Chlorella vulgaris Ameliorates Oxidative Stress and Improves the Muscle Regenerative Capacity of Young and Old Sprague-Dawley Rats
Abstract
1. Introduction
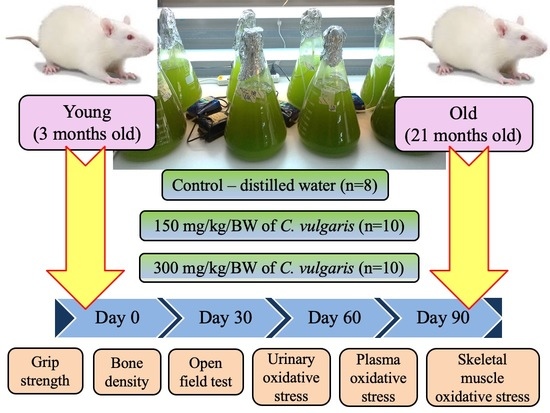
2. Materials and Methods
2.1. Animals
2.2. C. vulgaris Preparation
2.3. Grip Strength Test
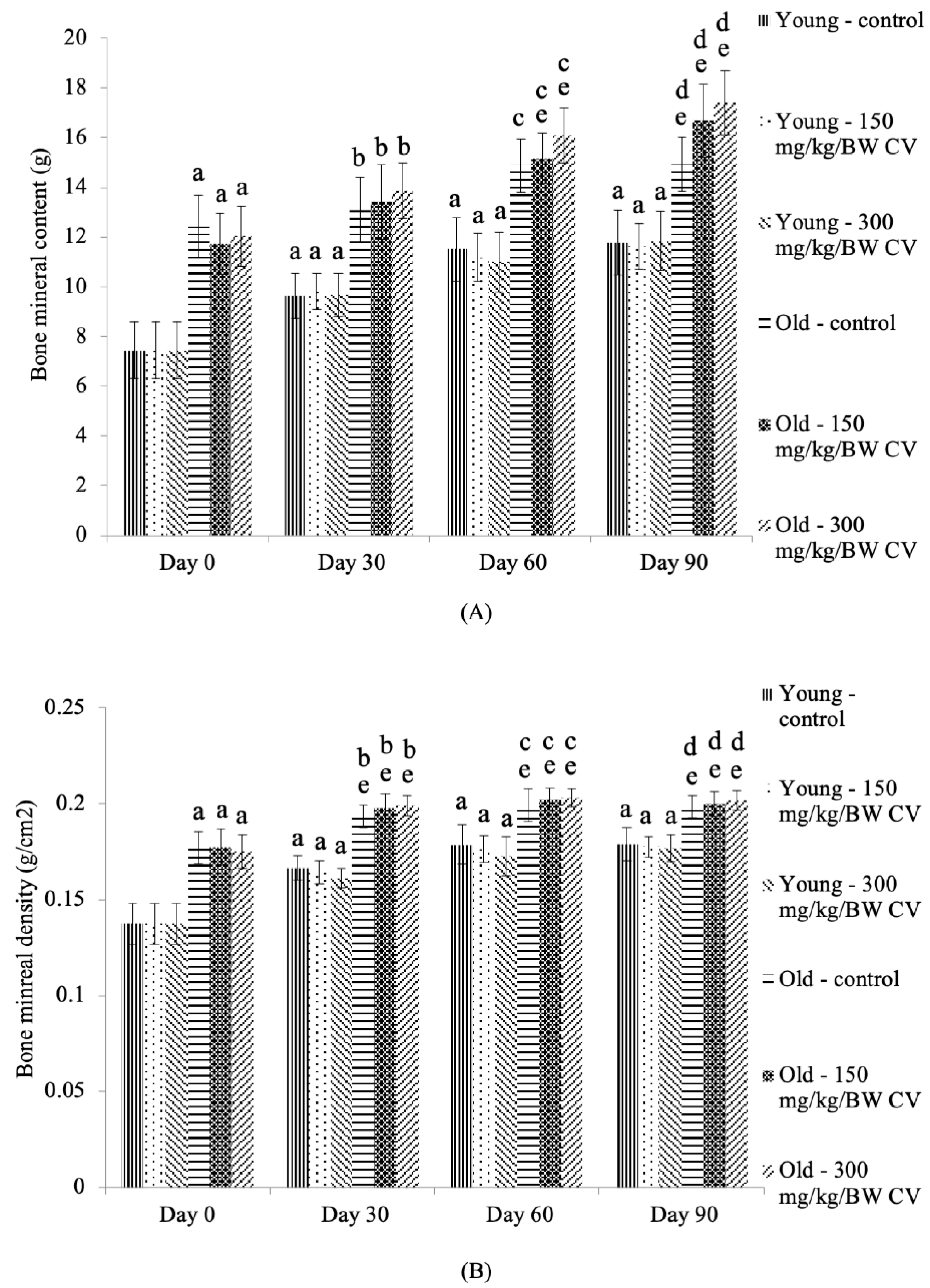
2.4. Measurement of Bone Density
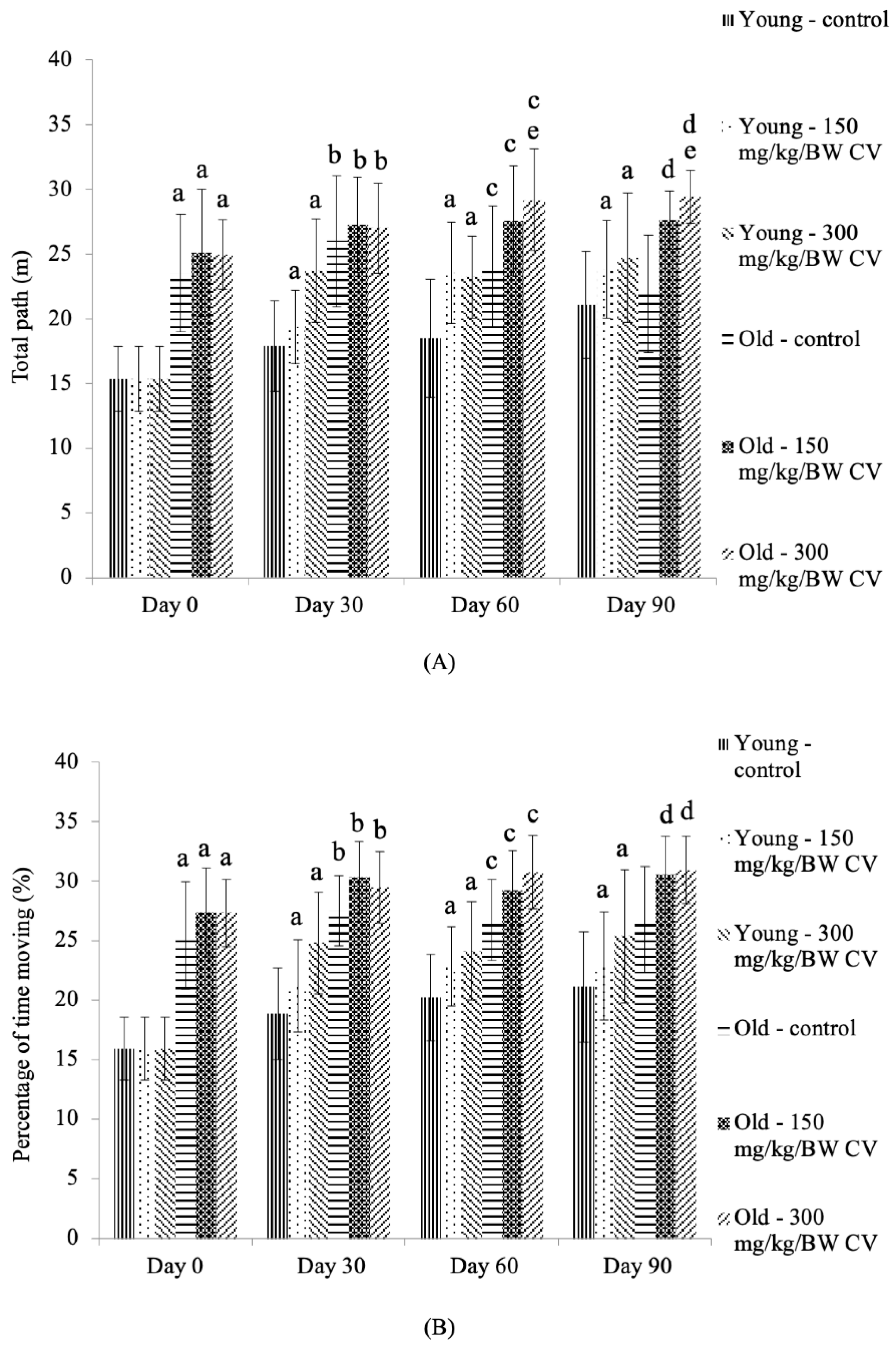
2.5. Open Field Test
2.6. Urine Collection
2.7. Blood Collection
2.8. Euthanization of Animals
2.9. Collection of Organs
2.10. Analysis of Urinary Oxidative Stress
2.11. Analysis of Plasma Oxidative Stress
2.12. Analysis of Skeletal Muscle Oxidative Stress
2.13. Statistical Analysis
3. Results
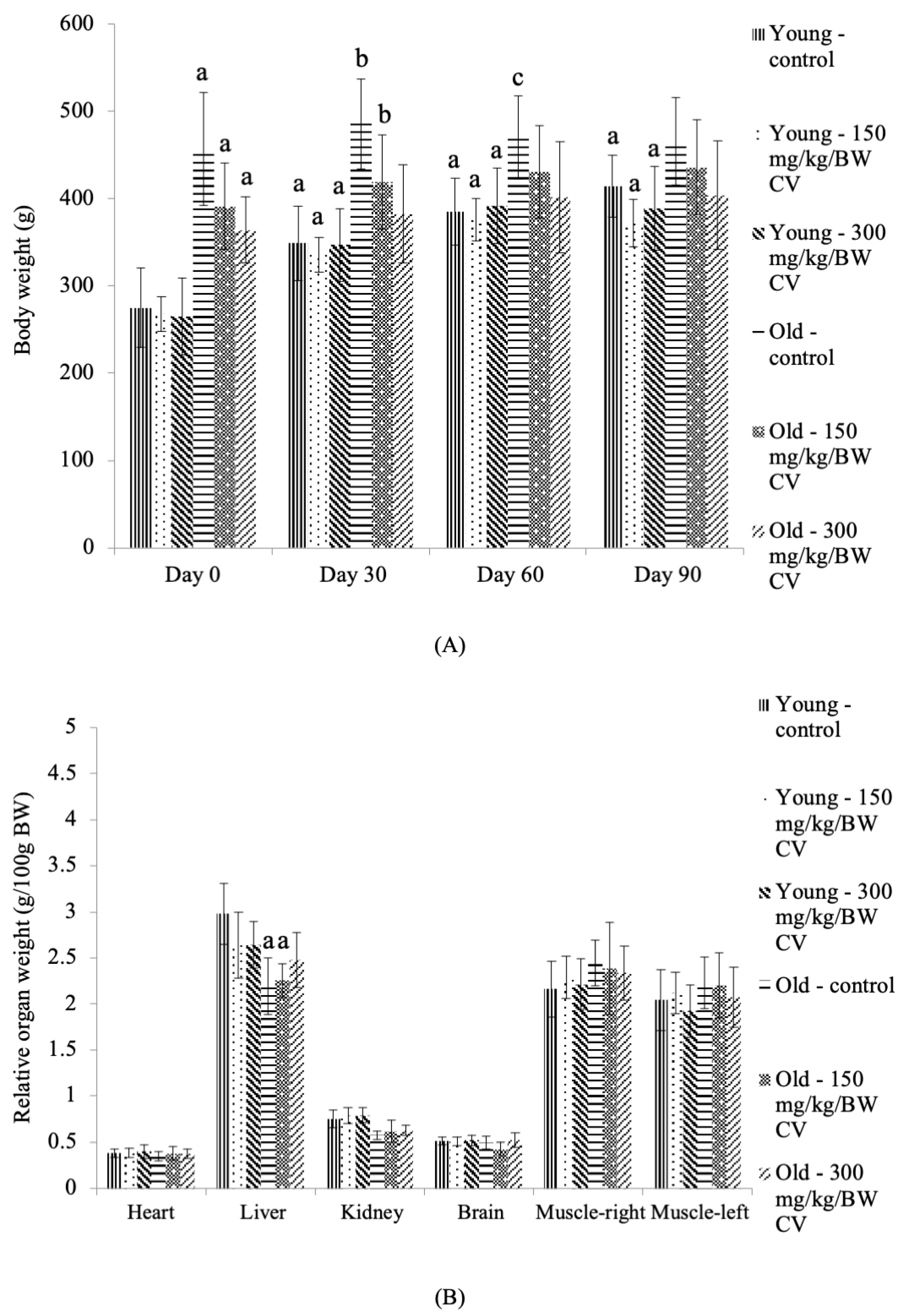
3.1. Body Weight and Relative Organ Weight
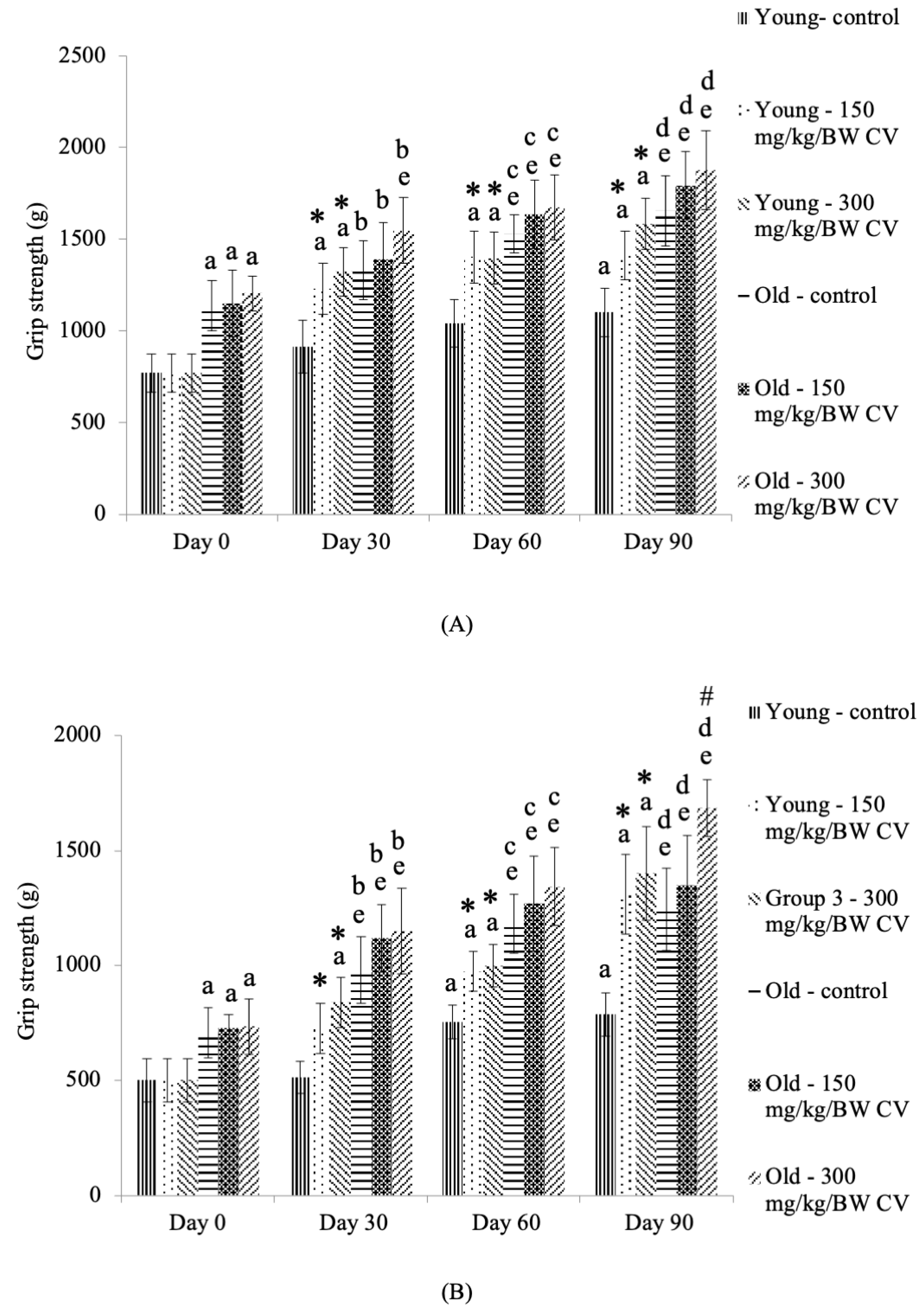
3.2. Measurement of Grip Strength
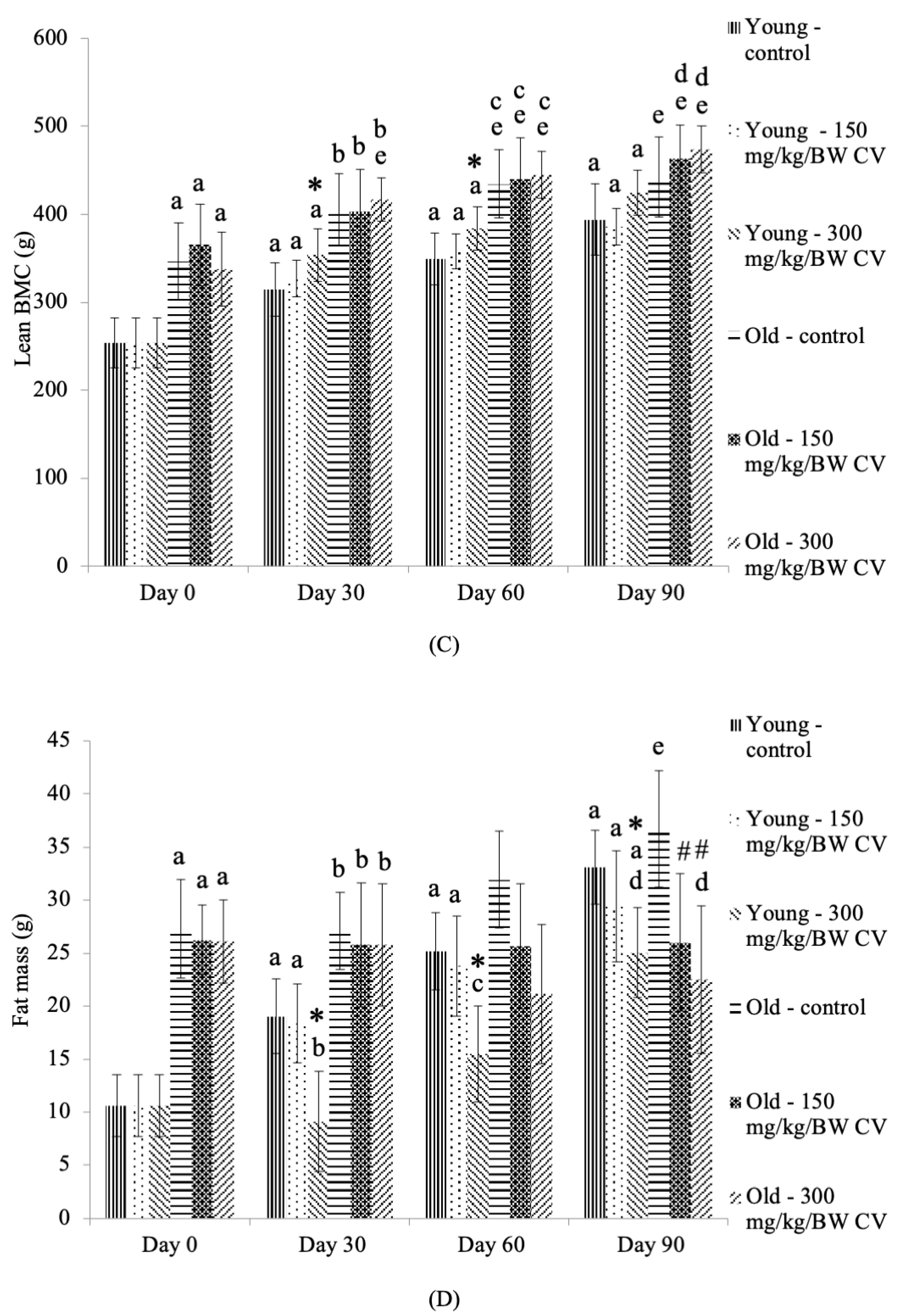
3.3. Measurement of Muscle and Bone Integrity
3.4. Measurement of Muscle Function
3.5. Levels of Oxidizing Stress Markers in the Tissue, Plasma, and Urine
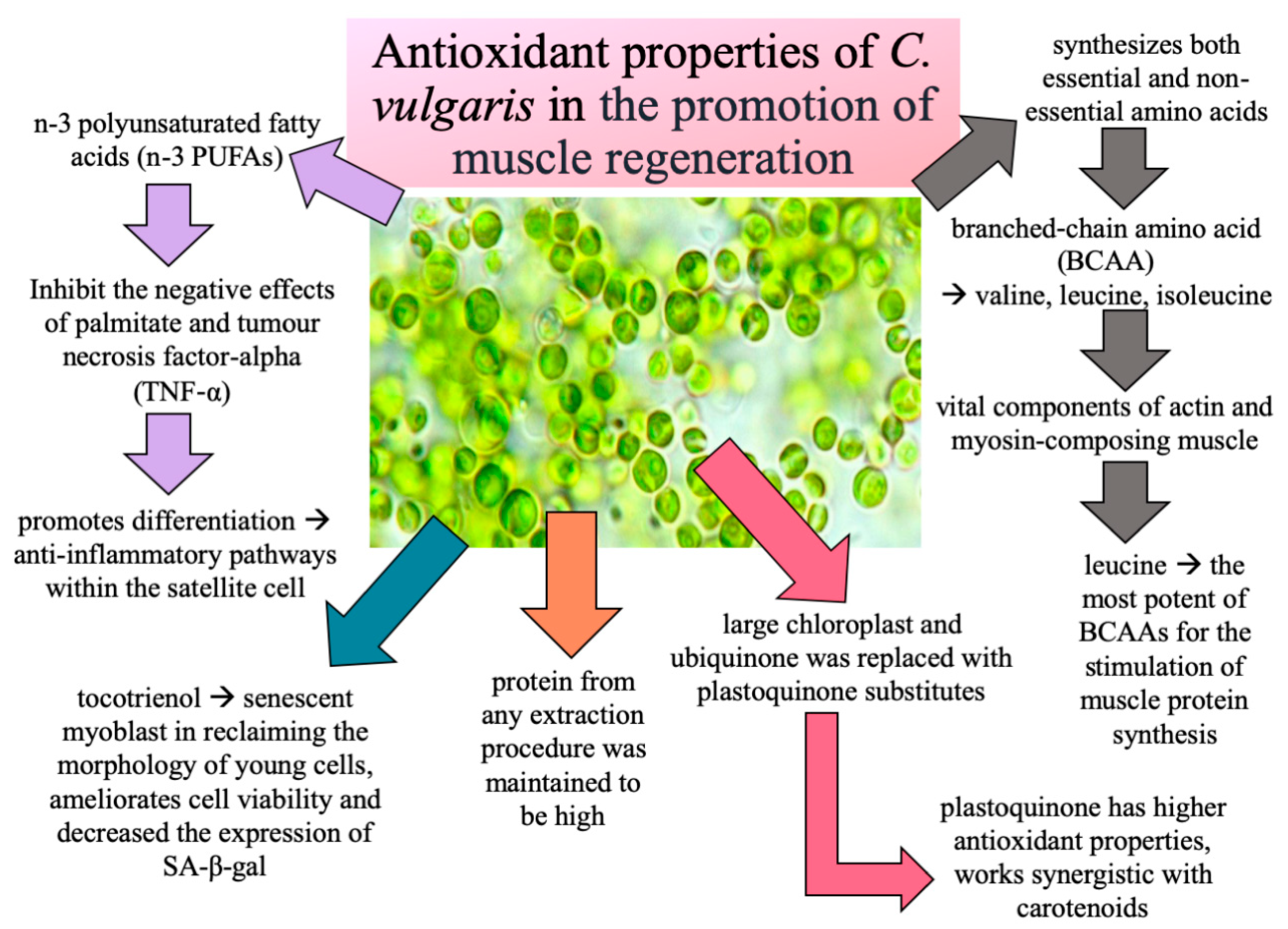
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2014, 96, 183–195. [Google Scholar] [CrossRef]
- Ziaaldini, M.M.; Marzetti, E.; Picca, A.; Murlasits, Z. Biochemical pathways of sarcopenia and their modulation by physical exercise: A narrative review. Front. Med. 2017, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J. Skeletal muscle loss: Cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 2010, 91, 1123S–1127S. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Anker, S.D.; Argiles, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.I.A.N.N.I.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by special interest groups “Cachexia-Anorexia in Chronic Wasting Diseases” and “Nutrition in Geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Atherton, P.J.; Williams, J.; Larvin, M.; Lund, J.N.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Ageing and Health; WHO: Geneva, Switzerland, 2015; pp. 1–260. [Google Scholar]
- Burton, L.A.; Sumukadas, D. Optimal management of sarcopenia. Clin. Interv. Aging 2010, 5, 217–228. [Google Scholar]
- Janssen, I. Evolution of sarcopenia research. Appl. Physiol. Nutr. Metab. 2010, 35, 707–712. [Google Scholar] [CrossRef]
