EEN Yesterday and Today … CDED Today and Tomorrow
Abstract
:1. Introduction
2. The Beginnings of EEN
3. Mechanisms of Diet on Inflammation
4. Mechanism and Efficacy of Exclusive Enteral Nutrition
5. Predictive Factors of Response to Exclusive Enteral Nutrition
6. Complications of Exclusive Enteral Nutrition
7. Quality of Life and Exclusive Enteral Nutrition
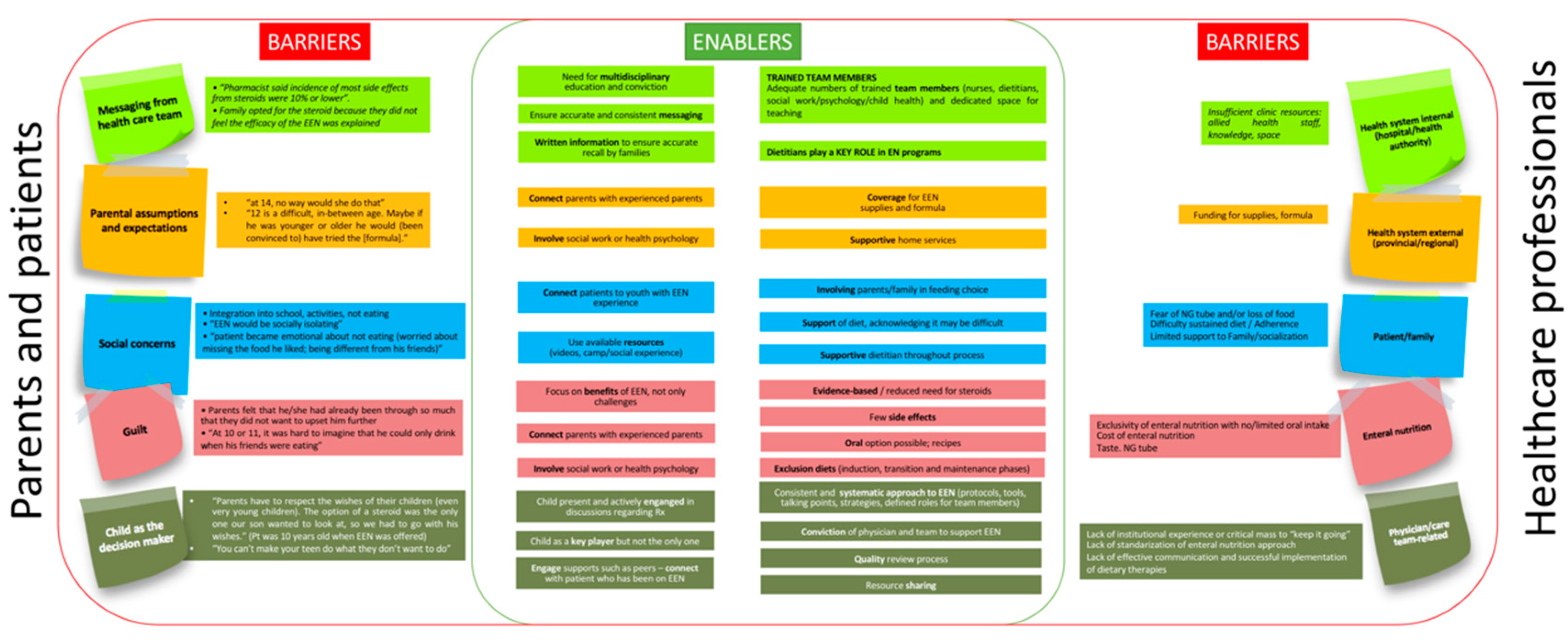
8. Barriers and Facilitating Elements to Use Exclusive Enteral Nutrition. Predictors of Non-Adherence to Exclusive Enteral Nutrition
9. Disadvantages or Points for Improvement of EEN
10. Efficacy of Partial Enteral Nutrition (PEN)
11. CDED: Today and Tomorrow
12. Efficacy of CDED
13. Advantages of CDED
14. Candidate Selection to CDED
15. Difficulties and Solutions when Applying CDED
16. Multidisciplinary Team: Role of the Dietitian
17. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ludvigsson, J.F.; Büsch, K.; Olén, O.; Askling, J.; Smedby, K.E.; Ekbom, A.; Lindberg, E.; Neovius, M. Prevalence of paediatric inflammatory bowel disease in Sweden: A nationwide population-based register study. BMC Gastroenterol. 2017, 17, 23. [Google Scholar] [CrossRef] [Green Version]
- Martin-de-Carpi, J.; Rodriguez, A.; Ramos, E.; Jimenez, S.; Martinez-Gomez, M.J.; Medina, E. Increasing incidence of pediatric inflammatory bowel disease in Spain (1996–2009): The SPIRIT Registry. Inflamm. Bowel Dis. 2013, 19, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Martin-de-Carpi, J.; Rodriguez, A.; Ramos, E.; Jimenez, S.; Martinez-Gomez, M.J.; Medina, E.; Navas-Lopez, V.M. The complete picture of changing pediatric inflammatory bowel disease incidence in Spain in 25 years (1985–2009): The EXPERIENCE registry. J. Crohns. Colitis 2014, 8, 763–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrascosa, A.; Fernández, J.; Fernández, M.; López-Siguero, J.; López, D.; Sánchez, E.; Colaborador, G. Estudios españoles de crecimiento 2010. Available online: http://www.estudiosdecrecimiento.es/estudio-transversal.html (accessed on 9 December 2020).
- del Rio, L.; Carrascosa, A.; Pons, F.; Gusinye, M.; Yeste, D.; Domenech, F.M. Bone mineral density of the lumbar spine in white Mediterranean Spanish children and adolescents: Changes related to age, sex, and puberty. Pediatr. Res. 1994, 35, 362–366. [Google Scholar] [PubMed] [Green Version]
- Navas-López, V.M.; Van Limbergen, J.E.; Martín-de-Carpi, J. Nutrición enteral en el paciente pediátrico con enfermedad de Crohn. Enferm inflam Intest dia 2016, 15, 112–122. [Google Scholar] [CrossRef]
- Critch, J.; Day, A.S.; Otley, A.; King-Moore, C.; Teitelbaum, J.E.; Shashidhar, H. Use of enteral nutrition for the control of intestinal inflammation in pediatric crohn disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Godin, J.P.; Martin, F.P.; Breton, I.; Schoepfer, A.; Nydegger, A. Total and activity-induced energy expenditure measured during a year in children with inflammatory bowel disease in clinical remission remain lower than in healthy controls. Clin. Nutr. 2020, 39, 3147–3152. [Google Scholar] [CrossRef]
- Timmer, A.; Behrens, R.; Buderus, S.; Findeisen, A.; Hauer, A.; Keller, K.-M.; Kliemann, G.; Lang, T.; Lohr, W.; Rzehak, P.; et al. Childhood onset inflammatory bowel disease: Predictors of delayed diagnosis from the CEDATA German-language pediatric inflammatory bowel disease registry. J. Pediatr. 2011, 158, 467–473.e2. [Google Scholar] [CrossRef]
- Ricciuto, A.; Aardoom, M.; Meyer, E.O.; Navon, D.; Carman, N.; Aloi, M.; Bronsky, J.; Däbritz, J.; Dubinsky, M.; Hussey, S.; et al. Predicting Outcomes in Pediatric Crohn’s Disease for Management Optimization: Systematic Review and Consensus Statements from PIBD-Ahead Program. Gastroenterology 2020, in press. [Google Scholar] [CrossRef]
- van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohn’s Colitis 2020. [Google Scholar] [CrossRef]
- Stephens, R.V.; Randall, H.T. Use of concentrated, balanced, liquid elemental diet for nutritional management of catabolic states. Ann. Surg. 1969, 170, 642–668. [Google Scholar] [CrossRef] [PubMed]
- Voitk, A.J.; Echave, V.; Feller, J.H.; Brown, R.A.; Gurd, F.N. Experience with elemental diet in the treatment of inflammatory bowel disease. Is this primary therapy? Arch. Surg. 1973, 107, 329–333. [Google Scholar] [CrossRef] [PubMed]
- O’Morain, C.; Segal, A.W.; Levi, A.J. Elemental diet as primary treatment of acute Crohn’s disease: A controlled trial. Br. Med. J. (Clin. Res. Ed.) 1984, 288, 1859–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, C.L.; Roulet, M.; Roy, C.C.; Weber, A. Continuous elemental enteral alimentation in children with Crohn’s disease and growth failure. Gastroenterology 1980, 79, 1205–1210. [Google Scholar] [CrossRef]
- Frivolt, K.; Schwerd, T.; Werkstetter, K.J.; Schwarzer, A.; Schatz, S.B.; Bufler, P.; Koletzko, S. Repeated exclusive enteral nutrition in the treatment of paediatric Crohn’s disease: Predictors of efficacy and outcome. Aliment. Pharmacol. Ther. 2014, 39, 1398–1407. [Google Scholar] [CrossRef]
- Cameron, F.L.; Gerasimidis, K.; Papangelou, A.; Missiou, D.; Garrick, V.; Cardigan, T.; Buchanan, E.; Barclay, A.R.; McGrogan, P.; Russell, R.K. Clinical progress in the two years following a course of exclusive enteral nutrition in 109 paediatric patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2013, 37, 622–629. [Google Scholar] [CrossRef]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: A randomized controlled open-label trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753. [Google Scholar] [CrossRef]
- Grover, Z.; Muir, R.; Lewindon, P. Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. J. Gastroenterol. 2014, 49, 638–645. [Google Scholar] [CrossRef]
- Connors, J.; Basseri, S.; Grant, A.; Giffin, N.; Mahdi, G.; Noble, A.; Rashid, M.; Otley, A.; Van Limbergena, J. Exclusive enteral nutrition therapy in paediatric Crohn’s disease results in long-term avoidance of corticosteroids: Results of a propensity-score matched cohort analysis. J. Crohn’s Colitis 2017, 11, 1063–1070. [Google Scholar] [CrossRef] [Green Version]
