Metabolic and Nutritional Issues Associated with Spinal Muscular Atrophy
Abstract
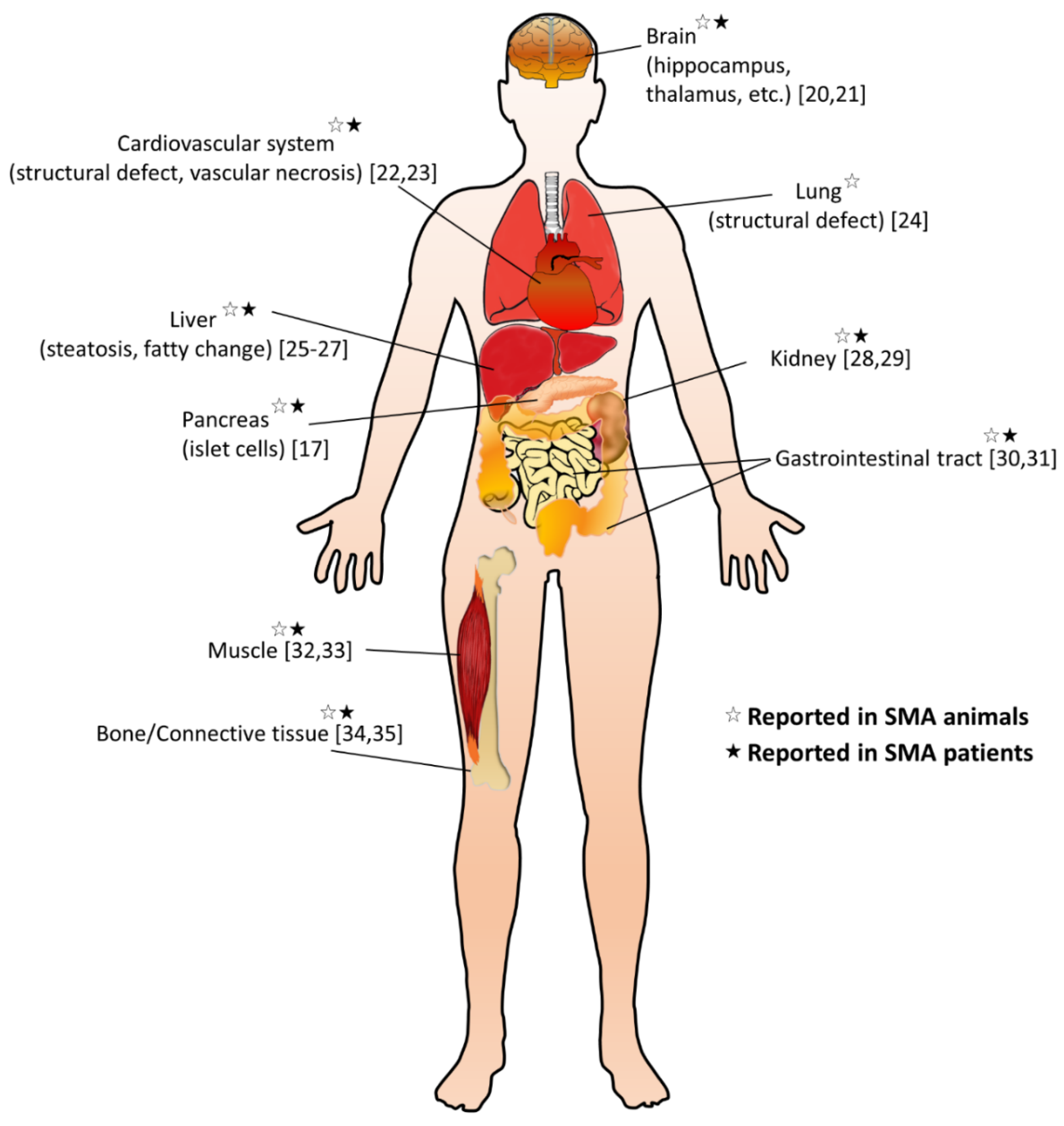
:1. Introduction
2. Lipid Metabolic Abnormalities in SMA
3. Glucose Metabolic Abnormalities in SMA
4. Altered Vitamin Level in SMA
5. Dietary Issues in SMA
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dubowitz, V. Ramblings in the history of spinal muscular atrophy. Neuromuscul. Disord. 2009, 19, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Darras, B.T. Spinal muscular atrophies. Pediatric Clin. N. Am. 2015, 62, 743–766. [Google Scholar] [CrossRef] [PubMed]
- Lunn, M.R.; Wang, C.H. Spinal muscular atrophy. Lancet 2008, 371, 2120–2133. [Google Scholar] [CrossRef]
- Farrar, M.A.; Park, S.B.; Vucic, S.; Carey, K.A.; Turner, B.J.; Gillingwater, T.H.; Swoboda, K.J.; Kiernan, M.C. Emerging therapies and challenges in spinal muscular atrophy. Ann. Neurol. 2017, 81, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Burglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Burghes, A.H. When is a deletion not a deletion? When it is converted. Am. J. Hum. Genet. 1997, 61, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Burghes, A.H.; Beattie, C.E. Spinal muscular atrophy: Why do low levels of survival motor neuron protein make motor neurons sick? Nat. Rev. Neurosci. 2009, 10, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Tisdale, S.; Pellizzoni, L. Disease mechanisms and therapeutic approaches in spinal muscular atrophy. J. Neurosci. 2015, 35, 8691–8700. [Google Scholar] [CrossRef] [Green Version]
- Wirth, B.; Karakaya, M.; Kye, M.J.; Mendoza-Ferreira, N. Twenty-five years of spinal muscular atrophy research: From phenotype to genotype to therapy, and what comes next. Annu. Rev. Genom. Hum. Genet. 2020, 21, 231–261. [Google Scholar] [CrossRef] [Green Version]
- Tu, W.Y.; Simpson, J.E.; Highley, J.R.; Heath, P.R. Spinal muscular atrophy: Factors that modulate motor neurone vulnerability. Neurobiol. Dis. 2017, 102, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.H. New and developing therapies in spinal muscular atrophy: From genotype to phenotype to treatment and where do we stand? Int. J. Mol. Sci. 2020, 21, 3297. [Google Scholar] [CrossRef]
- Nash, L.A.; Burns, J.K.; Chardon, J.W.; Kothary, R.; Parks, R.J. Spinal muscular atrophy: More than a disease of motor neurons? Curr. Mol. Med. 2016, 16, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.; Ramirez, A.; Bucchia, M.; Rinchetti, P.; Rideout, H.; Papadimitriou, D.; Re, D.B.; Corti, S. Is spinal muscular atrophy a disease of the motor neurons only: Pathogenesis and therapeutic implications? Cell Mol. Life Sci. 2016, 73, 1003–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, C.J.J.; Darras, B.T. Overturning the paradigm of spinal muscular atrophy as just a motor neuron disease. Pediatr. Neurol. 2020, 109, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018, 28, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.H.; Miller, E.A.; Zhang, R.Z.; Swoboda, K.J. Responses to fasting and glucose loading in a cohort of well children with spinal muscular atrophy type ii. J. Pediatrics 2015, 167, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Bowerman, M.; Swoboda, K.J.; Michalski, J.P.; Wang, G.S.; Reeks, C.; Beauvais, A.; Murphy, K.; Woulfe, J.; Screaton, R.A.; Scott, F.W.; et al. Glucose metabolism and pancreatic defects in spinal muscular atrophy. Ann. Neurol. 2012, 72, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Mehta, N.M.; Newman, H.; Tarrant, S.; Graham, R.J. Nutritional status and nutrient intake challenges in children with spinal muscular atrophy. Pediatric Neurol. 2016, 57, 80–83. [Google Scholar] [CrossRef]