- Noran, N.; Hairi, A.B.; Tee, G.H.; Izzuna, M. Sarcopenia in older people. Geriatrics 2012, 2012, 29–40. [Google Scholar]
- Børsheim, B.; Bui, Q.U.T.; Tissier, S.; Kobayashi, H.; Ferrando, A.A.; Wolfe, R.R. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin. Nutr. 2008, 27, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Ohno, N.; Tanaka, K.; Okuda, M.; Yadomae, T.; Nomoto, K.; Shoyama, Y. A new type of biological response modifier from Chlorella vulgaris which needs protein moiety to show an antitumour activity. Phytother. Res. 1998, 12, 309–319. [Google Scholar] [CrossRef]
- Shibata, S.; Natori, Y.; Nishihara, T.; Tomisaka, K.; Matsumoto, K.; Sansawa, H.; Nguyen, V.C. Antioxidant and anti-cataract effects of Chlorella on rats with streptozotocin-induced diabetes. J. Nutr. Sci. Vitaminol. 2003, 49, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Kitada, K.; Machmudah, S.; Sasaki, M.; Goto, M.; Nakashima, Y.; Kumamoto, S.; Hasegawa, T. Supercritical CO2 extraction of pigment components with pharmaceutical importance from Chlorella vulgaris. J. Chem. Technol. Biot. 2009, 84, 657–661. [Google Scholar] [CrossRef]
- Panahi, Y.; Pishgoo, B.; Jalalian, H.R.; Mohammadi, E.; Taghipour, H.R.; Sahebkar, A.; Abolhasani, E. Investigation of the effects of Chlorella vulgaris as an adjunctive therapy for dyslipidemia: Results of a randomised open-label clinical trial. Nutr. Diet. 2012, 69, 13–19. [Google Scholar] [CrossRef]
- Jeong, H.; Kwon, H.J.; Kim, M.K. Hypoglycemic effect of Chlorella vulgaris intake in type 2 diabetic Goto-Kakizaki and normal Wistar rats. Nutr. Res. Pract. 2009, 3, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Aizzat, O.; Yap, S.; Sopiah, H.; Madiha, M.; Hazreen, M.; Shailah, A.; Junizam, W.W.; Syaidah, A.N.; Srijit, D.; Musalmah, M.; et al. Modulation of oxidative stress by Chlorella vulgaris in streptozotocin (STZ) induced diabetic Sprague-Dawley rats. Adv. Med. Sci. 2010, 55, 281–288. [Google Scholar] [CrossRef]
- Ngah, W.Z.W.; Yusof, Y.A.M. Chemo preventive effect of Chlorella vulgaris in choline deficient diet and ethionine induced liver carcinogenesis in rats. Int. J. Cancer Res. 2006, 2, 234–241. [Google Scholar]