- Werkstetter, K.J.; Schatz, S.B.; Alberer, M.; Filipiak-Pittroff, B.; Koletzko, S. Influence of exclusive enteral nutrition therapy on bone density and geometry in newly diagnosed pediatric Crohn’s disease patients. Ann. Nutr. Metab. 2013, 63, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Afzal, N.A.; Van Der Zaag-Loonen, H.J.; Arnaud-Battandier, F.; Davies, S.; Murch, S.; Derkx, B.; Heuschkel, R.; Fell, J.M. Improvement in quality of life of children with acute Crohn’s disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Aliment. Pharmacol. Ther. 2004, 20, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Hojsak, I.; Pavić, A.M.; Mišak, Z.; Kolaček, S. Risk factors for relapse and surgery rate in children with Crohn’s disease. Eur. J. Pediatr. 2014, 173, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, M.; Giugliano, F.P.; Strisciuglio, C.; Urbonas, V.; Serban, D.E.; Banaszkiewicz, A.; Assa, A.; Hojsak, I.; Lerchova, T.; Navas-Lopez, V.M.; et al. Vaccinations and Immunization Status in Pediatric Inflammatory Bowel Disease: A Multicenter Study From the Pediatric IBD Porto Group of the ESPGHAN. Inflamm. Bowel Dis. 2019, 26, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Boneh, R.S.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 66, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Wine, E. Effects of enteral nutrition on Crohn’s disease: Clues to the impact of diet on disease pathogenesis. Inflamm. Bowel Dis. 2013, 19, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Haberman, Y.; Tickle, T.L.; Dexheimer, P.J.; Kim, M.-O.; Tang, D.; Karns, R.; Baldassano, R.N.; Noe, J.D.; Rosh, J.; Markowitz, J.; et al. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J. Clin. Invest. 2014, 124, 3617–3633. [Google Scholar] [CrossRef] [Green Version]
- Cleynen, I.; Boucher, G.; Jostins, L.; Schumm, L.P.; Zeissig, S.; Ahmad, T.; Andersen, V.; Andrews, J.M.; Annese, V.; Brand, S.; et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: A genetic association study. Lancet (Lond. Engl.) 2016, 387, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Menta, P.L.R.; Andrade, M.E.R.; Leocádio, P.C.L.; Fraga, J.R.; Dias, M.T.S.; Cara, D.C.; Cardoso, V.N.; Borges, L.F.; Capettini, L.S.A.; Aguilar, E.C.; et al. Wheat gluten intake increases the severity of experimental colitis and bacterial translocation by weakening of the proteins of the junctional complex. Br. J. Nutr. 2019, 121, 361–373. [Google Scholar] [CrossRef]
- Elamin, E.E.; Masclee, A.A.; Dekker, J.; Jonkers, D.M. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutr. Rev. 2013, 71, 483–499. [Google Scholar] [CrossRef]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef]
- Amit-Romach, E.; Uni, Z.; Cheled, S.; Berkovich, Z.; Reifen, R. Bacterial population and innate immunity-related genes in rat gastrointestinal tract are altered by vitamin A-deficient diet. J. Nutr. Biochem. 2009, 20, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Robles, H.; Castro-Ochoa, K.F.; Citalán-Madrid, A.F.; Schnoor, M. Beneficial effects of nutritional supplements on intestinal epithelial barrier functions in experimental colitis models in vivo. World J. Gastroenterol. 2019, 25, 4181–4198. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, G.J.; Aydemir, T.B.; Troche, C.; Martin, A.B.; Chang, S.M.; Cousins, R.J. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G171–G178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Liu, Z.; Li, H.; Cao, Z.; Li, W.; Song, Z.; Li, X.; Lu, A.; Lu, C.; Liu, Y. Naturally occurring TPE-CA maintains gut microbiota and bile acids homeostasis via FXR signaling modulation of the liver-gut axis. Front. Pharmacol. 2020, 11, 12. [Google Scholar] [CrossRef]
- Stevens, Y.; Van Rymenant, E.; Grootaert, C.; Van Camp, J.; Possemiers, S.; Masclee, A.; Jonkers, D. The intestinal fate of citrus flavanones and their effects on gastrointestinal health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef] [Green Version]
- Abulizi, N.; Quin, C.; Brown, K.; Chan, Y.K.; Gill, S.K.; Gibson, D.L. Gut mucosal proteins and bacteriome are shaped by the saturation index of dietary lipids. Nutrients 2019, 11, 428. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Jin, W.; Wu, R.; Li, J.; Park, S.A.; Tu, E.; Zanvit, P.; Xu, J.; Liu, O.; Cain, A.; et al. High Glucose Intake Exacerbates Autoimmunity through Reactive-Oxygen-Species-Mediated TGF-β Cytokine Activation. Immunity 2019, 51, 671–681.e5. [Google Scholar] [CrossRef]
- Zhang, D.M.; Jiao, R.Q.; Kong, L.D. High dietary fructose: Direct or indirect dangerous factors disturbing tissue and organ functions. Nutrients 2017, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic T reg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Gálvez, J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremonini, E.; Daveri, E.; Mastaloudis, A.; Adamo, A.M.; Mills, D.; Kalanetra, K.; Hester, S.N.; Wood, S.M.; Fraga, C.G.; Oteiza, P.I. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol. 2019, 26, 101269. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Pang, X.; Zhao, Y.; Wang, L.; Zhao, L. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012, 6, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-Fat Diet Determines the Composition of the Murine Gut Microbiome Independently of Obesity. Gastroenterology 2009, 137, 1716–1724.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silveira, A.L.M.; Ferreira, A.V.M.; de Oliveira, M.C.; Rachid, M.A.; da Cunha Sousa, L.F.; Dos Santos Martins, F.; Gomes-Santos, A.C.; Vieira, A.T.; Teixeira, M.M. Preventive rather than therapeutic treatment with high fiber diet attenuates clinical and inflammatory markers of acute and chronic DSS-induced colitis in mice. Eur. J. Nutr. 2017, 56, 179–191. [Google Scholar] [CrossRef]
- Hand, T.W.; Vujkovic-Cvijin, I.; Ridaura, V.K.; Belkaid, Y. Linking the Microbiota, Chronic Disease, and the Immune System. Trends Endocrinol. Metab. 2016, 27, 831–843. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chen, E.Z.; Baldassano, R.N.; Otley, A.R.; Griffiths, A.M.; Lee, D.; Bittinger, K.; Bailey, A.; Friedman, E.S.; Hoffmann, C.; et al. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn’s Disease. Cell Host Microbe 2017, 22, 247. [Google Scholar] [CrossRef]
- Alhagamhmad, M.H.; Lemberg, D.A.; Day, A.S.; Tan, L.-Z.; Ooi, C.Y.; Krishnan, U.; Gupta, N.; Munday, J.S.; Leach, S.T. Advancing nutritional therapy: A novel polymeric formulation attenuates intestinal inflammation in a murine colitis model and suppresses pro-inflammatory cytokine production in ex-vivo cultured inflamed colonic biopsies. Clin. Nutr. 2017, 36, 497–505. [Google Scholar] [CrossRef]
- Moore-Connors, J.M.; Dunn, K.A.; Bielawski, J.P.; Van Limbergen, J. Novel Strategies for Applied Metagenomics. Inflamm. Bowel Dis. 2016, 22, 709–718. [Google Scholar] [CrossRef]
- Hansen, R.; Russell, R.K.; Reiff, C.; Louis, P.; McIntosh, F.; Berry, S.H.; Mukhopadhya, I.; Bisset, W.M.; Barclay, A.R.; Bishop, J.; et al. Microbiota of de-novo pediatric IBD: Increased Faecalibacterium prausnitzii and reduced bacterial diversity in Crohn’s but not in ulcerative colitis. Am. J. Gastroenterol. 2012, 107, 1913–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quince, C.; Ijaz, U.Z.; Loman, N.; Eren, A.M.; Saulnier, D.; Russell, J.; Haig, S.J.; Calus, S.T.; Quick, J.; Barclay, A.; et al. Extensive Modulation of the Fecal Metagenome in Children With Crohn’s Disease During Exclusive Enteral Nutrition. Am. J. Gastroenterol. 2015, 110, 1718–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaakoush, N.O.; Day, A.S.; Leach, S.T.; Lemberg, D.A.; Nielsen, S.; Mitchell, H.M. Effect of exclusive enteral nutrition on the microbiota of children with newly diagnosed Crohn’s disease. Clin. Transl. Gastroenterol. 2015, 6, e71. [Google Scholar] [CrossRef] [PubMed]