- Lipnick, S.L.; Agniel, D.M.; Aggarwal, R.; Makhortova, N.R.; Finlayson, S.G.; Brocato, A.; Palmer, N.; Darras, B.T.; Kohane, I.; Rubin, L.L. Systemic nature of spinal muscular atrophy revealed by studying insurance claims. PLoS ONE 2019, 14, e0213680. [Google Scholar] [CrossRef]
- Wishart, T.M.; Huang, J.P.; Murray, L.M.; Lamont, D.J.; Mutsaers, C.A.; Ross, J.; Geldsetzer, P.; Ansorge, O.; Talbot, K.; Parson, S.H.; et al. Smn deficiency disrupts brain development in a mouse model of severe spinal muscular atrophy. Hum. Mol. Genet. 2010, 19, 4216–4228. [Google Scholar] [CrossRef] [Green Version]
- Mendonca, R.H.; Rocha, A.J.; Lozano-Arango, A.; Diaz, A.B.; Castiglioni, C.; Silva, A.M.S.; Reed, U.C.; Kulikowski, L.; Paramonov, I.; Cusco, I.; et al. Severe brain involvement in 5q spinal muscular atrophy type 0. Ann. Neurol. 2019, 86, 458–462. [Google Scholar] [CrossRef]
- Somers, E.; Lees, R.D.; Hoban, K.; Sleigh, J.N.; Zhou, H.; Muntoni, F.; Talbot, K.; Gillingwater, T.H.; Parson, S.H. Vascular defects and spinal cord hypoxia in spinal muscular atrophy. Ann. Neurol. 2016, 79, 217–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijngaarde, C.A.; Blank, A.C.; Stam, M.; Wadman, R.I.; van den Berg, L.H.; van der Pol, W.L. Cardiac pathology in spinal muscular atrophy: A systematic review. Orphanet J. Rare Dis. 2017, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Schreml, J.; Riessland, M.; Paterno, M.; Garbes, L.; Rossbach, K.; Ackermann, B.; Kramer, J.; Somers, E.; Parson, S.H.; Heller, R.; et al. Severe sma mice show organ impairment that cannot be rescued by therapy with the hdaci jnj-26481585. Eur. J. Hum. Genet. 2013, 21, 643–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deguise, M.-O.; Pileggi, C.; Beauvais, A.; Tierney, A.; Chehade, L.; De Repentigny, Y.; Michaud, J.; Llavero-Hurtado, M.; Lamont, D.; Atrih, A.J.B. A mouse model for spinal muscular atrophy provides insights into non-alcoholic fatty liver disease pathogenesis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Szunyogova, E.; Zhou, H.; Maxwell, G.K.; Powis, R.A.; Muntoni, F.; Gillingwater, T.H.; Parson, S.H. Survival motor neuron (smn) protein is required for normal mouse liver development. Sci. Rep. 2016, 6, 34635. [Google Scholar] [CrossRef]
- Deguise, M.O.; Baranello, G.; Mastella, C.; Beauvais, A.; Michaud, J.; Leone, A.; De Amicis, R.; Battezzati, A.; Dunham, C.; Selby, K.; et al. Abnormal fatty acid metabolism is a core component of spinal muscular atrophy. Ann. Clin. Transl. Neurol. 2019, 6, 1519–1532. [Google Scholar] [CrossRef] [Green Version]
- Nery, F.C.; Siranosian, J.J.; Rosales, I.; Deguise, M.O.; Sharma, A.; Muhtaseb, A.W.; Nwe, P.; Johnstone, A.J.; Zhang, R.; Fatouraei, M.; et al. Impaired kidney structure and function in spinal muscular atrophy. Neurol. Genet. 2019, 5, e353. [Google Scholar] [CrossRef] [Green Version]