- Azamai, E.S.M.; Sulaiman, S.; Habib, S.H.M.; Looi, M.L.; Das, S.; Hamid, N.A.A.; Ngah, W.Z.W.; Yusof, Y.A.M. Chlorella vulgaris triggers apoptosis in hepatocarcinogenesis-induced rats. J. Zhejiang Univ. Sci. B 2009, 10, 14–21. [Google Scholar] [CrossRef]
- Mukti, N.A.; Sulaiman, S.; Saad, S.M.; Basari, H. Chlorella vulgaris menunjukkan kesan antioksidan dan antitumor terhadap kanser hepar dalam kajian in vivo dan in vitro. Sains Malays. 2009, 38, 773–784. [Google Scholar]
- Makpol, S.; Yeoh, T.W.; Ruslam, F.C.; Arifin, K.T.; Yusof, Y.M. Comparative effect of Piper Betle, Chlorella vulgaris and tocotrienol-rich fraction on antioxidant enzymes activity in cellular ageing of human diploid fibroblasts. BMC Complement. Altern. Med. 2013, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saberbaghi, T.; Abbasian, F.; Yusof, Y.A.M.; Makpol, S. Modulation of cell cycle profile by Chlorella vulgaris prevents replicative senescence of human diploid fibroblasts. Evid. Based Complement. Altern. Med. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zainul Azlan, N.; Yusof, Y.A.M.; Alias, E.; Makpol, S. Chlorella vulgaris Improves the Regenerative Capacity of Young and Senescent Myoblasts and Promotes Muscle Regeneration. Oxid. Med. Cell. Longev. 2019, 2019, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zainul Azlan, N.; Yusof, Y.A.M.; Alias, E.; Makpol, S. Chlorella vulgaris Modulates Genes and Muscle-Specific microRNAs Expression to Promote Myoblast Differentiation in Culture. Evid. Based Complement. Altern. Med. 2019, 2019, 1–16. [Google Scholar]
- Andreollo, N.A.; dos Santos, E.F.; Araújo, M.R.; Lopes, L.R. Rat’s age versus human’s age: What is the relationship? ABCD Arq. Bras. Cir. Dig. 2012, 25, 49–51. [Google Scholar] [CrossRef]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624. [Google Scholar]
- Sengupta, P. A scientific review of age determination for a laboratory rat: How old is it in comparison with human age? Biomed. Int. 2015, 2, 81–89. [Google Scholar]
- Lee, H.S.; Park, H.J.; Kim, M.K. Effect of Chlorella vulgaris on lipid metabolism in Wistar rats fed high fat diet. Nutr. Res. Pract. 2008, 2, 204–210. [Google Scholar] [CrossRef]
- Cheong, L.K. Introduction to Techniques of Intravenous Inoculation and Anaesthesia in Rodents and Rabbit: An Illustration of Simple and Efficient Approaches; Universiti Kebangsaan Malaysia: Kuala Lumpur, Malaysia, 2015. [Google Scholar]
