Expression of Telomeric Repeat–Containing RNA Decreases in Sarcopenia and Increases after Exercise and Nutrition Intervention
Abstract
:1. Introduction
2. Method
2.1. Research Participants
2.2. Anthropometric Measurement
2.3. Grip Strength
2.4. Diagnosis of Sarcopenia
2.5. Cell Lines and Culture Conditions
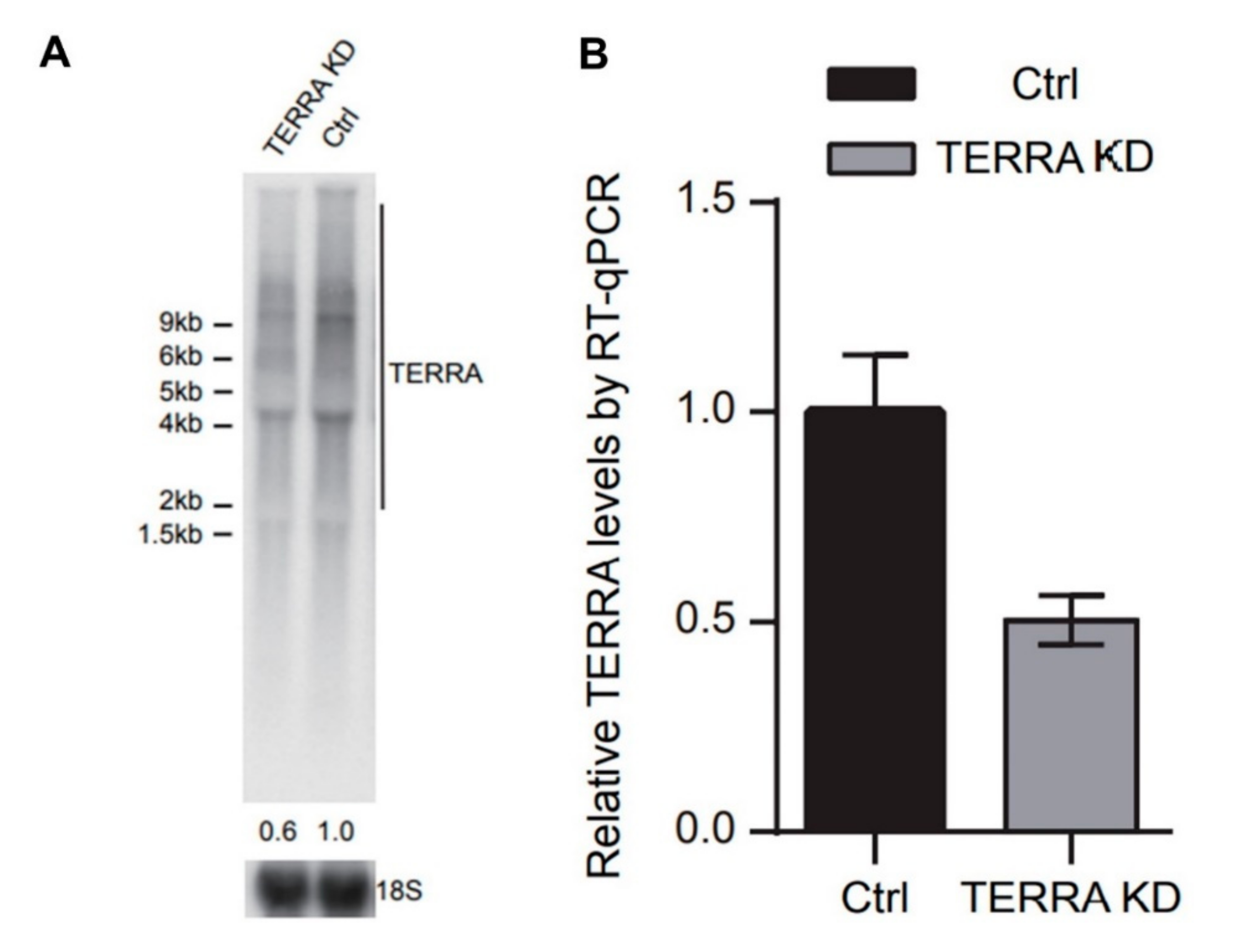
2.6. Northern Blot Analysis
2.7. Measurement of Telomere Length
2.8. RNA Isolation and Quantification of TERRA
2.9. Exercise and Nutritional Intervention
2.10. Statistical Analysis
3. Results
3.1. Basic Demographics
3.2. Telomere Length
3.3. TERRA Expression
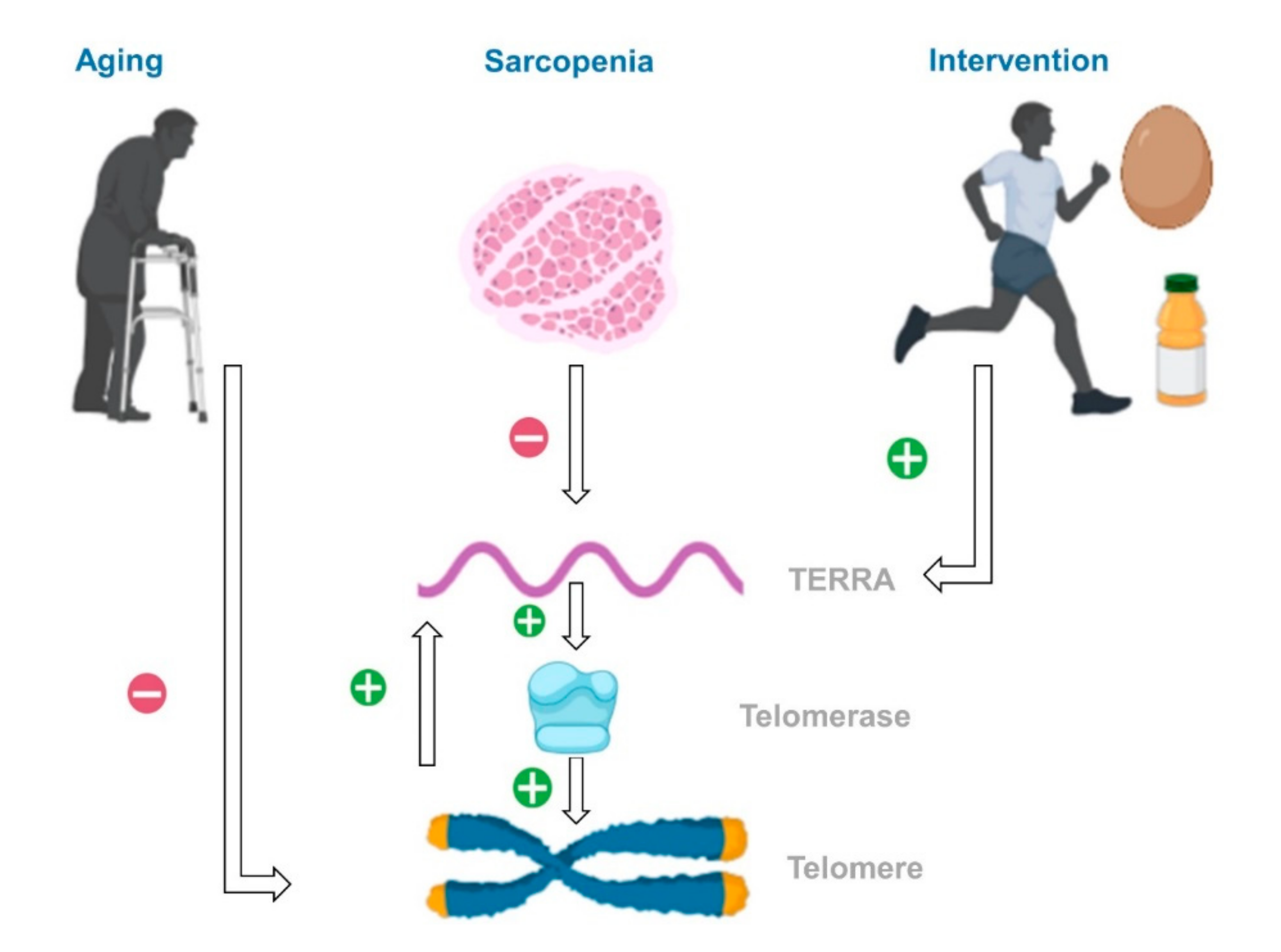
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chang, K.V.; Hsu, T.H.; Wu, W.T.; Huang, K.C.; Han, D.S. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing 2017, 46, 738–746. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.V.; Hsu, T.H.; Wu, W.T.; Huang, K.C.; Han, D.S. Association Between Sarcopenia and Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1164-e7–1164-e15. [Google Scholar] [CrossRef]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyere, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulou, S.K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Shammas, M.A. Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Zhou, J.; Dong, B. Effect of different levels of exercise on telomere length: A systematic review and meta-analysis. J. Rehabil. Med. 2019, 51, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Canudas, S.; Becerra-Tomas, N.; Hernandez-Alonso, P.; Galie, S.; Leung, C.; Crous-Bou, M.; De Vivo, I.; Gao, Y.; Gu, Y.; Meinila, J.; et al. Mediterranean Diet and Telomere Length: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 11, 1544–1554. [Google Scholar] [CrossRef]