- Gerasimidis, K.; Bertz, M.; Hanske, L.; Junick, J.; Biskou, O.; Aguilera, M.; Garrick, V.; Russell, R.K.; Blaut, M.; McGrogan, P.; et al. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn’s disease during enteral nutrition. Inflamm. Bowel Dis. 2014, 20, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.A.; Moore-Connors, J.; MacIntyre, B.; Stadnyk, A.W.; Thomas, N.A.; Noble, A.; Mahdi, G.; Rashid, M.; Otley, A.R.; Bielawski, J.P.; et al. Early Changes in Microbial Community Structure Are Associated with Sustained Remission after Nutritional Treatment of Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 2853–2862. [Google Scholar] [CrossRef] [PubMed]
- Logan, M.; Gkikas, K.; Svolos, V.; Nichols, B.; Milling, S.; Gaya, D.R.; Seenan, J.P.; Macdonald, J.; Hansen, R.; Ijaz, U.Z.; et al. Analysis of 61 exclusive enteral nutrition formulas used in the management of active Crohn’s disease-new insights into dietary disease triggers. Aliment. Pharmacol. Ther. 2020, 51, 935–947. [Google Scholar] [CrossRef] [Green Version]
- Swaminath, A.; Feathers, A.; Ananthakrishnan, A.N.; Falzon, L.; Li Ferry, S. Systematic review with meta-analysis: Enteral nutrition therapy for the induction of remission in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2017, 46, 645–656. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Chen, K.-C.; Chen, J. Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn’s disease: A meta-analysis. World J. Pediatr. 2019, 15, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Gerasimidis, K.; Talwar, D.; Duncan, A.; Moyes, P.; Buchanan, E.; Hassan, K.; O’Reilly, D.; McGrogan, P.; Edwards, C.A. Impact of exclusive enteral nutrition on body composition and circulating micronutrients in plasma and erythrocytes of children with active Crohn’s disease. Inflamm. Bowel Dis. 2012, 18, 1672–1681. [Google Scholar] [CrossRef]
- Narula, N.; Dhillon, A.; Zhang, D.; Sherlock, M.E.; Tondeur, M.; Zachos, M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 4, CD000542. [Google Scholar] [CrossRef] [PubMed]
- Navas-Lopez, V.M.; Martin-de-Carpi, J.; Segarra, O.; Garcia-Burriel, J.I.; Diaz-Martin, J.J.; Rodriguez, A.; Medina, E.; Juste, M. PRESENT; PREScription of Enteral Nutrition in pediaTric Crohn’s disease in Spain. Nutr. Hosp. 2014, 29, 537–546. [Google Scholar] [PubMed]
- Navas-López, V.M.; Blasco-Alonso, J.; Lacasa Maseri, S.; Girón Fernández-Crehuet, F.; Serrano Nieto, M.J.; Vicioso Recio, M.I.; Sierra Salinas, C. Exclusive enteral nutrition continues to be first line therapy for pediatric Crohn’s disease in the era of biologics. An. Pediatr. 2015, 83, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Rubio, A.; Pigneur, B.; Garnier-Lengline, H.; Talbotec, C.; Schmitz, J.; Canioni, D.; Goulet, O.; Ruemmele, F.M. The efficacy of exclusive nutritional therapy in paediatric Crohn’s disease, comparing fractionated oral vs. continuous enteral feeding. Aliment. Pharmacol. Ther. 2011, 33, 1332–1339. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, X.; Chen, H.; Li, M.; Wu, X.; Zhi, M.; Lan, P.; Hu, P. Efficacy of exclusive enteral nutrition in complicated Crohn’s disease. Scand. J. Gastroenterol. 2017, 52, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Ren, J.; Wang, G.; Li, G.; Liu, S.; Yan, D.; Gu, G.; Zhou, B.; Wu, X.; Chen, J.; et al. Exclusive enteral nutritional therapy can relieve inflammatory bowel stricture in Crohn’s disease. J. Clin. Gastroenterol. 2014, 48, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, I.R.; Udeen, S.; Davies, P.S.; Savage, M.O.; Walker-Smith, J.A. Remission induced by an elemental diet in small bowel Crohn’s disease. Arch. Dis. Child. 1987, 62, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Seidman, E.; Lohouses, M.; Turgeon, J.; Bouthillier, L.; Morin, C. Elemental diet versus prednisone as initial therapy in Crohn’s disease: Early and long term results. Gastroenterology 1991, 100, A150. [Google Scholar]
- Seidman, E.; Griffiths, A.; Jones, A.; Issenman, R. Semi-elemental (S-E) diet versus prednisone in pediatric Crohn´s disease. Gastroenterology 1993, 104, A778. [Google Scholar]
- Thomas, A.G.; Taylor, F.; Miller, V. Dietary intake and nutritional treatment in childhood Crohn’s disease. J. Pediatr. Gastroenterol. Nutr. 1993, 17, 75–81. [Google Scholar] [CrossRef]
- Beattie, R.M.; Schiffrin, E.J.; Donnet-Hughes, A.; Huggett, A.C.; Domizio, P.; MacDonald, T.T.; Walker-Smith, J.A. Polymeric nutrition as the primary therapy in children with small bowel Crohn’s disease. Aliment. Pharmacol. Ther. 1994, 8, 609–615. [Google Scholar] [CrossRef]
- Ruuska, T.; Savilahti, E.; Maki, M.; Ormala, T.; Visakorpi, J.K. Exclusive whole protein enteral diet versus prednisolone in the treatment of acute Crohn’s disease in children. J. Pediatr. Gastroenterol. Nutr. 1994, 19, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Akobeng, A.K.; Miller, V.; Stanton, J.; Elbadri, A.M.; Thomas, A.G. Double-blind randomized controlled trial of glutamine-enriched polymeric diet in the treatment of active Crohn’s disease. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.M.; Paintin, M.; Arnaud-Battandier, F.; Beattie, R.M.; Hollis, A.; Kitching, P.; Donnet-Hughes, A.; MacDonald, T.T.; Walker-Smith, J.A. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2000, 14, 281–289. [Google Scholar] [CrossRef]
- Phylactos, A.C.; Fasoula, I.N.; Arnaud-Battandier, F.; Walker-Smith, J.A.; Fell, J.M. Effect of enteral nutrition on antioxidant enzyme systems and inflammation in paediatric Crohn’s disease. Acta Paediatr. 2001, 90, 883–888. [Google Scholar]
- Terrin, G.; Berni Canani, R.; Ambrosini, A.; Viola, F.; De Mesquita, M.; Di Nardo, G.; Dito, L.; Cucchiara, S. A semielemental diet (Pregomin) as primary therapy for inducing remission in children with active Crohn’s disease. Ital. J. Pediatr. 2002, 28, 401–405. [Google Scholar]
- Ludvigsson, J.F.; Krantz, M.; Bodin, L.; Stenhammar, L.; Lindquist, B. Elemental versus polymeric enteral nutrition in paediatric Crohn’s disease: A multicentre randomized controlled trial. Acta Paediatr. Int. J. Paediatr. 2004, 93, 327–335. [Google Scholar] [CrossRef]
- Afzal, N.A.; Davies, S.; Paintin, M.; Arnaud-Battandier, F.; Walker-Smith, J.A.; Murch, S.; Heuschkel, R.; Fell, J. Colonic Crohn’s disease in children does not respond well to treatment with enteral nutrition if the ileum is not involved. Dig. Dis. Sci. 2005, 50, 1471–1475. [Google Scholar] [CrossRef]