- Tarantino, U.; Baldi, J.; Scimeca, M.; Piccirilli, E.; Piccioli, A.; Bonanno, E.; Gasbarra, E. The role of sarcopenia with and without fracture. Injury 2016, 47, S3–S10. [Google Scholar] [CrossRef]
- Lessard-Beaudoin, M.; Laroche, M.; Demers, M.J.; Grenier, G.; Graham, R.K. Characterization of age-associated changes in peripheral organ and brain region weights in C57bl/6 mice. Exp. Gerontol. 2015, 63, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Pötsch, M.S.; Tschirner, A.; Palus, S.; Von Haehling, S.; Doehner, W.; Beadle, J.; Coats, A.J.; Anker, S.D.; Springer, J. The anabolic catabolic transforming agent (Acta) espindolol increases muscle mass and decreases fat mass in old rats. J. Cachexia Sarcopenia Muscle 2014, 5, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Meyer, O.; Tilson, H.; Byrd, W.; Riley, M. Method for the routine assessment of fore- and hindlimb grip strength of rats and mice. Neurobehav. Toxicol. 1979, 1, 233–236. [Google Scholar] [PubMed]
- Zainul Azlan, N.; Abd Ghafar, N.; Yusof, Y.A.M.; Makpol, S. Toxicity study of Chlorella vulgaris water extract on female Sprague Dawley rats by using the Organization for Economic Cooperation and Development (OECD) Guideline 420. J. Appl. Phycol. 2020, 32, 3063–3075. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Gonzalez, M.C.; Schulzke, J.D.; Pirlich, M. Hand grip strength: Outcome predictor and marker of nutritional status. Clin. Nutr. 2011, 30, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.M.; Van Remmen, H.; Purkar, A.; Mahesula, S.; Gelfond, J.; Sabia, M.; Qi, W.; Lin, A.L.; Jaramillo, C.A.; Haskins, W.E. Microwave and magnetic (M2) proteomics of a mouse model of mild traumatic brain injury. Transl. Proteom. 2014, 3, 10–21. [Google Scholar] [CrossRef]
- Cohen, T.V.; Kollias, H.D.; Liu, N.; Ward, C.W.; Wagner, K.R. Genetic disruption of Smad7 impairs skeletal muscle growth and regeneration. J. Physiol. 2015, 593, 2479–2497. [Google Scholar] [CrossRef]
- Ablat, N.; Lv, D.; Ren, R.; Xiaokaiti, Y.; Ma, X.; Zhao, X.; Sun, Y.; Lei, H.; Xu, Y.; Ma, Y.; et al. Neuroprotective Effects of a standardized flavonoid extract from Safflower against a rotenone-induced rat model of Parkinson’s disease. Molecules 2016, 21, 1107. [Google Scholar] [CrossRef]