- Bernabeu-Wittel, M.; Gomez-Diaz, R.; Gonzalez-Molina, A.; Vidal-Serrano, S.; Diez-Manglano, J.; Salgado, F.; Soto-Martin, M.; Ollero-Baturone, M.; On Behalf Of The Proteo, R. Oxidative Stress, Telomere Shortening, and Apoptosis Associated to Sarcopenia and Frailty in Patients with Multimorbidity. J. Clin. Med. 2020, 9, 2669. [Google Scholar] [CrossRef]
- Rippberger, P.L.; Emeny, R.T.; Mackenzie, T.A.; Bartels, S.J.; Batsis, J.A. The association of sarcopenia, telomere length, and mortality: Data from the NHANES 1999–2002. Eur. J. Clin. Nutr. 2018, 72, 255–263. [Google Scholar] [CrossRef]
- Woo, J.; Yu, R.; Tang, N.; Leung, J. Telomere length is associated with decline in grip strength in older persons aged 65 years and over. AGE 2014, 36, 9711. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, L.; Lu, S. Role of TERRA in the regulation of telomere length. Int. J. Biol. Sci. 2015, 11, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Cusanelli, E.; Chartrand, P. Telomeric repeat-containing RNA TERRA: A noncoding RNA connecting telomere biology to genome integrity. Front. Genet. 2015, 6, 143. [Google Scholar] [CrossRef] [Green Version]
- Maicher, A.; Kastner, L.; Luke, B. Telomeres and disease: Enter TERRA. RNA Biol. 2012, 9, 843–849. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.V.; Wu, W.T.; Huang, K.C.; Han, D.S. Effectiveness of early versus delayed exercise and nutritional intervention on segmental body composition of sarcopenic elders—A randomized controlled trial. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Batra, R.; Nelles, D.A.; Pirie, E.; Blue, S.M.; Marina, R.J.; Wang, H.; Chaim, I.A.; Thomas, J.D.; Zhang, N.; Nguyen, V.; et al. Elimination of Toxic Microsatellite Repeat Expansion RNA by RNA-Targeting Cas9. Cell 2017, 170, 899–912.e10. [Google Scholar] [CrossRef]
- Chu, H.P.; Cifuentes-Rojas, C.; Kesner, B.; Aeby, E.; Lee, H.G.; Wei, C.; Oh, H.J.; Boukhali, M.; Haas, W.; Lee, J.T. TERRA RNA Antagonizes ATRX and Protects Telomeres. Cell 2017, 170, 86–101.e16. [Google Scholar] [CrossRef] [Green Version]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Hanley, J.A.; Negassa, A.; Edwardes, M.D.; Forrester, J.E. Statistical analysis of correlated data using generalized estimating equations: An orientation. Am. J. Epidemiol. 2003, 157, 364–375. [Google Scholar] [CrossRef]
- Marzetti, E.; Lorenzi, M.; Antocicco, M.; Bonassi, S.; Celi, M.; Mastropaolo, S.; Settanni, S.; Valdiglesias, V.; Landi, F.; Bernabei, R.; et al. Shorter telomeres in peripheral blood mononuclear cells from older persons with sarcopenia: Results from an exploratory study. Front. Aging Neurosci. 2014, 6, 233. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Arsenis, N.C.; You, T.; Ogawa, E.F.; Tinsley, G.M.; Zuo, L. Physical activity and telomere length: Impact of aging and potential mechanisms of action. Oncotarget 2017, 8, 45008–45019. [Google Scholar] [CrossRef] [Green Version]
