Decrease in Mucosal IL17A, IFNγ and IL10 Expressions in Active Crohn’s Disease Patients Treated with High-Dose Vitamin D Alone or Combined with Infliximab
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Approval and Conduction
2.2. Inclusion and Exclusion Criteria
2.3. Design and Intervention
- (1).
- Infliximab and vitamin D3 (Ifx + VitD);
- (2).
- Infliximab and placebo vitamin D3 (Ifx + placeboVitD);
- (3).
- Placebo infliximab and vitamin D3 (placeboIfx + VitD);
- (4).
- Placebo infliximab and placebo vitamin D3 (placeboIfx + placeboVitD).
2.4. Randomization and Blinding
2.5. Biopsies for Quantitative Polymerase Chain Reaction (qPCR)
2.6. Isolation of Ribonucleic Acid (RNA)
2.7. Quantitative PCR
2.8. Vitamin D Safety Markers
2.9. Statistical Analyses
3. Results
3.1. Patient Characteristics and Study Follow-Up
3.2. High-Dose Vitamin D and Infliximab Decrease Mucosal Th17-Related Cytokine Expression
3.3. High-Dose Vitamin D Treatment Decreases IL10 and INFγ Expression
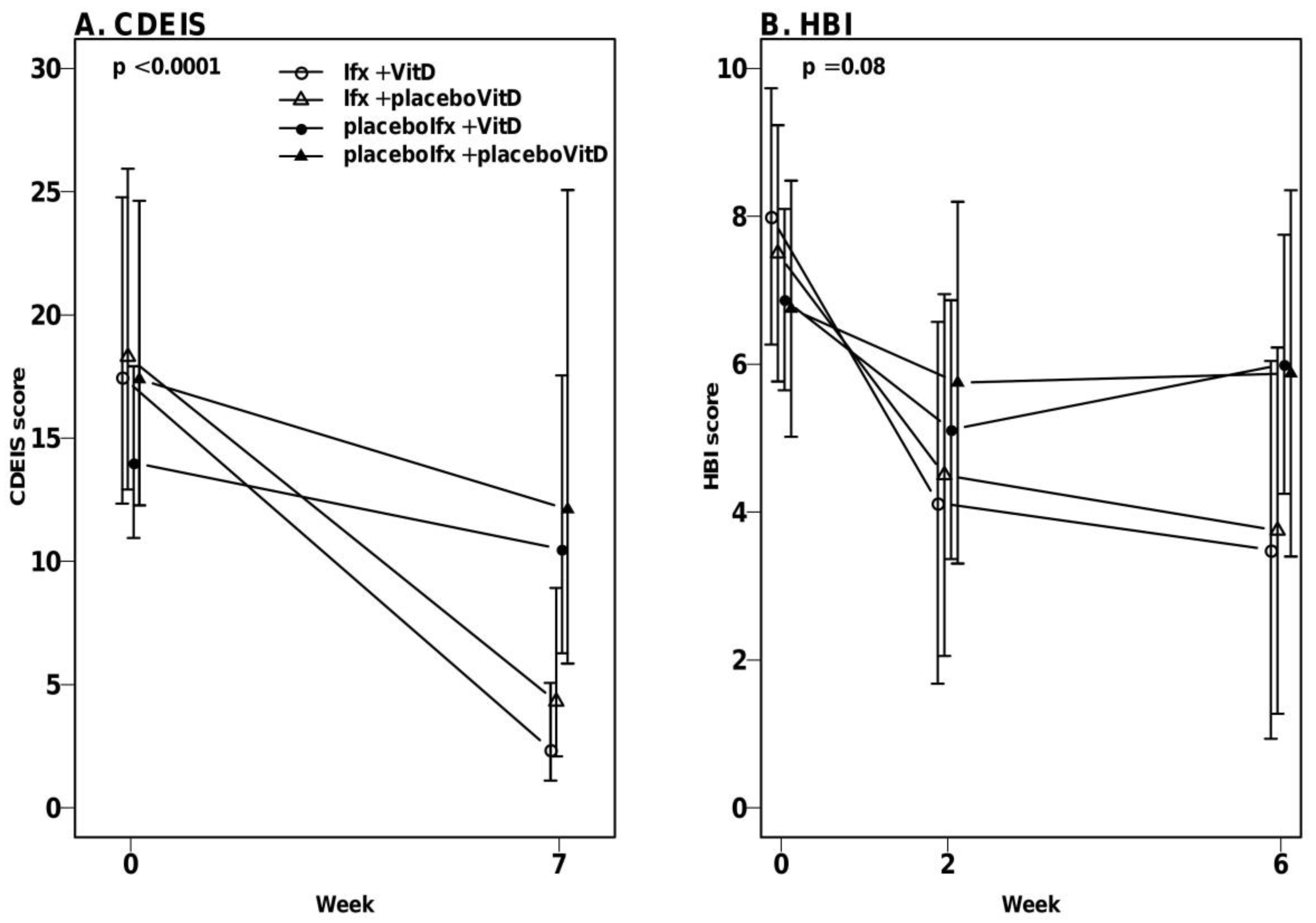
3.4. High-Dose Vitamin D Treatment Did Not Decrease Endoscopic Disease Activity
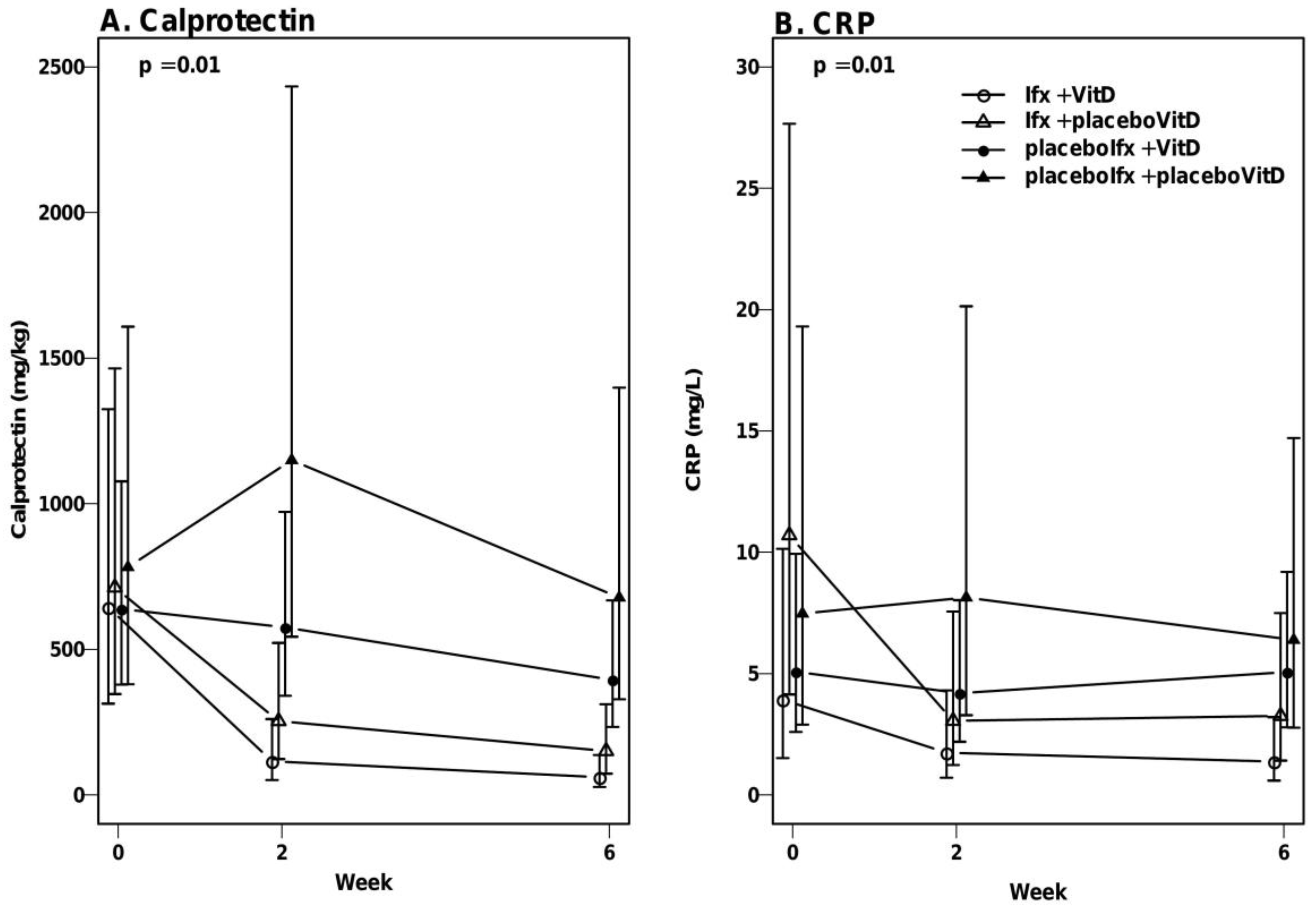
3.5. No Significant Effect of Vitamin D on Calprotectin and CRP
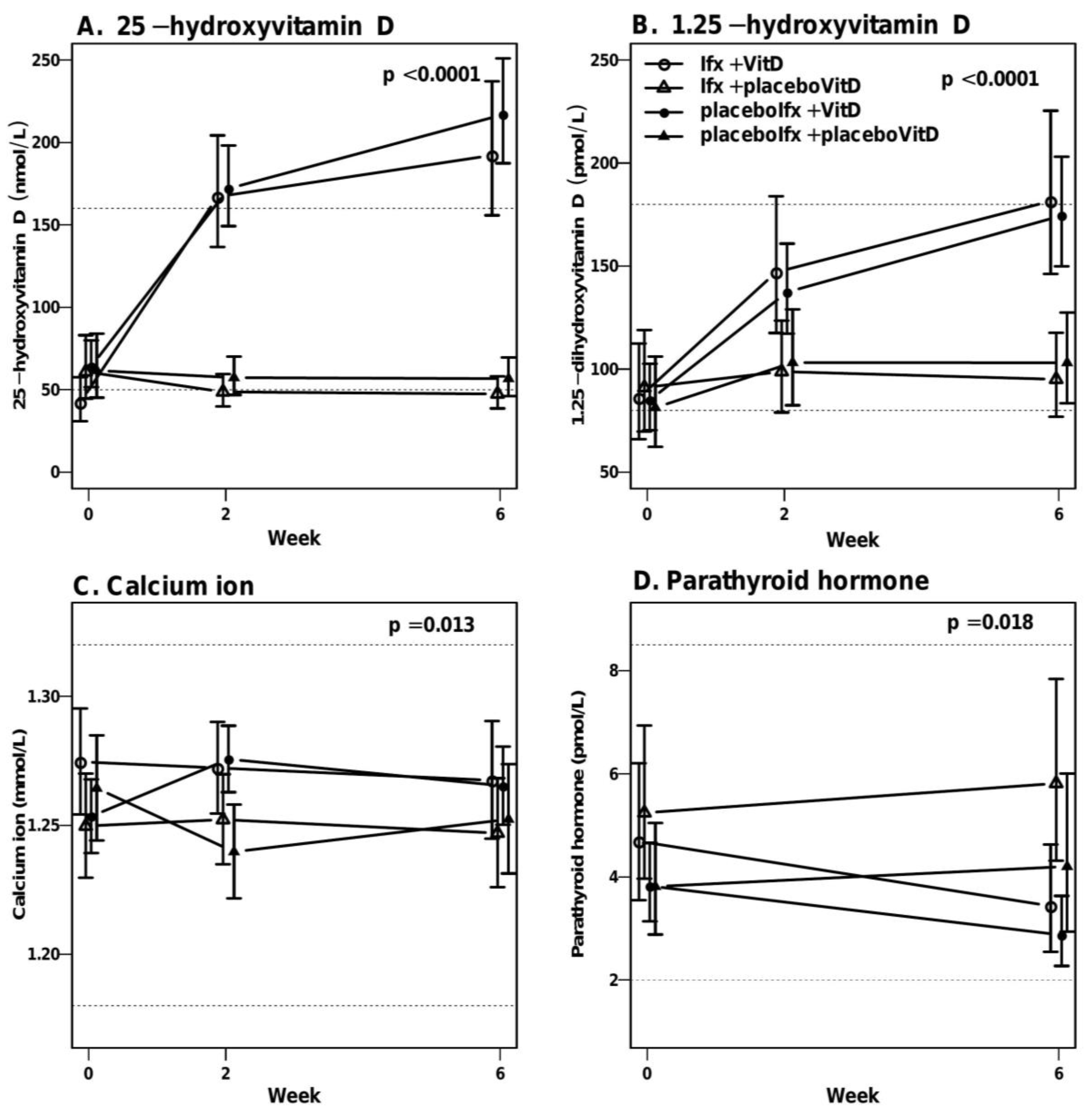
3.6. High-Dose Vitamin D Treatment Is Safe
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; d’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cruz, P.; Kamm, M.A.; Prideaux, L.; Allen, P.B.; Moore, G. Mucosal healing in Crohn’s disease: A systematic review. Inflamm. Bowel Dis. 2013, 19, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.T.; Hatton, R.D. Interplay between the TH17 and Treg cell lineages: A (co-)evolutionary perspective. Nat. Rev. Immunol. 