- Trueman, R.C.; Diaz, C.; Farr, T.D.; Harrison, D.J.; Fuller, A.; Tokarczuk, P.F.; Stewart, A.J.; Paisey, S.J.; Dunnett, S.B. Systematic and detailed analysis of behavioural tests in the rat middle cerebral artery occlusion model of stroke: Tests for long-term assessment. J. Cerebr. Blood F Met. 2017, 37, 1349–1361. [Google Scholar] [CrossRef]
- Alamri, F.F.; Al Shoyaib, A.; Biggers, A.; Jayaraman, S.; Guindon, J.; Karamyan, V.T. Applicability of the grip strength and automated Von Frey tactile sensitivity tests in the mouse photothrombotic model of stroke. Behav. Brain Res. 2018, 336, 250–255. [Google Scholar] [CrossRef]
- Hairi, N.N.; Cumming, R.G.; Naganathan, V.; Handelsman, D.J.; Le Couteur, D.G.; Creasey, H.; Waite, L.M.; Seibel, M.J.; Sambrook, P.N. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: The Concord Health and Ageing in Men Project. J. Am. Geriatr. Soc. 2010, 58, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Giusto, M.; Lattanzi, B.; Albanese, C.; Galtieri, A.; Farcomeni, A.; Giannelli, V.; Lucidi, C.; Di Martino, M.; Catalano, C.; Merli, M. Sarcopenia in liver cirrhosis: The role of computed tomography scan for the assessment of muscle mass compared with Dual-Energy X-Ray absorptiometry and anthropometry. Eur. J. Gastroenterol. Hepatol. 2015, 27, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Mazess, R.B.; Barden, H.S.; Bisek, J.P.; Hanson, J. Dual-energy X-Ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am. J. Clin. Nutr. 1990, 51, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The Health, Aging and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, K.B.; Thornton, J.C.; Heymsfield, S.B.; Gallagher, D. Greater lean tissue and skeletal muscle mass are associated with higher bone mineral content in children. Nutr. Metab. 2010, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Rahnert, J.A.; Luo, Q.; Balog, E.M.; Sokoloff, A.J.; Burkholder, T.J. Changes in growth-related kinases in head, neck and limb muscles with age. Exp. Gerontol. 2011, 46, 282–291. [Google Scholar] [CrossRef][Green Version]
- Xie, W.; Huang, X.; Chen, R.; Chen, R.; Li, T.; Wu, W.; Huang, Z. Esomeprazole alleviates the damage to stress ulcer in rats through not only its antisecretory effect but its antioxidant effect by inactivating the p38 MAPK and NF-κB signaling pathways. Drug Des. Dev. Ther. 2019, 13, 2969–2984. [Google Scholar] [CrossRef]