- Cusanelli, E.; Romero, C.A.; Chartrand, P. Telomeric noncoding RNA TERRA is induced by telomere shortening to nucleate telomerase molecules at short telomeres. Mol. Cell 2013, 51, 780–791. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.; Shukla, S.; Khan, S.; Farhan, M.; Kamal, M.A.; Meeran, S.M. Telomeric Repeat Containing RNA (TERRA): Aging and Cancer. CNS Neurol. Disord. Drug Targets 2015, 14, 936–946. [Google Scholar] [CrossRef]
- He, L.; Khanal, P.; Morse, C.I.; Williams, A.; Thomis, M. Differentially methylated gene patterns between age-matched sarcopenic and non-sarcopenic women. J. Cachexia Sarcopenia Muscle 2019, 10, 1295–1306. [Google Scholar] [CrossRef] [Green Version]
- Hsu, K.J.; Liao, C.D.; Tsai, M.W.; Chen, C.N. Effects of Exercise and Nutritional Intervention on Body Composition, Metabolic Health, and Physical Performance in Adults with Sarcopenic Obesity: A Meta-Analysis. Nutrients 2019, 11, 2163. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Lin, Z.; Li, S.; Liu, S.J. Effect of nutritional supplement combined with exercise intervention on sarcopenia in the elderly: A meta-analysis. Int. J. Nurs. Sci. 2017, 4, 389–401. [Google Scholar] [CrossRef]
- Grazioli, E.; Dimauro, I.; Mercatelli, N.; Wang, G.; Pitsiladis, Y.; Di Luigi, L.; Caporossi, D. Physical activity in the prevention of human diseases: Role of epigenetic modifications. BMC Genom. 2017, 18, 802. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Li, J.; Liu, Z.; Chuang, C.C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, B.S.; Kelleher, A.R.; Kimball, S.R. Regulation of muscle protein synthesis and the effects of catabolic states. Int. J. Biochem. Cell Biol. 2013, 45, 2147–2157. [Google Scholar] [CrossRef] [Green Version]