- Allardyce, H.; Kuhn, D.; Hernandez-Gerez, E.; Hensel, N.; Huang, Y.T.; Faller, K.; Gillingwater, T.H.; Quondamatteo, F.; Claus, P.; Parson, S.H. Renal pathology in a mouse model of severe spinal muscular atrophy is associated with downregulation of glial cell-line derived neurotrophic factor (gdnf). Hum. Mol. Genet. 2020, 29, 2365–2378. [Google Scholar] [CrossRef]
- Sintusek, P.; Catapano, F.; Angkathunkayul, N.; Marrosu, E.; Parson, S.H.; Morgan, J.E.; Muntoni, F.; Zhou, H. Histopathological defects in intestine in severe spinal muscular atrophy mice are improved by systemic antisense oligonucleotide treatment. PLoS ONE 2016, 11, e0155032. [Google Scholar] [CrossRef]
- Davis, R.H.; Godshall, B.J.; Seffrood, E.; Marcus, M.; LaSalle, B.A.; Wong, B.; Schroth, M.K.; Swoboda, K.J. Nutritional practices at a glance: Spinal muscular atrophy type i nutrition survey findings. J. Child. Neurol. 2014, 29, 1467–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripolone, M.; Ronchi, D.; Violano, R.; Vallejo, D.; Fagiolari, G.; Barca, E.; Lucchini, V.; Colombo, I.; Villa, L.; Berardinelli, A.; et al. Impaired muscle mitochondrial biogenesis and myogenesis in spinal muscular atrophy. JAMA Neurol. 2015, 72, 666–675. [Google Scholar] [CrossRef]
- Deguise, M.O.; Boyer, J.G.; McFall, E.R.; Yazdani, A.; De Repentigny, Y.; Kothary, R. Differential induction of muscle atrophy pathways in two mouse models of spinal muscular atrophy. Sci. Rep. 2016, 6, 28846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vai, S.; Bianchi, M.L.; Moroni, I.; Mastella, C.; Broggi, F.; Morandi, L.; Arnoldi, M.T.; Bussolino, C.; Baranello, G. Bone and spinal muscular atrophy. Bone 2015, 79, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Shanmugarajan, S.; Tsuruga, E.; Swoboda, K.J.; Maria, B.L.; Ries, W.L.; Reddy, S.V. Bone loss in survival motor neuron (smn(−/−) smn2) genetic mouse model of spinal muscular atrophy. J. Pathol. 2009, 219, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, F.; Hussain, G.; Dupuis, L.; Loeffler, J.P.; Henriques, A. A plural role for lipids in motor neuron diseases: Energy, signaling and structure. Front. Cell Neurosci. 2014, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Harpey, J.P.; Charpentier, C.; Paturneau-Jouas, M.; Renault, F.; Romero, N.; Fardeau, M. Secondary metabolic defects in spinal muscular atrophy type ii. Lancet 1990, 336, 629–630. [Google Scholar] [CrossRef]
- Kelley, R.I.; Sladky, J.T. Dicarboxylic aciduria in an infant with spinal muscular atrophy. Ann. Neurol. 1986, 20, 734–736. [Google Scholar] [CrossRef]
- Butchbach, M.E.; Rose, F.F., Jr.; Rhoades, S.; Marston, J.; McCrone, J.T.; Sinnott, R.; Lorson, C.L. Effect of diet on the survival and phenotype of a mouse model for spinal muscular atrophy. Biochem. Biophys. Res. Commun. 2010, 391, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Sproule, D.M.; Montes, J.; Montgomery, M.; Battista, V.; Koenigsberger, D.; Shen, W.; Punyanitya, M.; De Vivo, D.C.; Kaufmann, P. Increased fat mass and high incidence of overweight despite low body mass index in patients with spinal muscular atrophy. Neuromuscul. Disord. Nmd 2009, 19, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Tein, I.; Sloane, A.E.; Donner, E.J.; Lehotay, D.C.; Millington, D.S.; Kelley, R.I. Fatty acid oxidation abnormalities in childhood-onset spinal muscular atrophy: Primary or secondary defect(s)? Pediatric Neurol. 1995, 12, 21–30. [Google Scholar] [CrossRef]