2009, 9, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lopes, J.E.; Chong, M.M.; Ivanov, I.I.; Min, R.; Victora, G.D.; Shen, Y.; Du, J.; Rubtsov, Y.P.; Rudensky, A.Y.; et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 2008, 453, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, K.; Ogawa, A.; Mizoguchi, E.; Shimomura, Y.; Andoh, A.; Bhan, A.K.; Blumberg, R.S.; Xavier, R.J.; Mizoguchi, A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Investig. 2008, 118, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Powell, N.; Pantazi, E.; Pavlidis, P.; Tsakmaki, A.; Li, K.; Yang, F.; Parker, A.; Pin, C.; Cozzetto, D.; Minns, D.; et al. Interleukin-22 orchestrates a pathological endoplasmic reticulum stress response transcriptional programme in colonic epithelial cells. Gut 2020, 69, 578–590. [Google Scholar] [CrossRef] [Green Version]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin D in autoimmunity: Molecular mechanisms and therapeutic potential. Front. Immunol. 2016, 7, 697. [Google Scholar] [CrossRef] [Green Version]
- Frigstad, S.O.; Høivik, M.; Jahnsen, J.; Dahl, S.R.; Cvancarova, M.; Grimstad, T.; Berset, I.P.; Huppertz-Hauss, G.; Hovde, Ø.; Torp, R.; et al. Vitamin D deficiency in inflammatory bowel disease: Prevalence and predictors in a Norwegian outpatient population. Scand. J. Gastroenterol. 2017, 52, 100–106. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D deficiency and the pathogenesis of Crohn’s disease. J. Steroid Biochem. Mol. Biol. 2018, 175, 23–28. [Google Scholar] [CrossRef]