- Knight, C.; El-Matary, W.; Spray, C.; Sandhu, B.K. Long-term outcome of nutritional therapy in paediatric Crohn’s disease. Clin. Nutr. 2005, 24, 775–779. [Google Scholar] [CrossRef]
- Day, A.S.; Whitten, K.E.; Lemberg, D.A.; Clarkson, C.; Vitug-Sales, M.; Jackson, R.; Bohane, T.D. Exclusive enteral feeding as primary therapy for Crohn’s disease in Australian children and adolescents: A feasible and effective approach. J. Gastroenterol. Hepatol. 2006, 21, 1609–1614. [Google Scholar] [CrossRef]
- Johnson, T.; Macdonald, S.; Hill, S.M.; Thomas, A.; Murphy, M.S. Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: A randomised controlled trial. Gut 2006, 55, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Berni Canani, R.; Terrin, G.; Borrelli, O.; Romano, M.T.; Manguso, F.; Coruzzo, A.; D’Armiento, F.; Romeo, E.F.; Cucchiara, S. Short- and long-term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn’s disease. Dig. Liver Dis. 2006, 38, 381–387. [Google Scholar] [CrossRef]
- Rodrigues, A.F.; Johnson, T.; Davies, P.; Murphy, M.S. Does polymeric formula improve adherence to liquid diet therapy in children with active Crohn’s disease? Arch. Dis. Child. 2007, 92, 767–770. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, E.; Gaunt, W.W.; Cardigan, T.; Garrick, V.; McGrogan, P.; Russell, R.K. The use of exclusive enteral nutrition for induction of remission in children with Crohn’s disease demonstrates that disease phenotype does not influence clinical remission. Aliment. Pharmacol. Ther. 2009, 30, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Whitten, K.E.; Leach, S.T.; Bohane, T.D.; Woodhead, H.J.; Day, A.S. Effect of exclusive enteral nutrition on bone turnover in children with Crohn’s disease. J. Gastroenterol. 2010, 45, 399–405. [Google Scholar] [CrossRef]
- Grogan, J.L.; Casson, D.H.; Terry, A.; Burdge, G.C.; El-Matary, W.; Dalzell, A.M. Enteral feeding therapy for newly diagnosed pediatric Crohn’s disease: A double-blind randomized controlled trial with two years follow-up. Inflamm. Bowel Dis. 2012, 18, 246–253. [Google Scholar] [CrossRef]
- Lambert, B.; Lemberg, D.A.; Leach, S.T.; Day, A.S. Longer-term outcomes of nutritional management of Crohn’s disease in children. Dig. Dis. Sci. 2012, 57, 2171–2177. [Google Scholar] [CrossRef]
- de Bie, C.; Kindermann, A.; Escher, J. Use of exclusive enteral nutrition in paediatric Crohn’s disease in The Netherlands. J. Crohns. Colitis 2013, 7, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Soo, J.; Malik, B.A.; Turner, J.M.; Persad, R.; Wine, E.; Siminoski, K.; Huynh, H.Q. Use of exclusive enteral nutrition is just as effective as corticosteroids in newly diagnosed pediatric Crohn’s disease. Dig. Dis. Sci. 2013, 58, 3584–3591. [Google Scholar] [CrossRef]
- Levine, A.; Turner, D.; Pfeffer Gik, T.; Amil Dias, J.; Veres, G.; Shaoul, R.; Staiano, A.; Escher, J.; Kolho, K.L.; Paerregaard, A.; et al. Comparison of outcomes parameters for induction of remission in new onset pediatric Crohn’s disease: Evaluation of the porto IBD group “growth relapse and outcomes with therapy” (GROWTH CD) study. Inflamm. Bowel Dis. 2014, 20, 278–285. [Google Scholar] [CrossRef]
- Lee, D.; Baldassano, R.N.; Otley, A.R.; Albenberg, L.; Griffiths, A.M.; Compher, C.; Chen, E.Z.; Li, H.; Gilroy, E.; Nessel, L.; et al. Comparative Effectiveness of Nutritional and Biological Therapy in North American Children with Active Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yu, J.; Zhao, H.; Lou, J.; Chen, F.; Peng, K.; Chen, J. Short-Term Efficacy of Exclusive Enteral Nutrition in Pediatric Crohn’s Disease: Practice in China. Gastroenterol. Res. Pract. 2015, 2015, 428354. [Google Scholar] [CrossRef] [PubMed]
- Navas-López, V.M. Eficacia de la Nutrición Enteral Exclusiva en la enfermedad de Crohn pediátrica: Factores predictivos de respuesta y de mantenimiento de la remisión. Doctoral dissertation, Universidad de Málaga, Málaga, Spain, 2015. [Google Scholar]
- Kim, H.J.; Kim, Y.; Cho, J.M.; Oh, S.H.; Kim, K.M. Therapeutic Efficacy of Oral Enteral Nutrition in Pediatric Crohn’s Disease: A Single Center Non-Comparative Retrospective Study. Yonsei Med. J. 2016, 57, 1185–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafferty, L.; Tuohy, M.; Carey, A.; Sugrue, S.; Hurley, M.; Hussey, S. Outcomes of exclusive enteral nutrition in paediatric Crohn’s disease. Eur. J. Clin. Nutr. 2017, 71, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yu, J.; Lou, J.; Fang, Y.; Chen, J. Exclusive Enteral Nutrition versus Infliximab in Inducing Therapy of Pediatric Crohn’s Disease. Gastroenterol. Res. Pract. 2017, 2017, 6595048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen-Dolev, N.; Sladek, M.; Hussey, S.; Turner, D.; Veres, G.; Koletzko, S.; Martin de Carpi, J.; Staiano, A.; Shaoul, R.; Lionetti, P.; et al. Differences in Outcomes Over Time With Exclusive Enteral Nutrition Compared With Steroids in Children With Mild to Moderate Crohn’s Disease: Results From the GROWTH CD Study. J. Crohns. Colitis 2018, 12, 306–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pigneur, B.; Lepage, P.; Mondot, S.; Schmitz, J.; Goulet, O.; Dore, J.; Ruemmele, F.M. Mucosal Healing and Bacterial Composition in Response to Enteral Nutrition vs. Steroid-based Induction Therapy-A Randomised Prospective Clinical Trial in Children With Crohn’s Disease. J. Crohns. Colitis 2019, 13, 846–855. [Google Scholar] [CrossRef] [Green Version]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef] [Green Version]
- Logan, M.; Clark, C.M.; Ijaz, U.Z.; Gervais, L.; Duncan, H.; Garrick, V.; Curtis, L.; Buchanan, E.; Cardigan, T.; Armstrong, L.; et al. The reduction of faecal calprotectin during exclusive enteral nutrition is lost rapidly after food re-introduction. Aliment. Pharmacol. Ther. 2019, 50, 664–674. [Google Scholar] [CrossRef]
- Kang, Y.; Park, S.; Kim, S.; Kim, S.Y.; Koh, H. Therapeutic Efficacy of Exclusive Enteral Nutrition with Specific Polymeric Diet in Pediatric Crohn’s Disease. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Rolandsdotter, H.; Jonsson-Videsater, K.; Fagerberg, U.L.; Finkel, Y.; Eberhardson, M. Exclusive Enteral Nutrition: Clinical Effects and Changes in Mucosal Cytokine Profile in Pediatric New Inflammatory Bowel Disease. Nutrients 2019, 11, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpato, E.; Strisciuglio, C.; Martinelli, M.; Russo, M.; Cenni, S.; Casertano, M.; Serra, M.R.; Staiano, A.; Miele, E. Exclusive enteral nutrition effect on the clinical course of pediatric Crohn’s disease: A single center experience. Eur. J. Pediatr. 2020, 179, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Hart, L.; Farbod, Y.; Szamosi, J.C.; Yamamoto, M.; Britz-McKibbin, P.; Halgren, C.; Zachos, M.; Pai, N. Effect of Exclusive Enteral Nutrition and Corticosteroid Induction Therapy on the Gut Microbiota of Pediatric Patients with Inflammatory Bowel Disease. Nutrients 2020, 12, 1961. [Google Scholar] [CrossRef] [PubMed]