- Fetahu, I.S.; Hobaus, J.; Kallay, E. Vitamin D and the epigenome. Front. Physiol. 2014, 5, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saum, K.U.; Dieffenbach, A.K.; Muezzinler, A.; Muller, H.; Holleczek, B.; Stegmaier, C.; Butterbach, K.; Schick, M.; Canzian, F.; Stammer, H.; et al. Frailty and telomere length: Cross-sectional analysis in 3537 older adults from the ESTHER cohort. Exp. Gerontol. 2014, 58, 250–255. [Google Scholar] [CrossRef]
- Sameer, A.S.; Nissar, S.; Aziz, R. Telomeres and Estrogens: The Unholy Nexus in Pathogenesis of Atherosclerosis. Cardiol. Res. 2014, 5, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Liang, Z.A.; Sandford, A.J.; Xiong, X.Y.; Yang, Y.Y.; Ji, Y.L.; He, J.Q. Selection of suitable housekeeping genes for real-time quantitative PCR in CD4(+) lymphocytes from asthmatics with or without depression. PLoS ONE 2012, 7, e48367. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Umemoto, T.; Tanaka, Y.; Okano, T.; Yamato, M. β2-Microglobulin is an appropriate reference gene for RT-PCR-based gene expression analysis of hematopoietic stem cells. Regen. Ther. 2015, 1, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, B.K.; Choi, Y.; Bae, J.; Lee, W.M.; Hoh, J.K.; Choi, J.S. Increased amounts and stability of telomeric repeat-containing RNA (TERRA) following DNA damage induced by etoposide. PLoS ONE 2019, 14, e0225302. [Google Scholar] [CrossRef]
- Feretzaki, M.; Renck Nunes, P.; Lingner, J. Expression and differential regulation of human TERRA at several chromosome ends. RNA 2019, 25, 1470–1480. [Google Scholar] [CrossRef]