- Jørgensen, S.P.G.; Agnholt, J.; Glerup, H.; Lyhne, S.; Villadsen, G.; Hvas, C.L.; Bartels, L.E.; Kelsen, J.; Christensen, L.A.; Dahlerup, J.F.; et al. Clinical trial: Vitamin D3 treatment in Crohn’s disease—A randomised double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2010, 32, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Zullow, S.; Jambaulikar, G.; Rustgi, A.; Quezada, S.; Cross, R.K. Risk factors for vitamin D deficiency and impact of repletion in a tertiary care inflammatory bowel disease population. Dig. Dis. Sci. 2017, 62, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.W.; Collins, E.; Cao, B.; Carrellas, M.; Crowell, A.M.; Korzenik, J.R. Higher 25-hydroxyvitamin D levels are associated with greater odds of remission with anti-tumour necrosis factor-alpha medications among patients with inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2017, 45, 653–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zator, Z.A.; Cantu, S.M.; Konijeti, G.G.; Nguyen, D.D.; Sauk, J.; Yajnik, V.; Ananthakrishnan, A.N. Pretreatment 25-hydroxyvitamin D levels and durability of anti-tumor necrosis factor-alpha therapy in inflammatory bowel diseases. J. Parenter. Enteral Nutr. 2014, 38, 385–391. [Google Scholar] [CrossRef]
- Schaffler, H.; Schmidt, M.; Huth, A.; Reiner, J.; Glass, A.; Lamprecht, G. Clinical factors are associated with vitamin D levels in IBD patients: A retrospective analysis. J. Dig. Dis. 2018, 19, 24–32. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Yang, L.; Weaver, V.; Smith, J.P.; Bingaman, S.; Hartman, T.J.; Cantorna, M.T. Therapeutic effect of vitamin d supplementation in a pilot study of Crohn’s patients. Clin. Transl. Gastroenterol. 2013, 4, e33. [Google Scholar] [CrossRef]