- Hojsak, I.; Matic, K.; Sila, S.; Trivić, I.; Mišak, Z.; Kolaček, S. Characteristics of polymeric formula and route of delivery of exclusive enteral nutrition have no effect on disease outcome and weight gain in pediatric Crohn’s disease. Clin. Nutr. 2020, 39, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Moriczi, M.; Pujol-Muncunill, G.; Martín-Masot, R.; Jiménez Treviño, S.; Segarra Cantón, O.; Ochoa Sangrador, C.; Peña Quintana, L.; González Santana, D.; Rodríguez Martínez, A.; Rosell Camps, A.; et al. Predictors of Response to Exclusive Enteral Nutrition in Newly Diagnosed Crohn´s Disease in Children: PRESENCE Study from SEGHNP. Nutrients 2020, 12, 1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giaffer, M.H.; North, G.; Holdsworth, C.D. Controlled trial of polymeric versus elemental diet in treatment of active Crohn’s disease. Lancet (Lond. Engl.) 1990, 335, 816–819. [Google Scholar] [CrossRef]
- Malchow, H.; Steinhardt, H.J.; Lorenz-Meyer, H.; Strohm, W.D.; Rasmussen, S.; Sommer, H.; Jarnum, S.; Brandes, J.W.; Leonhardt, H.; Ewe, K. Feasibility and effectiveness of a defined-formula diet regimen in treating active Crohn’s disease. European Cooperative Crohn’s Disease Study III. Scand. J. Gastroenterol. 1990, 25, 235–244. [Google Scholar] [CrossRef]
- Lochs, H.; Steinhardt, H.J.; Klaus-Wentz, B.; Zeitz, M.; Vogelsang, H.; Sommer, H.; Fleig, W.E.; Bauer, P.; Schirrmeister, J.; Malchow, H. Comparison of enteral nutrition and drug treatment in active Crohn’s disease. Results of the European Cooperative Crohn’s Disease Study. IV. Gastroenterology 1991, 101, 881–888. [Google Scholar] [CrossRef]
- Rigaud, D.; Cosnes, J.; Le Quintrec, Y.; Rene, E.; Gendre, J.P.; Mignon, M. Controlled trial comparing two types of enteral nutrition in treatment of active Crohn’s disease: Elemental versus polymeric diet. Gut 1991, 32, 1492–1497. [Google Scholar] [CrossRef] [Green Version]
- Park, R.; Galloway, A.; Danesh, B.; Russell, R. Double-blind controlled trial of elemental and polymeric diets as primary therapy in active Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 1991, 3, 483–490. [Google Scholar]
- Raouf, A.H.; Hildrey, V.; Daniel, J.; Walker, R.J.; Krasner, N.; Elias, E.; Rhodes, J.M. Enteral feeding as sole treatment for Crohn’s disease: Controlled trial of whole protein v amino acid based feed and a case study of dietary challenge. Gut 1991, 32, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Fleming, C.R.; Burnes, J.U.; Nelson, J.K.; Ilstrup, D.M. A randomized prospective trial comparing a defined formula diet, corticosteroids, and a defined formula diet plus corticosteroids in active Crohn’s disease. Mayo Clin. Proc. 1992, 67, 328–333. [Google Scholar] [CrossRef]
- Gonzalez-Huix, F.; de Leon, R.; Fernandez-Banares, F.; Esteve, M.; Cabre, E.; Acero, D.; Abad-Lacruz, A.; Figa, M.; Guilera, M.; Planas, R. Polymeric enteral diets as primary treatment of active Crohn’s disease: A prospective steroid controlled trial. Gut 1993, 34, 778–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorard, D.A.; Hunt, J.B.; Payne-James, J.J.; Palmer, K.R.; Rees, R.G.; Clark, M.L.; Farthing, M.J.; Misiewicz, J.J.; Silk, D.B. Initial response and subsequent course of Crohn’s disease treated with elemental diet or prednisolone. Gut 1993, 34, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Royall, D.; Jeejeebhoy, K.N.; Baker, J.P.; Allard, J.P.; Habal, F.M.; Cunnane, S.C.; Greenberg, G.R. Comparison of amino acid v peptide based enteral diets in active Crohn’s disease: Clinical and nutritional outcome. Gut 1994, 35, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Katsumata, T.; Yokoyama, K.; Takahashi, H.; Igarashi, M.; Saigenji, K. A randomized controlled study of total parenteral nutrition and enteral nutrition by elemental and polymeric diet as primary therapy in active phase of Crohn’s disease. Nihon Shokakibyo Gakkai Zasshi 1998, 95, 1212–1221. [Google Scholar]
- Verma, S.; Brown, S.; Kirkwood, B.; Giaffer, M.H. Polymeric versus elemental diet as primary treatment in active Crohn’s disease: A randomized, double-blind trial. Am. J. Gastroenterol. 2000, 95, 735–739. [Google Scholar] [CrossRef]
- Sakurai, T.; Matsui, T.; Yao, T.; Takagi, Y.; Hirai, F.; Aoyagi, K.; Okada, M. Short-term efficacy of enteral nutrition in the treatment of active Crohn’s disease: A randomized, controlled trial comparing nutrient formulas. JPEN J. Parenter. Enteral Nutr. 2002, 26, 98–103. [Google Scholar] [CrossRef]
- Gassull, M.A.; Fernandez-Banares, F.; Cabre, E.; Papo, M.; Giaffer, M.H.; Sanchez-Lombrana, J.L.; Richart, C.; Malchow, H.; Gonzalez-Huix, F.; Esteve, M. Fat composition may be a clue to explain the primary therapeutic effect of enteral nutrition in Crohn’s disease: Results of a double blind randomised multicentre European trial. Gut 2002, 51, 164–168. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, R.; Zhu, W.; Gong, J.; Zhang, W.; Li, Y.; Gu, L.; Li, N.; Li, J. Effect of exclusive enteral nutrition on health-related quality of life for adults with active Crohn’s disease. Nutr. Clin. Pract. 2013, 28, 499–505. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, J.-N.; Gong, J.-F.; Wang, H.-G.; Li, Y.; Zhang, L.; Zuo, L.-G.; Feng, Y.; Gu, L.-L.; Li, N.; et al. Impact of enteral nutrition on energy metabolism in patients with Crohn’s disease. World J. Gastroenterol. 2015, 21, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Zhang, H.; Wang, X.; Xu, D.; Jin, D.; Li, P.; Ye, J.; Yu, Q.; Chen, Y. Efficacy predictors of a 2-month exclusive enteral nutrition for inducing remission of active Crohn’s disease. Eur. J. Clin. Nutr. 2018, 72, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, Z.; Huang, L.; Gong, J.; Li, Y.; Gu, L.; Shen, W.; Zhu, W. A nomogram for predicting the response to exclusive enteral nutrition in adult patients with isolated colonic Crohn’s disease. Therap. Adv. Gastroenterol. 2019, 12, 1756284819881301. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gupta, A.; Kedia, S.; Agarwal, S.; Singh, N.; Goyal, S.; Jain, S.; Gupta, V.; Sahu, P.; Vuyyuru, S.K.; et al. Efficacy and tolerability of exclusive enteral nutrition in adult patients with complicated Crohn’s disease. Intest. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E.; Duncan, H.; Buchanan, E.; Cardigan, T.; Garrick, V.; Curtis, L.; Gervais, L.; Barclay, A.; Haddock, G.; Hansen, R.; et al. Prehabilitation: The Impact of Preoperative Exclusive Enteral Nutrition on Paediatric Patients With Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Bessa, I.; Lago, P.; Santos, M.D.; Leite, J.; Castro-Pocas, F. Preoperative Enteral Nutrition and Surgical Outcomes in Adults with Crohn’s Disease: A Systematic Review. GE Port. J. Gastroenterol. 