- Tatem, K.S.; Quinn, J.L.; Phadke, A.; Yu, Q.; Gordish-Dressman, H.; Nagaraju, K. Behavioral and locomotor measurements using an open field activity monitoring system for skeletal muscle diseases. Jove J. Vis. Exp. 2014, 91, e51785. [Google Scholar] [CrossRef]
- Yu, Q.; Sali, A.; Van Der Meulen, J.; Creeden, B.K.; Gordish-Dressman, H.; Rutkowski, A.; Rayavarapu, S.; Uaesoontrachoon, K.; Huynh, T.; Nagaraju, K.; et al. Omigapil treatment decreases fibrosis and improves respiratory rate in Dy2j mouse model of congenital muscular dystrophy. PLoS ONE 2013, 8, e65468. [Google Scholar] [CrossRef]
- Arthur, S.T.; Cooley, I.D. The effect of physiological stimuli on sarcopenia; Impact of Notch and Wnt signaling on impaired aged skeletal muscle repair. Int. J. Biol. Sci. 2012, 8, 731–760. [Google Scholar] [CrossRef]
- Powers, S.K.; Morton, A.B.; Ahn, B.; Smuder, A.J. Redox control of skeletal muscle atrophy. Free Radic. Biol. Med. 2016, 98, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, E.S.; Mancinelli, R.; Pietrangelo, T.; La Rovere, R.M.L.; Quattrocelli, M.; Sampaolesi, M.; Fulle, S. Myomir dysregulation and reactive oxygen species in aged human satellite cells. Biochem. Biophys. Res. Commun. 2016, 473, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Klawitter, J.; Haschke, M.; Shokati, T.; Klawitter, J.; Christians, U. Quantification of 15-F2t-isoprostane in human plasma and urine: Results from enzyme-linked immunoassay and liquid chromatography/tandem mass spectrometry cannot be compared. Rapid Commun. Mass Spectrom. 2011, 25, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, G.; Mescka, C.P.; Sitta, A.; Donida, B.; Marchetti, D.; Hammerschmidt, T.; Faverzani, J.; Coelho Dde, M.; Wajner, M.; Dutra-Filho, C.S.; et al. Urinary biomarkers of oxidative damage in maple syrup urine disease: The L-carnitine role. Int. J. Dev. Neurosci. 2015, 42, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.J.; Morrow, J.D. Measurement of F 2-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 2011, 28, 505–513. [Google Scholar] [CrossRef]