- Crawford, T.O.; Sladky, J.T.; Hurko, O.; Besner-Johnston, A.; Kelley, R.I. Abnormal fatty acid metabolism in childhood spinal muscular atrophy. Ann. Neurol. 1999, 45, 337–343. [Google Scholar] [CrossRef]
- Poruk, K.E.; Davis, R.H.; Smart, A.L.; Chisum, B.S.; Lasalle, B.A.; Chan, G.M.; Gill, G.; Reyna, S.P.; Swoboda, K.J. Observational study of caloric and nutrient intake, bone density, and body composition in infants and children with spinal muscular atrophy type i. Neuromuscul. Disord. 2012, 22, 966–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkel, R.S.; Crawford, T.O.; Swoboda, K.J.; Kaufmann, P.; Juhasz, P.; Li, X.; Guo, Y.; Li, R.H.; Trachtenberg, F.; Forrest, S.J.; et al. Candidate proteins, metabolites and transcripts in the biomarkers for spinal muscular atrophy (bforsma) clinical study. PLoS ONE 2012, 7, e35462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulroy, E.; Gleeson, S.; Furlong, M.J. Stress-induced ketoacidosis in spinal muscular atrophy: An under-recognized complication. J. Neuromuscul. Dis. 2016, 3, 419–423. [Google Scholar] [CrossRef]
- Lakkis, B.; El Chediak, A.; Hashash, J.G.; Koubar, S.H. Severe ketoacidosis in a patient with spinal muscular atrophy. Cen Case Rep. 2018, 7, 292–295. [Google Scholar] [CrossRef]
- Shababi, M.; Lorson, C.L.; Rudnik-Schoneborn, S.S. Spinal muscular atrophy: A motor neuron disorder or a multi-organ disease? J. Anat. 2014, 224, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Zolkipli, Z.; Sherlock, M.; Biggar, W.D.; Taylor, G.; Hutchison, J.S.; Peliowski, A.; Alman, B.A.; Ling, S.C.; Tein, I. Abnormal fatty acid metabolism in spinal muscular atrophy may predispose to perioperative risks. Eur. J. Paediatr. Neurol. 2012, 16, 549–553. [Google Scholar] [CrossRef]
- Feldman, A.G.; Parsons, J.A.; Dutmer, C.M.; Veerapandiyan, A.; Hafberg, E.; Maloney, N.; Mack, C.L. Subacute liver failure following gene replacement therapy for spinal muscular atrophy type 1. J. Pediatr. 2020, 225, 252–258.e1. [Google Scholar] [CrossRef]
- Hua, Y.; Sahashi, K.; Rigo, F.; Hung, G.; Horev, G.; Bennett, C.F.; Krainer, A.R. Peripheral smn restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature 2011, 478, 123–126. [Google Scholar] [CrossRef]
- Ferreira, G.C.; McKenna, M.C. L-carnitine and acetyl-l-carnitine roles and neuroprotection in developing brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.L.; McDonald, D.A.; Borum, P.R. Acylcarnitines: Role in brain. Prog. Lipid Res. 2010, 49, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.; Lanza, G.; Cantone, M.; D’Amico, E.; Fisicaro, F.; Puglisi, V.; Vinciguerra, L.; Bella, R.; Vicari, E.; Malaguarnera, G. Acetyl-l-carnitine in dementia and other cognitive disorders: A critical update. Nutrients 2020, 12, 1389. [Google Scholar] [CrossRef] [PubMed]
- Czyzewski, K.; Stern, L.Z.; Sadeh, M.; Bahl, J.J. Changes in muscle carnitine during regeneration. Exp. Neurol. 1984, 86, 73–80. [Google Scholar] [CrossRef]