- Correale, J.; Ysrraelit, M.C.; Gaitan, M.I. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain 2009, 132, 1146–1160. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Qureshi, O.S.; Gardner, D.; Hou, T.Z.; Briggs, Z.; Soskic, B.; Baker, J.; Raza, K.; Sansom, D.M. Vitamin D antagonises the suppressive effect of inflammatory cytokines on CTLA-4 expression and regulatory function. PLoS ONE 2015, 10, e0131539. [Google Scholar] [CrossRef] [Green Version]
- Palmer, M.T.; Lee, Y.K.; Maynard, C.L.; Oliver, J.R.; Bikle, D.D.; Jetten, A.M.; Weaver, C.T. Lineage-specific effects of 1,25-dihydroxyvitamin D(3) on the development of effector CD4 T cells. J. Biol. Chem. 2011, 286, 997–1004. [Google Scholar] [CrossRef] [Green Version]
- Raftery, T.; Martineau, A.R.; Greiller, C.L.; Ghosh, S.; McNamara, D.; Bennett, K.; Meddings, J.; O’Sullivan, M. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: Results from a randomised double-blind placebo-controlled study. United Eur. Gastroenterol. J. 2015, 3, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raftery, T.; Martineau, A.R.; Greiller, C.L.; Ghosh, S.; McNamara, D.; Bennett, K.; Meddings, J.; O’Sullivan, M. IL-23/IL-17 immunity as a hallmark of Crohn’s disease. Inflamm. Bowel Dis. 2008, 14, 1175–1184. [Google Scholar]
- Jiang, W.; Su, J.; Zhang, X.; Cheng, X.; Zhou, J.; Shi, R.; Zhang, H. Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflamm. Res. 2014, 63, 943–950. [Google Scholar] [CrossRef]
- Colin, E.M.; Asmawidjaja, P.S.; van Hamburg, J.P.; Mus, A.M.C.; van Driel, M.; Hazes, J.M.W.; Van Leeuwen, J.P.T.M.; Lubberts, E. 1,25-dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 2010, 62, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Pantalena, L.C.; Liu, X.K.; Gaffen, S.L.; Liu, H.; Rohowsky-Kochan, C.; Ichiyama, K.; Yoshimura, A.; Steinman, L.; Christakos, S.; et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol. Cell