2019, 26, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Ruemmele, F.M.; Veres, G.; Kolho, K.L.; Griffiths, A.; Levine, A.; Escher, J.C.; Amil Dias, J.; Barabino, A.; Braegger, C.P.; Bronsky, J.; et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J. Crohn’s Colitis 2014, 8, 1179–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Limbergen, J.; Haskett, J.; Griffiths, A.M.; Critch, J.; Huynh, H.; Ahmed, N.; deBruyn, J.C.; Issenman, R.; El-Matary, W.; Walters, T.D.; et al. Toward enteral nutrition for the treatment of pediatric Crohn disease in Canada: A workshop to identify barriers and enablers. Can. J. Gastroenterol. Hepatol. 2015, 29, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Lawley, M.; Wu, J.W.; Navas-Lopez, V.M.; Huynh, H.Q.; Carroll, M.W.; Chen, M.; Medvedev, P.; Day, A.S.; Hussey, S.; Sigall-Boneh, R.; et al. Global Variation in Use of Enteral Nutrition for Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2018, 67, e22–e29. [Google Scholar] [CrossRef] [PubMed]
- Svolos, V.; Gerasimidis, K.; Buchanan, E.; Curtis, L.; Garrick, V.; Hay, J.; Laird, S.; Munro, J.; Gaya, D.R.; Russell, R.K.; et al. Dietary treatment of Crohn’s disease: Perceptions of families with children treated by exclusive enteral nutrition, a questionnaire survey. BMC Gastroenterol. 2017, 17, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, P.; Pan, Z.; Furuta, G.T.; Kim, D.Y.; de Zoeten, E. Parent Perspectives on Exclusive Enteral Nutrition for the Treatment of Pediatric Crohn’s Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Mutsekwa, R.N.; Edwards, J.T.; Angus, R.L. Exclusive enteral nutrition in the management of Crohn’s disease: A qualitative exploration of experiences, challenges and enablers in adult patients. J. Hum. Nutr. Diet. 2020. [Google Scholar] [CrossRef]
- Faiman, A.; Mutalib, M.; Moylan, A.; Morgan, N.; Crespi, D.; Furman, M.; Kader, A. Standard versus rapid food reintroduction after exclusive enteral nutritional therapy in paediatric Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2014, 26, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Pascual Pérez, A.I.; Pujol Muncunill, G.; Domínguez Sánchez, P.; Feo Ortega, S.; Martín de Carpi, J. Duration of sustained remission after treatment by induction with exclusive enteral nutrition and azathioprine in patients with Crohn’s disease. An. Pediatr. (Barc.) 2020. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Zhang, D.; Gordon, M.; MacDonald, J.K. Enteral nutrition for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 8, CD005984. [Google Scholar] [CrossRef]
- El-Matary, W.; Otley, A.; Critch, J.; Abou-Setta, A.M. Enteral Feeding Therapy for Maintaining Remission in Crohn’s Disease: A Systematic Review. JPEN J. Parenter. Enteral Nutr. 2017, 41, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Wilschanski, M.; Sherman, P.; Pencharz, P.; Davis, L.; Corey, M.; Griffiths, A. Supplementary enteral nutrition maintains remission in paediatric Crohn’s disease. Gut 1996, 38, 543–548. [Google Scholar] [CrossRef]
- Belli, D.C.; Seidman, E.; Bouthillier, L.; Weber, A.M.; Roy, C.C.; Pletincx, M.; Beaulieu, M.; Morin, C.L. Chronic intermittent elemental diet improves growth failure in children with Crohn’s disease. Gastroenterology 1988, 94, 603–610. [Google Scholar] [CrossRef]
- Schulman, J.M.; Pritzker, L.; Shaoul, R. Maintenance of Remission with Partial Enteral Nutrition Therapy in Pediatric Crohn’s Disease: A Retrospective Study. Can. J. Gastroenterol. Hepatol. 2017, 2017, 5873158. [Google Scholar] [CrossRef]
- Gupta, K.; Noble, A.; Kachelries, K.E.; Albenberg, L.; Kelsen, J.R.; Grossman, A.B.; Baldassano, R.N. A novel enteral nutrition protocol for the treatment of pediatric Crohn’s disease. Inflamm. Bowel Dis. 2013, 19, 1374–1378. [Google Scholar] [CrossRef]
- Levine, A.; El-Matary, W.; Van Limbergen, J. A Case-Based Approach to New Directions in Dietary Therapy of Crohn’s Disease: Food for Thought. Nutrients 2020, 12, 880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Sigall Boneh, R.; Van Limbergen, J.; Wine, E.; Assa, A.; Shaoul, R.; Milman, P.; Cohen, S.; Kori, M.; Peleg, S.; On, A.; et al. Dietary Therapies Induce Rapid Response and Remission in Pediatric Patients With Active Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- De Bie, C.I.; Hummel, T.Z.; Kindermann, A.; Kokke, F.T.M.; Damen, G.M.; Kneepkens, C.M.F.; Van Rheenen, P.F.; Schweizer, J.J.; Hoekstra, J.H.; Norbruis, O.F.; et al. The duration of effect of infliximab maintenance treatment in paediatric Crohn’s disease is limited. Aliment. Pharmacol. Ther. 2011, 33, 243–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- deBruyn, J.C.; Jacobson, K.; El-Matary, W.; Carroll, M.; Wine, E.; Wrobel, I.; Van Woudenberg, M.; Huynh, H.Q. Long-term Outcomes of Infliximab Use for Pediatric Crohn Disease: A Canadian Multicenter Clinical Practice Experience. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Sigall Boneh, R.; Sarbagili Shabat, C.; Yanai, H.; Chermesh, I.; Ben Avraham, S.; Boaz, M.; Levine, A. Dietary Therapy With the Crohn’s Disease Exclusion Diet is a Successful Strategy for Induction of Remission in Children and Adults Failing Biological Therapy. J. Crohns. Colitis 2017, 11, 1205–1212. [Google Scholar] [CrossRef] [Green Version]
- Middleton, G.; Golley, R.; Patterson, K.; Le Moal, F.; Coveney, J. What can families gain from the family meal? A mixed-papers systematic review. Appetite 2020, 153, 104725. [Google Scholar] [CrossRef]
- Gavin, J.; Marino, L.V.; Ashton, J.J.; Beattie, R.M. Patient, parent and professional perception of the use of maintenance enteral nutrition in Paediatric Crohn’s Disease. Acta Paediatr. 2018, 107, 2199–2206. [Google Scholar] [CrossRef]
- Green, T.J.; Issenman, R.M.; Jacobson, K. Patients’ diets and preferences in a pediatric population with inflammatory bowel disease. Can. J. Gastroenterol. 1998, 12, 544–549. [Google Scholar] [CrossRef] [Green Version]
- Zallot, C.; Quilliot, D.; Chevaux, J.-B.; Peyrin-Biroulet, C.; Guéant-Rodriguez, R.M.; Freling, E.; Collet-Fenetrier, B.; Williet, N.; Ziegler, O.; Bigard, M.-A.; et al. Dietary beliefs and behavior among inflammatory bowel disease patients. Inflamm. Bowel Dis. 2013, 19, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Pituch-Zdanowska, A.; Kowalska-Duplaga, K.; Jarocka-Cyrta, E.; Stawicka, A.; Dziekiewicz, M.; Banaszkiewicz, A. Dietary Beliefs and Behaviors among Parents of Children with Inflammatory Bowel Disease. J. Med. Food 2019, 22, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Agricultura, Pesca y Alimentación. Informe del consumo alimentario en España 2019. Available online: https://www.mapa.gob.es/eu/alimentacion/temas/consumo-tendencias/informe2019_vf_tcm35-540250.pdf (accessed on 9 December 2020).