- Liu, W.; Morrow, J.D.; Yin, H. Quantification of F2-isoprostanes as a reliable index of oxidative stress in vivo using Gas Chromatography–Mass Spectrometry (GC-MS) method. Free Radic. Biol. Med. 2009, 47, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Czerska, M.; Mikolajewska, K.; Zielinski, M.; Gromadzinska, J.; Wasowicz, W. Today’s oxidative stress markers. Med. Pr. 2015, 66, 393–405. [Google Scholar] [CrossRef]
- Kumar, V.; Gill, K.D. To measure activity of creatine kinase in serum. In Basic Concepts in Clinical Biochemistry: A Practical Guide, 1st ed.; Springer: Singapore, 2018; pp. 131–133. [Google Scholar]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N. The effect of gender and menstrual phase on serum creatine kinase activity and muscle soreness following downhill running. Antioxidants 2017, 6, 16. [Google Scholar] [CrossRef]
- Kasapoglu, M.; Özben, T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp. Gerontol. 2001, 36, 209–220. [Google Scholar] [CrossRef]
- Bouzid, M.A.; Hammouda, O.; Matran, R.; Robin, S.; Fabre, C. Changes in oxidative stress markers and biological markers of muscle injury with aging at rest and in response to an exhaustive exercise. PLoS ONE 2014, 9, e90420. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Romano, A.D.; Buglio, A.L.; Castriotta, V.; Guglielmi, G.; Greco, A.; Serviddio, G.; Vendemiale, G. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas 2018, 109, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Ohsawa, I.; Nishimaki, K.; Kumamoto, S.; Maruyama, I.; Suzuki, Y.; Ohta, S. Preventive effects of Chlorella on skeletal muscle atrophy in muscle-specific mitochondrial aldehyde dehydrogenase 2 activity-deficient mice. BMC Complement. Altern. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Makpol, S.; Yaacob, N.; Zainuddin, A.; Yusof, Y.A.M.; Ngah, W.Z.W. Chlorella vulgaris modulates hydrogen peroxide-induced DNA damage and telomere shortening of human fibroblasts derived from different aged individuals. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Tokuşoglu, Ö.; Üunal, M. Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J. Food Sci. 2003, 68, 1144–1148. [Google Scholar] [CrossRef]
- Dreyer, H.C.; Volpi, E. Role of protein and amino acids in the pathophysiology and treatment of sarcopenia. J. Am. Coll. Nutr. 2005, 24, 140S–145S. [Google Scholar] [CrossRef]