Biol. 2011, 31, 3653–3669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.; Pantalena, L.C.; Liu, X.K.; Gaffen, S.L.; Liu, H.; Rohowsky-Kochan, C.; Ichiyama, K.; Yoshimura, A.; Steinman, L.; Christakos, S.; et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: Unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012, 61, 1693–1700. [Google Scholar]
- Gubatan, J.; Mitsuhashi, S.; Longhi, M.S.; Zenlea, T.; Rosenberg, L.; Robson, S.; Moss, A.C. Higher serum vitamin D levels are associated with protective serum cytokine profiles in patients with ulcerative colitis. Cytokine 2018, 103, 38–45. [Google Scholar] [CrossRef]
- Vrablicova, Z.; Soltys, K.; Krajcovicova, A.; Sturdik, I.; Koller, T.; Huorka, M.; Payer, J.; Stuchlik, S.; Killinger, Z.; Jackuliak, P.; et al. Influence of vitamin D on the expression of mRNA of cytokines in the mucosa of inflammatory bowel disease patients. Bratisl. Lek. Listy 2018, 119, 408–415. [Google Scholar] [CrossRef]
- Tan, B.; Li, P.; Lv, H.; Yang, H.; Li, Y.; Li, J.; Wang, O.; Qian, J.M. Treatment of vitamin D deficiency in Chinese inflammatory bowel disease patients: A prospective, randomized, open-label, pilot study. J. Dig. Dis. 2018, 19, 215–224. [Google Scholar] [CrossRef]
- Bak, N.F.; Bendix, M.; Hald, S.; Reinert, L.; Magnusson, M.K.; Agnholt, J. High-dose vitamin D3 supplementation decreases the number of colonic CD103+ dendritic cells in healthy subjects. Eur. J. Nutr. 2017, 57, 2607–2619. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, M.; Saneei, P.; Siassi, F.; Esmaillzadeh, A. Vitamin D status in relation to Crohn’s disease: Meta-analysis of observational studies. Nutrition 2016, 32, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Bendix, M.; Greisen, S.; Dige, A.; Hvas, C.L.; Bak, N.; Jørgensen, S.P.; Dahlerup, J.F.; Deleuran, B.; Agnholt, J. Vitamin D increases programmed death receptor-1 expression in Crohn’s disease. Oncotarget 2017, 8, 24177–24186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
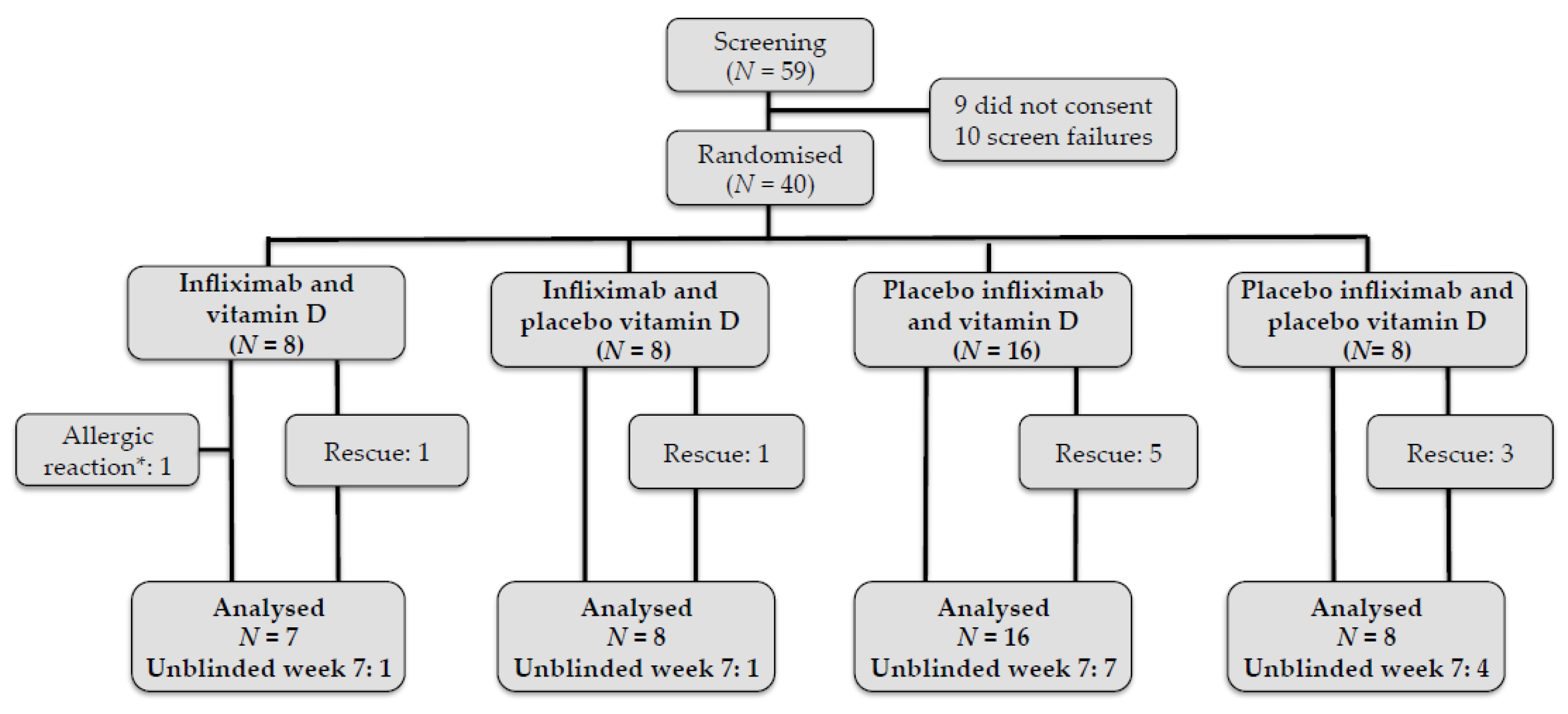
| Ifx + VitD | Ifx + PlaceboVitD | Placeboifx + VitD | PlaceboIfx + placebovitd | |
|---|---|---|---|---|
| n | 8 | 8 | 16 | 8 |
| BMI | 25.0 (21.5–29.9) | 22.8 (20.5–44.3) | 24.8 (18.7–32.9) | 28.8 (23.1–34.2) |
| Years since diagnosis | 2.3 | 3.3 | 1.2 | 1.9 |
| Smoking | 3 (37.5%) | 1 (12.5%) | 1 (6.3%) | 3 (37.5%) |
| Family history of CD or UC | 1 | 2 | 3 | 1 |
| Former or present stenosis | 3 (37.5%) | 3 (37.5%) | 1 (6.3%) | 3 (37.5%) |
| Former abdominal surgery | 1 (12.5%) | 0 | 3 (18.8%) | 1 (12.5%) |
| Former or present fistula | 0 | 1 (12.5%) | 1 (6.3%) | 0 |
| Former or present abscess | 0 | 2 (25%) | 2 (12.5%) | 1 (12.5%) |
| Former or present fissure | 0 | 0 | 1 (6.3%) | 0 |
| Other autoimmune diseases | 0 | 2 (25%) | 2 (12.5%) | 1 (12.5%) |
| Extra intestinal manifestations | 2 (25%) | 2 (25%) | 5 (31.3%) | 0 |
| Fatigue | 4 (50%) | 5 (62.5%) | 13 (81.25%) | 4 (50%) |
| Former treatment with infliximab | 2 (25%) | 3 (37.5%) | 4 (25%) | 1 (12.5%) |
| Former treatment with Adalimumab | 0 | 0 | 2 (12.5%) | 1 (12.5%) |
| Former treatment with other biologicals | 0 | 0 | 1 (6.3%) | 0 |
| Budesonide users (3 mg/day) | 0 | 1 (12.5%) | 0 | 0 |
| Azathioprine users | 3 (37.5%) | 6 (75%) | 3 (18.8%) | 1 (12.5%) |
| HBI | 7 (5–14) | 6.5 (5–16) | 7 (5–11) | 5 (5–10) |
| calprotectin, mg/kg | 884 (114–2174) | 718 (163–3366) | 895 (113–2094) | 714 (256–6000) |