- Brito, N.B.; Célix, M.S.; Jiménez, O.M.; García, L.C.; Trenco, P.Á. Situación del Dietista-Nutricionista en el Sistema Nacional de Salud Español: Documento de posicionamiento del Grupo de Especialización en Nutrición Clínica y Dietética de la Academia Española de Nutrición y Dietética. Rev. Esp. Nutr. Hum. Diet. 2020, 24, 278–288. [Google Scholar] [CrossRef]
| Dietary Component | Reference | Model | Effects |
|---|---|---|---|
| Natural Components of the Diet | |||
| Gluten | Menta (2019) [29] | Female C57BL/6 mice | In mice with dextran sulfate sodium (DSS)-induced colitis: involvement of the desmosomes, adherent zonule and direct damage to the colon mucosa. |
| Alcohol | Elamin (2013) [30] | In vitro | Direct damage to the epithelium, increasing intestinal permeability. |
| Dietary salt | Miranda (2018) [31] | C57BL/6 mice | Decrease in Lactobacillus sp. Decrease in butyrate production Increased expression of pro-inflammatory genes such as Rac1, Map2k1, Map2k6, Atf2. Suppression of the expression of genes such as Ccl3, Ccl4, Cxcl2, Cxcr4, Ccr7 |
| Vitamin A | Amit-Romach (2009) [32] | Wistar Rats | Increase of mucus, defensin-6 and TLR |
| Vitamin D | Vargas-Robles (2019) [33] | WT mice | In mice with colitis induced by dextran sulfate sodium (DSS): maintains the expression of TJ proteins (expression of ZO-1, occludin and claudin-1) and improved barrier function, decreased FITC-dextran permeability and levels circulating LPS. |
| Zinc | Guthrie (2015) [34] | ZIP14 KO mice | Decreased expression of phosphorylated occludin and claudin-1, and increased claudin-2, maintaining intestinal barrier function. |
| Flavanones | Liu (2020) [35] | C57BL/6J mice | Increase in ZO-1 and proteins associated with occludin and reduction in serum endotoxin. FXR stimulation with reduced hepatic synthesis of bile acids. |
| Stevens (2019) [36] | In vitro | Increase in TEER and decrease in the flow of FITC-dextran, improving intestinal barrier function. | |
| Corn oil | Abulizi (2019) [37] | C57BL/6 mice | Decreases the kinase linked to the integrin, which is essential for barrier function, and decreased expression of various TJ proteins of the intestinal barrier. |
| High glucose diet | Zhang (2017) [38] | C57BL/6 mice | Increases Th17 differentiation and activation of cytokines. |
| Fructose | Zhang (2017) [39] | C57BL/6 mice | Mitochondrial dysfunction, increased inflammatory cytokines and intestinal barrier dysfunction. |
| Aryl hydrocarbon receptor, derived from the digestion of vegetables from the Brasicaceae family | Gao (2018) [40] | Animal models | Necessary for activation and production of Il-22 through innate lymphoid cells type 3 (ILC3) and gamma delta intraepithelial T cells in the intestinal barrier |
| Smith (2013) [41] Gálvez (2014) [42] | SPF, ASF and GF mice | Reduction of SCFA with reduction of colonic regulatory T cells, especially Th17, important in the pathogenesis of IBD. | |
| Food Additives | |||
| Anthocyanins | Cremonini (2019) [43] | Male C57BL/6J mice Caco-2 cells | Decreases endotoxin levels, increases GLP-2 levels and MUC2 expression. |
| Dietary Component | Reference | Model | Effects |
|---|---|---|---|
| Natural Components of the Diet | |||
| High fat + high sugar diet | Zhang (2012) [44] | C57BL/6J mice | Alteration of the microbiota with a decrease in diversity and an increase in opportunistic pathogens. |
| Fat | Le Chatelier (2013) [45] Hildebrandt (2009) [46] | Human | Increase in Proteobacteria and Firmicutes and decrease in Bacteroidetes |
| High fiber diet | Silveira (2017) [47] | BALB/c female mice | High fiber diet protected from acute colitis |
| Low fiber diet | Hand (2016) [48] | Human | Decrease in intestinal diversity with a predominance of Gram-negatives (Bacteroides, Proteobacteria, Verrucomicrobia) and increase in lipopolysaccharides levels. |
| Reference (Year of Publication) (Ref) | N | Formula | T | Remission Criteria | R a |
|---|---|---|---|---|---|
| Morin (1980) [15] | 4 | E | 6 | CDAI < 150 | 100% |
| Sanderson (1987) [68] | 8 | E | 6 | Improvement of LSI | 88% |
| Seidman (1991) [69] | 10 | E | 3 | CDAI < 150 | 80% |
| Seidman (1993) [70] | 24 | SE | 4 | CDAI < 150 | 86% |
| Thomas (1993) [71] | 12 | E | 4 | Improvement of LSI | 100% |
| Beattie (1994) [72] | 7 | P | 8 | Improvement of LSI | 100% |
| Ruuska (1994) [73] | 10 | P | 8 | PCDAI ≤ 10 | 90% |
| Akobeng (2000) [74] | 16 | P | 4 | PCDAI < 10 | 50% |
| Fell (2000) [75] | 29 | P | 8 | PCDAI ≤ 10 | 79% |
| Phylactos (2001) [76] | 14 | P | 8 | PCDAI ≤ 10 | 93% |
| Terrin (2002) [77] | 10 | SE | 8 | PCDAI < 10 | 90% |
| Ludvigsson (2004) [78] | 17 | P | 6 | PCDAI < 10 or decrease 45% or 15 points from baseline | 82% |
| 16 | E | 6 | 69% | ||
| Afzal (2005) [79] | 26 | P | 8 | PCDAI < 20 | 88% |
| Knight (2005) [80] | 40 | E | 6 | CDAI | 90% |
| 4 | P | ||||
| Day (2006) [81] | 27 | P | 6–8 | PCDAI ≤15 | 70% |
| Borrelli (2006) [18] | 19 | P | 10 | PCDAI ≤10 | 79% |
| Johnson (2006) [82] | 24 | E | 6 | PCDAI < 10 | 41% |
| Berni Canani (2006) [83] | 12 | E | 8 | PCDAI < 10 | 87% |
| 13 | SE | ||||
| 12 | P | ||||
| Rodrigues (2007) [84] | 53 | E | 6 | Not specified b | 64% |
| 45 | P | 44% | |||
| Buchanan (2009) [85] | 110 | P/E | 8 | Clinical and biochemical response | 80% |
| Whitten (2010) [86] | 23 | P | 8 | PCDAI < 15 | 69% |
| Rubio (2011) [65] | 106 | P | 8 | PCDAI < 10 | 81% |