- Fujita, S.; Volpi, E. Amino acids and muscle loss with aging. J. Nutr. 2006, 136, 277S–280S. [Google Scholar] [CrossRef]
- Ursu, A.V.; Marcati, A.; Sayd, T.; Sante-Lhoutellier, V.; Djelveh, G.; Michaud, P. Extraction, fractionation and functional properties of proteins from the microalgae Chlorella vulgaris. Bioresour. Technol. 2014, 157, 134–139. [Google Scholar] [CrossRef]
- Bosaeus, I.; Rothenberg, E. Nutrition and physical activity for the prevention and treatment of age-related sarcopenia. Proc. Nutr. Soc. 2016, 75, 174–180. [Google Scholar] [CrossRef]
- Khor, S.C.; Razak, A.M.; Ngah, W.Z.W.; Yusof, Y.A.M.; Karim, N.A.; Makpol, S. The tocotrienol-rich fraction is superior to tocopherol in promoting myogenic differentiation in the prevention of replicative senescence of myoblasts. PLoS ONE 2016, 11, e0149265. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Bhavan, P.; Seenivasan, C.; Muralisankar, T. Nutritional profile of Spirulina platensis, Chlorella vulgaris and Azolla pinnata to novel protein source for aquaculture feed formulation. Austin J. Aquac. Mar. Biol. 2017, 2, 1–8. [Google Scholar]
- Tachtsis, B.; Camera, D.; Lacham-Kaplan, O. Potential Roles of n-3 PUFAs during Skeletal Muscle Growth and Regeneration. Nutrients 2018, 10, 309. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainul Azlan, N.; Mohd Yusof, Y.A.; Makpol, S. Chlorella vulgaris Ameliorates Oxidative Stress and Improves the Muscle Regenerative Capacity of Young and Old Sprague-Dawley Rats. Nutrients 2020, 12, 3752. https://doi.org/10.3390/nu12123752
Zainul Azlan N, Mohd Yusof YA, Makpol S. Chlorella vulgaris Ameliorates Oxidative Stress and Improves the Muscle Regenerative Capacity of Young and Old Sprague-Dawley Rats. Nutrients. 2020; 12(12):3752. https://doi.org/10.3390/nu12123752
Chicago/Turabian StyleZainul Azlan, Nurhazirah, Yasmin Anum Mohd Yusof, and Suzana Makpol. 2020. "Chlorella vulgaris Ameliorates Oxidative Stress and Improves the Muscle Regenerative Capacity of Young and Old Sprague-Dawley Rats" Nutrients 12, no. 12: 3752. https://doi.org/10.3390/nu12123752
APA StyleZainul Azlan, N., Mohd Yusof, Y. A., & Makpol, S. (2020). Chlorella vulgaris Ameliorates Oxidative Stress and Improves the Muscle Regenerative Capacity of Young and Old Sprague-Dawley Rats. Nutrients, 12(12), 3752. https://doi.org/10.3390/nu12123752