- Ahmad, S.; Heraclides, A.; Sun, Q.; Elgzyri, T.; Ronn, T.; Ling, C.; Isomaa, B.; Eriksson, K.F.; Groop, L.; Franks, P.W.; et al. Telomere length in blood and skeletal muscle in relation to measures of glycaemia and insulinaemia. Diabet. Med. 2012, 29, e377–e381. [Google Scholar] [CrossRef]
- Hiam, D.; Smith, C.; Voisin, S.; Denham, J.; Yan, X.; Landen, S.; Jacques, M.; Alvarez-Romero, J.; Garnham, A.; Woessner, M.N.; et al. Aerobic capacity and telomere length in human skeletal muscle and leukocytes across the lifespan. Aging 2020, 12, 359–369. [Google Scholar] [CrossRef] [PubMed]

| Sarcopenia (−) (N = 36) | Sarcopenia (+) (N = 36) | p Value | |
|---|---|---|---|
| Demographics and anthropometrics measurements | |||
| Age (year) | 75.69 ± 5.50 (73.83 to 77.55) | 74.86 ± 6.67 (72.60 to 77.11) | 0.565 |
| Female gender (number, %) | 28, 77.77% | 28, 77.77% | 1.000 |
| Height (cm) | 155.09 ± 7.62 (152.51 to 157.66) | 154.51 ± 7.18 (152.08 to 156.95) | 0.744 |
| Weight (kg) | 61.82 ± 9.76 (58.52 to 65.12) | 51.39 ± 7.97 (48.69 to 54.09) | <0.001 * |
| Body mass index (kg/m2) | 25.64 ± 2.88 (24.66 to 26.61) | 21.45 ± 2.31 (20.67 to 22.23) | <0.001 * |
| Physical performance | |||
| Handgrip strength (kg) | 19.86 ± 6.52 (17.65 to 22.06) | 16.91 ± 4.96 (15.23 to 18.59) | 0.035 * |
| Body Composition | |||
| Skeletal muscle index (kg/m2) | 6.63 ± 0.70 (6.39 to 6.86) | 5.57 ± 0.52 (5.39 to 5.75) | <0.001 * |
| Laboratory Measurement | |||
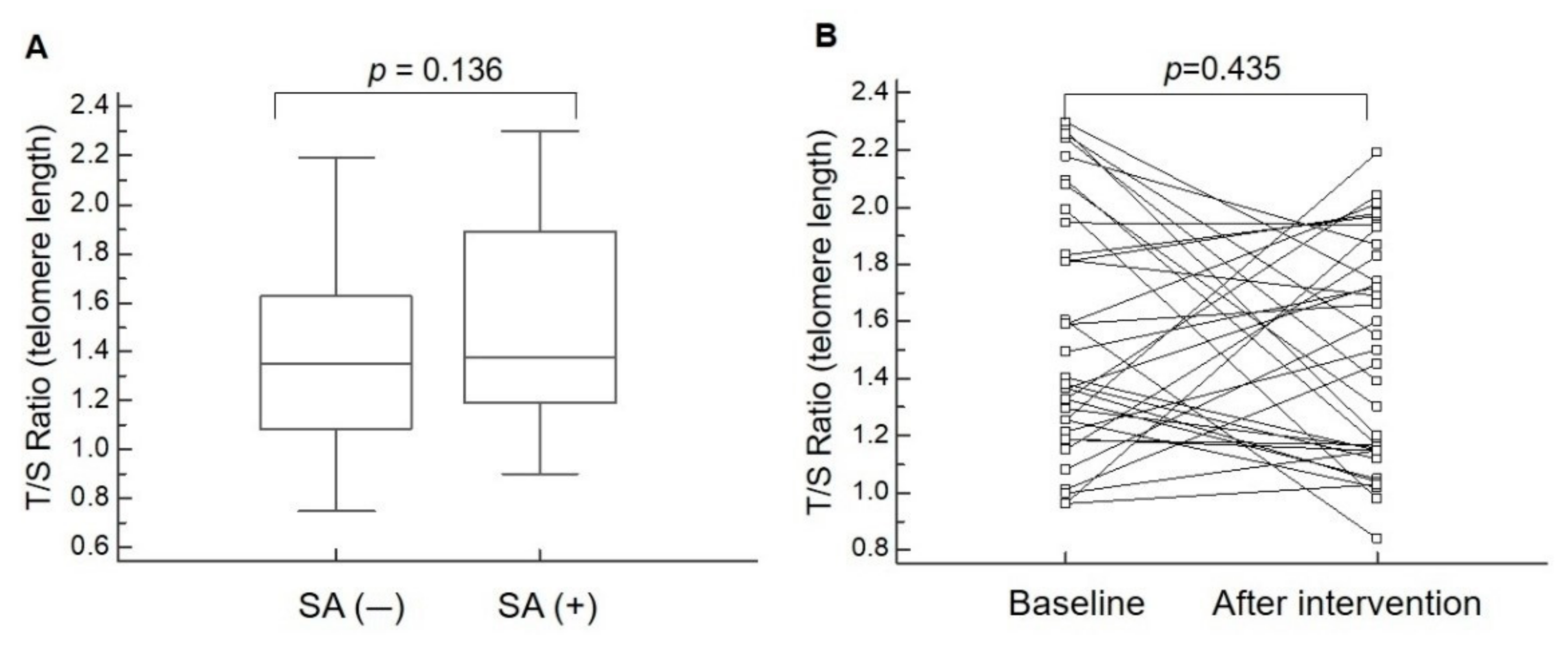
| Relative telomere length | 1.38 ± 0.37 (1.25 to 1.50) | 1.52 ± 0.43 (1.37 to 1.67) | 0.136 |
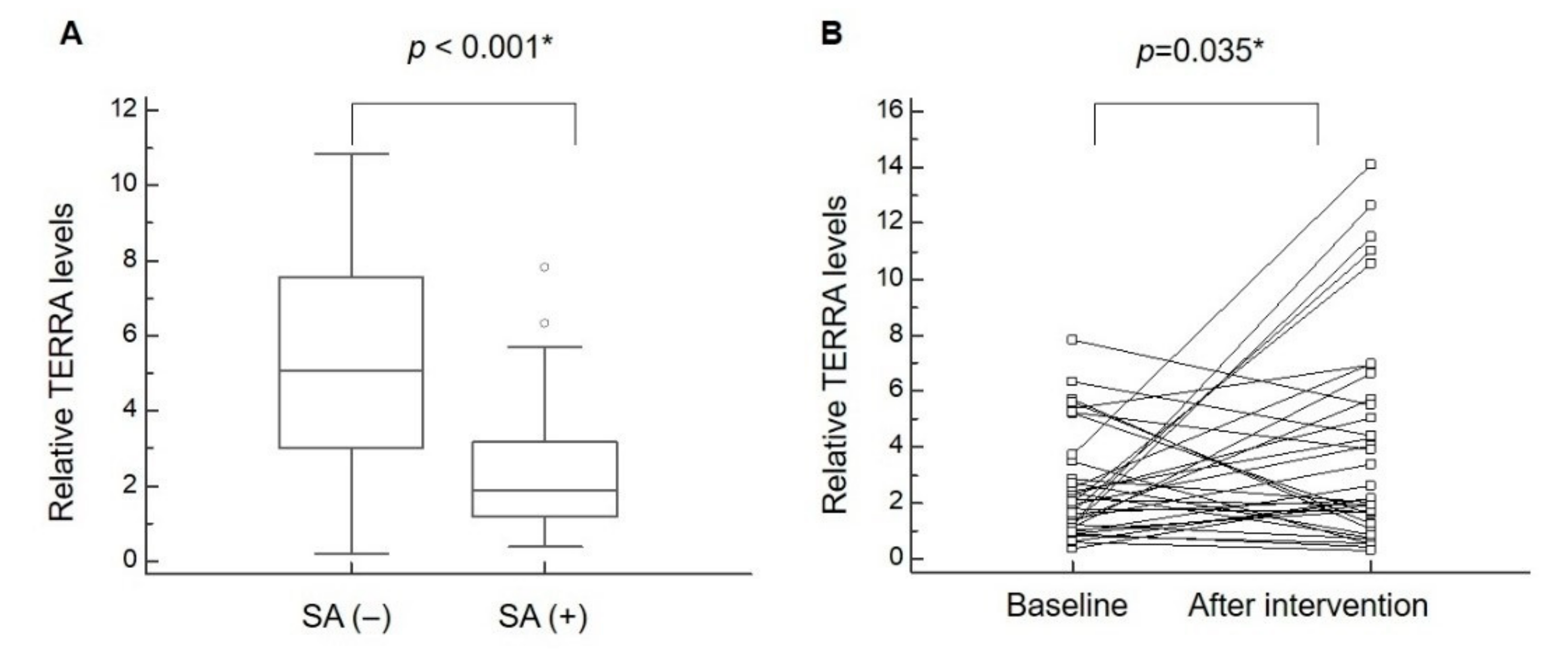
| TERRA expression | 5.18 ± 2.98 (4.17 to 6.19) | 2.51 ± 1.89 (1.86 to 3.15) | <0.001 * |
