; Jørgensen, N.O.; Kido, K.; Larsen, J.K.; de Wendt, C.; Al-Hasani, H.; Wojtaszewski, J.F. TBC1D4 is Necessary for Enhancing Muscle Insulin Sensitivity in Response to AICAR and Contraction. Diabetes 2019, 68, 1756–1766. [Google Scholar] [CrossRef]
- Pilegaard, H.; Osada, T.; Andersen, L.T.; Helge, J.W.; Saltin, B.; Neufer, P.D. Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism 2005, 54, 1048–1055. [Google Scholar] [CrossRef]
- Cluberton, L.J.; McGee, S.L.; Murphy, R.M.; Hargreaves, M. Effect of carbohydrate ingestion on exercise-induced alterations in metabolic gene expression. J. Appl. Physiol. (1985) 2005, 99, 1359–1363. [Google Scholar] [CrossRef]
- Banner, C.D.; Gottlicher, M.; Widmark, E.; Sjovall, J.; Rafter, J.J.; Gustafsson, J.A. A systematic analytical chemistry/cell assay approach to isolate activators of orphan nuclear receptors from biological extracts: Characterization of peroxisome proliferator-activated receptor activators in plasma. J. Lipid Res. 1993, 34, 1583–1591. [Google Scholar] [PubMed]
- Watt, M.J.; Southgate, R.J.; Holmes, A.G.; Febbraio, M.A. Suppression of plasma free fatty acids upregulates peroxisome proliferator-activated receptor (PPAR) alpha and delta and PPAR coactivator 1alpha in human skeletal muscle, but not lipid regulatory genes. J. Mol. Endocrinol. 2004, 33, 533–544. [Google Scholar] [CrossRef]
- Tanaka, T.; Yamamoto, J.; Iwasaki, S.; Asaba, H.; Hamura, H.; Ikeda, Y.; Watanabe, M.; Magoori, K.; Ioka, R.X.; Tachibana, K.; et al. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc. Natl. Acad. Sci. USA 2003, 100, 15924–15929. [Google Scholar] [CrossRef]
- Fan, W.; Waizenegger, W.; Lin, C.S.; Sorrentino, V.; He, M.X.; Wall, C.E.; Li, H.; Liddle, C.; Ruth, T.Y.; Atkins, A.R.; et al. PPARdelta promotes running endurance by preserving glucose. Cell Metab. 2017, 25, 1186–1193. [Google Scholar] [CrossRef]
- Barres, R.; Yan, J.; Egan, B.; Treebak, J.T.; Rasmussen, M.; Fritz, T.; Caidahl, K.; Krook, A.; O’Gorman, D.J.; Zierath, J.R. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012, 15, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Hénique, C.; Mansouri, A.; Vavrova, E.; Lenoir, V.; Ferry, A.; Esnous, C.; Ramond, E.; Girard, J.; Bouillaud, F.; Prip-Buus, C.; et al. Increasing mitochondrial muscle fatty acid oxidation induces skeletal muscle remodeling toward an oxidative phenotype. FASEB J. 2015, 29, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundsgaard, A.-M.; Fritzen, A.M.; Kiens, B. The Importance of Fatty Acids as Nutrients during Post-Exercise Recovery. Nutrients 2020, 12, 280. https://doi.org/10.3390/nu12020280
Lundsgaard A-M, Fritzen AM, Kiens B. The Importance of Fatty Acids as Nutrients during Post-Exercise Recovery. Nutrients. 2020; 12(2):280. https://doi.org/10.3390/nu12020280
Chicago/Turabian StyleLundsgaard, Anne-Marie, Andreas M. Fritzen, and Bente Kiens. 2020. "The Importance of Fatty Acids as Nutrients during Post-Exercise Recovery" Nutrients 12, no. 2: 280. https://doi.org/10.3390/nu12020280
APA StyleLundsgaard, A.-M., Fritzen, A. M., & Kiens, B. (2020). The Importance of Fatty Acids as Nutrients during Post-Exercise Recovery. Nutrients, 12(2), 280. https://doi.org/10.3390/nu12020280