- Czyzewski, K.; Stern, L.Z.; Sadeh, M.; Bahl, J.J. Altered rat skeletal muscle carnitine with age and after denervation. Muscle Nerve 1985, 8, 34–37. [Google Scholar] [CrossRef]
- Bresolin, N.; Freddo, L.; Tegazzin, V.; Bet, L.; Armani, M.; Angelini, C. Carnitine and acyltransferase in experimental neurogenic atrophies: Changes with treatment. J. Neurol. 1984, 231, 170–175. [Google Scholar] [CrossRef]
- Carrasco, P.; Jacas, J.; Sahun, I.; Muley, H.; Ramirez, S.; Puisac, B.; Mezquita, P.; Pie, J.; Dierssen, M.; Casals, N. Carnitine palmitoyltransferase 1c deficiency causes motor impairment and hypoactivity. Behav. Brain Res. 2013, 256, 291–297. [Google Scholar] [CrossRef]
- Rinaldi, C.; Schmidt, T.; Situ, A.J.; Johnson, J.O.; Lee, P.R.; Chen, K.L.; Bott, L.C.; Fado, R.; Harmison, G.H.; Parodi, S.; et al. Mutation in cpt1c associated with pure autosomal dominant spastic paraplegia. JAMA Neurol. 2015, 72, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Lu, Y.; Neubert, T.A.; Resh, M.D. Mass spectrometric analysis of gap-43/neuromodulin reveals the presence of a variety of fatty acylated species. J. Biol. Chem. 2002, 277, 33032–33040. [Google Scholar] [CrossRef] [Green Version]
- Fallini, C.; Donlin-Asp, P.G.; Rouanet, J.P.; Bassell, G.J.; Rossoll, W. Deficiency of the survival of motor neuron protein impairs mrna localization and local translation in the growth cone of motor neurons. J. Neurosci. 2016, 36, 3811–3820. [Google Scholar] [CrossRef]
- Fuller, H.R.; Gillingwater, T.H.; Wishart, T.M. Commonality amid diversity: Multi-study proteomic identification of conserved disease mechanisms in spinal muscular atrophy. Neuromuscul Disord. 2016, 26, 560–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okun, J.G.; Kolker, S.; Schulze, A.; Kohlmuller, D.; Olgemoller, K.; Lindner, M.; Hoffmann, G.F.; Wanders, R.J.; Mayatepek, E. A method for quantitative acylcarnitine profiling in human skin fibroblasts using unlabelled palmitic acid: Diagnosis of fatty acid oxidation disorders and differentiation between biochemical phenotypes of mcad deficiency. Biochim. Biophys. Acta 2002, 1584, 91–98. [Google Scholar] [CrossRef]
- Aguer, C.; McCoin, C.S.; Knotts, T.A.; Thrush, A.B.; Ono-Moore, K.; McPherson, R.; Dent, R.; Hwang, D.H.; Adams, S.H.; Harper, M.E. Acylcarnitines: Potential implications for skeletal muscle insulin resistance. FASEB J. 2015, 29, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Bruce, A.K.; Jacobsen, E.; Dossing, H.; Kondrup, J. Hypoglycaemia in spinal muscular atrophy. Lancet 1995, 346, 609–610. [Google Scholar] [PubMed]
- Orngreen, M.C.; Zacho, M.; Hebert, A.; Laub, M.; Vissing, J. Patients with severe muscle wasting are prone to develop hypoglycemia during fasting. Neurology 2003, 61, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Berti, B.; Onesimo, R.; Leone, D.; Palermo, C.; Giorgio, V.; Buonsenso, D.; Pane, M.; Mercuri, E. Hypoglycaemia in patients with type 1 sma: An underdiagnosed problem? Arch. Dis. Child. 2020, 105, 707. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, N.H.; Golden, L.; John, R.M.; Naini, A.; Vivo, D.C.; Sproule, D.M. Diabetic ketoacidosis in an adult patient with spinal muscular atrophy type ii: Further evidence of extraneural pathology due to survival motor neuron 1 mutation? J. Child. Neurol. 2013, 28, 1517–1520. [Google Scholar] [CrossRef]