| Grogan (2012) [87] | 20 | E | 6 | PCDAI ≤ 10 | 70% |
| 21 | P | 71% | |||
| Lambert (2012) [88] | 31 | P | 6–8 | PCDAI < 15 | 84% |
| de Bie (2013) [89] | 77 | P | 6 | Clinical response | 53% |
| Soo (2013) [90] | 36 | P/SE | 6 | PCDAI ≤10 | 89% |
| Cameron (2013) [17] | 109 | P/E | 8 | PCDAI ≤10 | 60% |
| Frivolt (2014) [16] | 40 | P/E | 6–8 | wPCDAI < 12.5 | 95% |
| Levine (2014) [91] | 43 | P | 6–8 | PCDAI < 10 | 72% |
| Grover (2014) [19] | 28 | P | 6 | PCDAI < 10 | 79% |
| Hojsak (2014) [23] | 57 | P | 6–8 | PCDAI < 10 | 84% |
| Lee (2015) [92] | 22 | P/E | 8 | PCDAI ≤ 10 | 59% |
| Luo (2015) [93] | 13 | P | 8 | PCDAI < 10 | 69% |
| Navas (2015) [94] | 50 | P | 6–8 | wPCDAI < 12.5 | 84% |
| Kim (2016) [95] | 66 | E | 6 | PCDAI < 10 | 88% |
| Connors (2017) [20] | 76 | P | 8–16 | PCDAI < 7.5 | 87% |
| Lafferty (2017) [96] | 28 | P/E | 6–8 | PCDAI ≤ 10 | 85% |
| Luo (2017) [97] | 13 | P | 8 | PCDAI ≤ 10 | 83% |
| Cohen–Dolev (2018) [98] | 60 | P | 6–8 | PCDAI < 10 | 63% |
| Pigneur (2019) [99] | 13 | P | 8 | Harvey-Bradshaw | 100% |
| Levine (2019) [100] | 34 | P | 6 | PCDAI ≤ 10 | 59% |
| Logan (2019) [101] | 66 | P | 8 | wPCDAI < 12.5 | 62% |
| Kang (2019) [102] | 19 | P | 8 | PCDAI < 10 | 65% |
| Rolandsdotter (2019) [103] | 13 | P | 6 | PCDAI ≤ 10 | 77% |
| Chan (2020) [95] | 13 | P | 8 | PCDAI < 10 | 69% |
| Scarpato (2020) [104] | 47 | P | 6–8 | PCDAI ≤ 10 | 68% |
| Hart (2020) [105] | 16 | SE | 8 | PCDAI ≤ 10 | 93% |
| Hojsak (2020) [106] | 92 | P | 6–8 | PCDAI ≤ 10 | 77% |
| Moriczi (2020) [107] | 222 | P | 6–8 | wPCDAI < 12.5 | 83% |
| Total | 2016 | IC (95%) | 75.7% (73.8–77.5) |
| Reference (Year of Publication) (Ref) | N | Formula | T | Remission Criteria | R |
|---|---|---|---|---|---|
| O’Morain 1 (1984) [14] | 11 | E | 4 | H-B | 80% |
| Giaffer 2 (1990) [108] | 14 | P | 4 | CDAI | 36% |
| 16 | E | 75% | |||
| Malchow 1 (1990) [109] | 51 | P | 3–6 | CDAI | 41% |
| Lochs 1 (1991) [110] | 52 | P | 4–6 | CDAI | 55% |
| Rigaud 2 (1991) [111] | 15 | P | 4 | CDAI | 73% |
| 15 | E | 66% | |||
| Park 2 (1991) [112] | 7 | P | 4 | Simple activity index | 73% |
| 7 | E | 69% | |||
| Raouf 2 (1991) [113] | 11 | P | 3 | Simple activity index | 71% |
| 13 | E | 29% | |||
| Lindor 1 (1992) [114] | 9 | E | 4 | CDAI | 33% |
| González-Huix 1 (1993) [115] | 15 | P | 4 | Van Hess index | 80% |
| Gorard 1 (1993) [116] | 22 | E | 4 | Simple activity index | 32% |
| Royall 2 (1994) [117] | 19 | E | 3 | CDAI | 84% |
| 21 | O | 75% | |||
| Mansfield 2 (1995) [118] | 22 | O | 4 | CDAI | 36% |
| 22 | E | 36% | |||
| Verma 2 (2000) [119] | 11 | P | 4 | CDAI | 55% |
| 10 | E | 80% | |||
| Sakurai (2002) [120] | 36 | P | 6 | CDAI | 70% |
| Gassull 1 (2002) [121] | 43 | P | 4 | CDAI | 36% |
| Guo (2013) [122] | 13 | P | 4 | CDAI | 85% |
| Hu (2014) [67] | 59 | O | 12 | CDAI | 81% |
| Zhao (2015) [123] | 40 | P/O | 4 | CDAI | 52% |
| Yang (2017) [66] | 41 | NE | 12 | CDAI | 80% |
| Xue (2018) [124] | 67 | NE | 8 | CDAI | 68% |
| Xu (2019) [125] | 104 | NE | H-B | 52% | |
| Sharma (2020) [126] | 31 | P/S-E | 2–6 | CDAI | 80% |
| Total | 797 | IC (95%) | 60.1% (56.6–63.4) |
| Reference | Study | Patients (n) | Remission at Week 6 |
|---|---|---|---|
| Sigall-Boneh R (2014) [144] | Retrospective | 47 | 24/33 children 9/14 adults |
| Sigall-Boneh R (2017) [148] | Retrospective | 21 | 6/10 children 7/11 adults |
| Levine A (2019) [100] | RCT | 40 CDED | 30/40 (75%) |
| Levine A (2020) [143] | Cases series | 4 | 3/3 children/adolescents |
| CDED Phase 1 | CDED Phase 2 | CDED Maintenance Phase | |
|---|---|---|---|
| Breakfast | Modulen ®(250 mL) 3 Banana pancakes (1 banana + 1 egg) | Modulen®(250 mL) Wholewheat bread (1 slice) with olive oil and tomato slices | Modulen®(250 mL) Wholewheat bread (1 slice) with olive oil and tomato slices |
| Snack | Modulen® (350 mL) | Modulen® (250 mL) Carrot oat muffins (1 egg) | Modulen® (250 mL) 1 pear |
| Lunch | Homemade potato chips Chicken meatballs (100 g) with homemade tomato sauce 1 banana | Chickpeas (20 g) salad with tuna (1 can), 1 boiled egg, avocado (1/3) and sweet potato (1/2) 1 banana | Quinoa salad (20 g) with tomato and onion Grilled salmon (120 g) 1 apple |
| Snack | Smoothie: Modulen® (350 mL) and apple | Sliced apple with almond butter (10 g) | Yogurt (125 g) |
| Dinner | Baked chicken breast (150 g) Baked potato and carrot | Homemade beef burger (100 g) Homemade chips potato (1 potato) 1 banana | Spanish omelette (1 egg, 1 potato, onion) Roasted peppers 1 banana |
| Price | €3.06 | €3.93 | €3.95 |
| Modulen IBD® | €20 | €10 | €10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrador-López, M.; Martín-Masot, R.; Navas-López, V.M. EEN Yesterday and Today … CDED Today and Tomorrow. Nutrients 2020, 12, 3793. https://doi.org/10.3390/nu12123793
Herrador-López M, Martín-Masot R, Navas-López VM. EEN Yesterday and Today … CDED Today and Tomorrow. Nutrients. 2020; 12(12):3793. https://doi.org/10.3390/nu12123793
Chicago/Turabian StyleHerrador-López, Marta, Rafael Martín-Masot, and Víctor Manuel Navas-López. 2020. "EEN Yesterday and Today … CDED Today and Tomorrow" Nutrients 12, no. 12: 3793. https://doi.org/10.3390/nu12123793
APA StyleHerrador-López, M., Martín-Masot, R., & Navas-López, V. M. (2020). EEN Yesterday and Today … CDED Today and Tomorrow. Nutrients, 12(12), 3793. https://doi.org/10.3390/nu12123793