| 25-hydroxyvitamin D, nmol/L | 45 (11–83) | 73 (33–88) | 66.5 (32–94) | 72.5 (21–90) |
| CRP, mg/L | 5.3 (0.6–25.6) | 16.5 (0.6–38.5) | 8.8 (0.3–25) | 6.3 (0.8–45.9) |
| Leucocytes, 109/L | 7.67 (5–11.6) | 7.1 (5.6–9.4) | 7.6 (5.0–11.5) | 8.2 (3.7–15.8) |
| Haemoglobin, mmol/L | 8.7 (7.5–10.2) | 8.6 (6.4–9.5) | 8.5 (6.9–9.7) | 8.5 (6.6–10) |
| SH score | 21 (11–38) | 20 (3–33) | 14.5 (4–36) | 21 (5–33) |
| Endoscopic CDEIS score | 13 (11–32) | 18 (7–33) | 14 (6–29) | 19 (6–49) |
| 1. Ifx + VitD | 2. Ifx + PlaceboVitD | Group 1 vs. 2 | 3. PlaceboIfx + VitD | 4. PlaceboIfx + PlaceboVitD | Group 3 vs. 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week 0: N = 7 Week 7: N = 7 | Week 0: N = 7 Week 7: N = 8 | Week 0: N = 14 Week 7: N = 16 | Week 0: N = 8 Week 7: N = 5 | |||||||
| Gene | Median ratio (95%CI) | p | Median ratio (95%CI) | p | p | Median ratio (95%CI) | p | Median ratio (95%CI) | p | p |
| IL22 | 0.03 (0.005–0.12) | 0.00001 | 0.12 (0.03–0.46) | 0.002 | 0.15 | 0.47 (0.17–1.26) | 0.13 | 0.57 (0.12–2.62) | 0.47 | 0.83 |
| VDR | 0.7 (0.34–1.45) | 0.34 | 0.83 (0.42–1.67) | 0.61 | 0.34 | 1.37 (0.84–2.22) | 0.21 | 0.72 (0.32–1.62) | 0.42 | 0.18 |
| IL17F | 0.25 (0.11–0.6) | 0.002 | 0.36 (0.16–0.81) | 0.013 | 0.55 | 0.92 (0.5–1.69) | 0.78 | 0.97 (0.39–2.44) | 0.95 | 0.92 |
| IL17A | 0.19 (0.07–0.55) | 0.002 | 0.39 (0.14–1.07) | 0.07 | 0.34 | 0.45 (0.22–0.95) | 0.04 | 0.61 (0.19–1.94) | 0.40 | 0.67 |
| 1. Iflx + VitD | 2. Iflx + placeboVitD | Group 1 vs. 2 | 3. PlaceboIfx + VitD | 4. PlaceboIfx + PlaceboVitD | Group 3 vs. 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week 0: N = 7 Week 7: N = 7 | Week 0: N = 7 Week 7: N = 8 | Week 0: N = 14 Week 7: N = 16 | Week 0: N = 8 Week 7: N = 5 | |||||||