- Moon, S.S. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the korean population: The korea national health and nutrition examination survey (knhanes) 2009–2010. Endocr. J. 2014, 61, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Brener, A.; Sagi, L.; Shtamler, A.; Levy, S.; Fattal-Valevski, A.; Lebenthal, Y. Insulin-like growth factor-1 status is associated with insulin resistance in young patients with spinal muscular atrophy. Neuromuscul Disord. 2020, 30, 888–896. [Google Scholar] [CrossRef]
- Kolbel, H.; Hauffa, B.P.; Wudy, S.A.; Bouikidis, A.; Della Marina, A.; Schara, U. Hyperleptinemia in children with autosomal recessive spinal muscular atrophy type i-iii. PLoS ONE 2017, 12, e0173144. [Google Scholar]
- Bowerman, M.; Michalski, J.P.; Beauvais, A.; Murray, L.M.; DeRepentigny, Y.; Kothary, R. Defects in pancreatic development and glucose metabolism in smn-depleted mice independent of canonical spinal muscular atrophy neuromuscular pathology. Hum. Mol. Genet. 2014, 23, 3432–3444. [Google Scholar] [CrossRef] [Green Version]
- Tsai, L.K.; Chen, C.L.; Ting, C.H.; Lin-Chao, S.; Hwu, W.L.; Dodge, J.C.; Passini, M.A.; Cheng, S.H. Systemic administration of a recombinant aav1 vector encoding igf-1 improves disease manifestations in sma mice. Mol. Ther. 2014, 22, 1450–1459. [Google Scholar] [CrossRef] [Green Version]
- Millino, C.; Fanin, M.; Vettori, A.; Laveder, P.; Mostacciuolo, M.L.; Angelini, C.; Lanfranchi, G. Different atrophy-hypertrophy transcription pathways in muscles affected by severe and mild spinal muscular atrophy. BMC Med. 2009, 7, 14. [Google Scholar] [CrossRef]
- Friesen, W.J.; Massenet, S.; Paushkin, S.; Wyce, A.; Dreyfuss, G. Smn, the product of the spinal muscular atrophy gene, binds preferentially to dimethylarginine-containing protein targets. Mol. Cell 2001, 7, 1111–1117. [Google Scholar] [CrossRef]
- Majumder, A.; Behera, J.; Jeremic, N.; Tyagi, S.C. Hypermethylation: Causes and consequences in skeletal muscle myopathy. J. Cell Biochem. 2017, 118, 2108–2117. [Google Scholar] [CrossRef]
- Khatri, I.A.; Chaudhry, U.S.; Seikaly, M.G.; Browne, R.H.; Iannaccone, S.T. Low bone mineral density in spinal muscular atrophy. J. Clin. Neuromuscul. Dis. 2008, 10, 11–17. [Google Scholar] [CrossRef]
- Martinez, E.E.; Quinn, N.; Arouchon, K.; Anzaldi, R.; Tarrant, S.; Ma, N.S.; Griffin, J.; Darras, B.T.; Graham, R.J.; Mehta, N.M. Comprehensive nutritional and metabolic assessment in patients with spinal muscular atrophy: Opportunity for an individualized approach. Neuromuscul. Disord. 2018, 28, 512–519. [Google Scholar] [CrossRef]
- Aton, J.; Davis, R.H.; Jordan, K.C.; Scott, C.B.; Swoboda, K.J. Vitamin d intake is inadequate in spinal muscular atrophy type i cohort: Correlations with bone health. J. Child. Neurol. 2014, 29, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, H.M.; Hornung, L.N.; Stenger, P.J.; Rutter, M.M.; Wong, B.L.; Rybalsky, I.; Khoury, J.C.; Kalkwarf, H.J. Low bone mineral density and fractures are highly prevalent in pediatric patients with spinal muscular atrophy regardless of disease severity. Neuromuscul. Disord. 2017, 27, 331–337. [Google Scholar] [CrossRef]