| Before Intervention (N = 36) | After Intervention (N = 36) | p Value | |
|---|---|---|---|
| T/S ratio (telomere length) | 1.55 ± 0.44 (1.40 to 1.70) | 1.48 ± 0.39 (1.34 to 1.61) | 0.435 |
| T/S ratio (TERRA expression) | 2.53 ± 1.94 (1.87 to 3.19) | 4.09 ± 3.87 (2.77 to 5.40) | 0.035 * |
| Age (Year) | Sarcopenia | Intervention | Female Gender | |
|---|---|---|---|---|
| Telomere length | −0.017 (−0.02 to −0.005) | −0.131 (−0.31 to 0.04) | 0.048 (−0.13 to 0.22) | −0.020 (−0.20 to 0.16) |
| p = 0.004 * | p = 0.154 | p = 0.605 | p = 0.840 | |
| TERRA expression | −0.034 (−0.13 to 0.06) | −2.705 (−3.85 to −1.55) | 1.599 (0.22 to 2.97) | −0.632 (−1.84 to 0.57) |
| p = 0.492 | p < 0.001 * | p = 0.023 * | p = 0.306 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-V.; Chen, Y.-C.; Wu, W.-T.; Shen, H.-J.; Huang, K.-C.; Chu, H.-P.; Han, D.-S. Expression of Telomeric Repeat–Containing RNA Decreases in Sarcopenia and Increases after Exercise and Nutrition Intervention. Nutrients 2020, 12, 3766. https://doi.org/10.3390/nu12123766
Chang K-V, Chen Y-C, Wu W-T, Shen H-J, Huang K-C, Chu H-P, Han D-S. Expression of Telomeric Repeat–Containing RNA Decreases in Sarcopenia and Increases after Exercise and Nutrition Intervention. Nutrients. 2020; 12(12):3766. https://doi.org/10.3390/nu12123766
Chicago/Turabian StyleChang, Ke-Vin, Yu-Chen Chen, Wei-Ting Wu, Hong-Jhin Shen, Kuo-Chin Huang, Hsueh-Ping Chu, and Der-Sheng Han. 2020. "Expression of Telomeric Repeat–Containing RNA Decreases in Sarcopenia and Increases after Exercise and Nutrition Intervention" Nutrients 12, no. 12: 3766. https://doi.org/10.3390/nu12123766