- Chen, Y.S.; Shih, H.H.; Chen, T.H.; Kuo, C.H.; Jong, Y.J. Prevalence and risk factors for feeding and swallowing difficulties in spinal muscular atrophy types ii and iii. J. Pediatrics 2012, 160, 447–451. [Google Scholar] [CrossRef]
- Deguise, M.O.; Chehade, L.; Tierney, A.; Beauvais, A.; Kothary, R. Low fat diets increase survival of a mouse model of spinal muscular atrophy. Ann. Clin. Transl. Neurol. 2019, 6, 2340–2346. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Pera, M.C.; Scoto, M.; Finkel, R.; Muntoni, F. Spinal muscular atrophy—Insights and challenges in the treatment era. Nat. Rev. Neurol. 2020, 16, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Butchbach, M.E.; Singh, J.; Gurney, M.E.; Burghes, A.H. The effect of diet on the protective action of d156844 observed in spinal muscular atrophy mice. Exp. Neurol. 2014, 256, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narver, H.L.; Kong, L.; Burnett, B.G.; Choe, D.W.; Bosch-Marce, M.; Taye, A.A.; Eckhaus, M.A.; Sumner, C.J. Sustained improvement of spinal muscular atrophy mice treated with trichostatin a plus nutrition. Ann. Neurol. 2008, 64, 465–470. [Google Scholar] [CrossRef]
- Bertoli, S.; De Amicis, R.; Mastella, C.; Pieri, G.; Giaquinto, E.; Battezzati, A.; Leone, A.; Baranello, G. Spinal muscular atrophy, types i and ii: What are the differences in body composition and resting energy expenditure? Clin. Nutr. 2017, 36, 1674–1680. [Google Scholar] [CrossRef] [Green Version]
| Altered Metabolic Aspect | Reference | Study Design | Study Aim | Enrollment | Patient Features | Main Findings |
|---|---|---|---|---|---|---|
| Lipid (fatty acid), carnitine | Kelley et al. (1986) [38] | Case report | To describe an SMA infant with elevated certain urinary organic acids, suggesting a defect of fatty acid metabolism. | SMA: 1 | SMA type 1; age 9 months old |
|
| Lipid (fatty acid), carnitine, acylcarnitine | Harpey et al. (1990) [37] | Cross-sectional | assess the metabolic defects of fatty acids and carnitine/acylcarnitine among patients with SMA | SMA: 14 | SMA type 2: 100%; age range 1–11.5 years old |
|
| Lipid (fatty acid), carnitine, acylcarnitine | Tein et al. (1995) [41] | Cross-sectional | To identify and quantify the FA oxidation abnormalities in SMA and to correlate these with disease severity and to identify specific underlying defects. | SMA: 15 | SMA type 1: 20%, type 2: 53%, type 3: 27%; age range 2 months old–20 years old |
|
| Lipid (fatty acid), carnitine | Crawford et al. (1999) [42] |
| To evaluate fasting and non-fasting lipid profiles in urine and plasma in infants and children with SMA. | SMA: 50 healthy controls: 22 disease controls: 6 SMA: 13 healthy control: 23 | SMA type 1: 66%, type 2/3: 34% Disease controls: non-SMA denervation disorders (n = 6) Healthy controls: age 8–11 months old (n = 4), age 1–6 years old (n = 19) |
|
| Lipid (fatty acid), carnitine, acylcarnitine, ketone, glucose | Zolkipli et al. (2012) [48] | Case report | Describing a type 2 SMA children with catabolic crisis related to possibly impaired intramitochondrial β-oxidation. | SMA: 1 | SMA type 2; age 15 years old |
|
| Lipid (fatty acid), ketone, glucose | Mulroy et al. (2016) [45] | Case report | Describing a type 2 SMA adult presented with severe ketoacidosis with mild hypoglycemia | SMA: 1 | SMA type 2; age: 50 years old; BMI: 16.4 kg/m2 |
|
| Lipid (fatty acid), ketone | Lakkis et al. (2018) [46] | Case report | Describing a type 3 SMA adult presented with severe ketoacidosis with normal serum glucose | SMA: 1 | SMA type 3; age: 36 years old; BMI: 23 kg/m2 |
|
| Lipid (fatty acid), glucose | Deguise et al. (2019) [27] | Cross-sectional |
| SMA: 72 | SMA type 1 20%, type 2 72%, type 3 8%; median age 3.8 years old |
|
| Altered Metabolic Aspect | Reference | Study Design | Study Aim | Enrollment | Patient Features | Main Findings |
|---|---|---|---|---|---|---|
| Glucose, ketone | Bruce et al. (1995) [64] | Case study | To describe a phenomenon of hypoglycemia in patients with SMA. | SMA: 2 | SMA type 2 100%; age 14 years old and 20 years old, respectively |
|
| Glucose | Orngreen et al. (2003) [65] | Two or more single-arm study | To investigate the effect of 23 h of fasting on plasma glucose and other metabolites, glucose turnover, and hormonal changes in NMD patients with low muscle mass. | SMA: 4 Healthy controls: 6 | SMA type 2 100%; mean age 25 years old; average body weight 29.8 kg Controls: mean age 24 years old; average body weight 69.5 kg |
|
| Glucose, insulin | Bowerman et al. (2012) [17] | Cross-sectional | To describe glucose metabolism and pancreatic developmental defects in SMA. | SMA: 6 | SMA type 1 100%; age range 7–35 months old. Control: age range 4–36 months old. |
|
| Glucose, ketone | Lamarca et al. (2013) [67] | Case study | To describe a phenomenon of DM and diabetic ketoacidosis in a patient with type 2 SMA. | SMA: 1 | SMA typ 2, age 29 years old, BMI: 10.2 kg/m2 |
|
| Glucose, insulin, ketone | Davies et al. (2015) [16] | Case series | To examine the impact of fasting and glucose tolerance in an SMA type 2 population. | SMA: 6 | SMA type 2 100%; mean age 8.9 ± 1.7 years old (range 7–10 years old) |
|
| Glucose | Berti et al. (2020) [66] | Cross-sectional | To describe the incidence of hypoglycemia in type 1 SMA patients after short-term fasting (> 4 h but <6 h) | SMA:45 | SMA type 1: 100%; median age: 42 months old (hypoglycemic) vs. 21.5 months old (non-hypoglycemic); BMI: −2.19 kg/m2 |
|
| Glucose, insulin, IGF-1 | Brener et al. (2020) [69] | Cross-sectional | To determine the IGF-1 status in SMA patients and its association with insulin resistance. | SMA: 34 | SMA type 1: 47%, type 2: 29%, type 3: 24%; mean age: 7.1 years old; mean BMI: −1.60 kg/m2 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-J.; Chen, T.-H.; Wu, Y.-Z.; Tseng, Y.-H. Metabolic and Nutritional Issues Associated with Spinal Muscular Atrophy. Nutrients 2020, 12, 3842. https://doi.org/10.3390/nu12123842
Li Y-J, Chen T-H, Wu Y-Z, Tseng Y-H. Metabolic and Nutritional Issues Associated with Spinal Muscular Atrophy. Nutrients. 2020; 12(12):3842. https://doi.org/10.3390/nu12123842
Chicago/Turabian StyleLi, Yang-Jean, Tai-Heng Chen, Yan-Zhang Wu, and Yung-Hao Tseng. 2020. "Metabolic and Nutritional Issues Associated with Spinal Muscular Atrophy" Nutrients 12, no. 12: 3842. https://doi.org/10.3390/nu12123842
APA StyleLi, Y.-J., Chen, T.-H., Wu, Y.-Z., & Tseng, Y.-H. (2020). Metabolic and Nutritional Issues Associated with Spinal Muscular Atrophy. Nutrients, 12(12), 3842. https://doi.org/10.3390/nu12123842