| Gene | Median ratio (95%CI) | p | Median ratio (95%CI) | p | p | Median ratio (95%CI) | p | Median ratio (95%CI) | p | p |
| TGF-β | 0.82 (0.45–1.52) | 0.53 | 0.73 (0.41–1.29) | 0.28 | 0.77 | 0.70 (0.47–1.05) | 0.09 | 1.17 (0.59–2.31) | 0.65 | 0.21 |
| IL10 | 0.49 (0.27–0.88) | 0.02 | 0.75 (0.43–1.3) | 0.31 | 0.3 | 0.63 (0.43–0.93) | 0.02 | 0.92 (0.48–1.75) | 0.80 | 0.33 |
| IFNγ | 0.21 (0.07–0.59) | 0.003 | 0.37 (0.14–1.00) | 0.05 | 0.43 | 0.47 (0.23–0.95) | 0.03 | 0.96 (0.30–3.02) | 0.94 | 0.3 |
| TNF-α | 0.61 (0.32–1.13) | 0.12 | 0.47 (0.25–0.85) | 0.01 | 0.55 | 0.68 (0.45–1.03) | 0.07 | 1.20 (0.59–2.45) | 0.62 | 0.18 |
| CAMP | 0.39 (0.16–0.93) | 0.03 | 0.49 (0.21–1.12) | 0.09 | 0.71 | 1.05 (0.59–1.86) | 0.88 | 1.26 (0.47–3.37) | 0.64 | 0.75 |
| CYP27B1 * | 0.03 (0.005–0.14) | 0.0001 | 0.05 (0.01–0.20) | 0.0001 | 0.57 | 0.52 (0.20–1.39) | 0.2 | 1.16 (0.25–5.33) | 0.84 | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendix, M.; Dige, A.; Jørgensen, S.P.; Dahlerup, J.F.; Bibby, B.M.; Deleuran, B.; Agnholt, J. Decrease in Mucosal IL17A, IFNγ and IL10 Expressions in Active Crohn’s Disease Patients Treated with High-Dose Vitamin D Alone or Combined with Infliximab. Nutrients 2020, 12, 3699. https://doi.org/10.3390/nu12123699
Bendix M, Dige A, Jørgensen SP, Dahlerup JF, Bibby BM, Deleuran B, Agnholt J. Decrease in Mucosal IL17A, IFNγ and IL10 Expressions in Active Crohn’s Disease Patients Treated with High-Dose Vitamin D Alone or Combined with Infliximab. Nutrients. 2020; 12(12):3699. https://doi.org/10.3390/nu12123699
Chicago/Turabian StyleBendix, Mia, Anders Dige, Søren Peter Jørgensen, Jens Frederik Dahlerup, Bo Martin Bibby, Bent Deleuran, and Jørgen Agnholt. 2020. "Decrease in Mucosal IL17A, IFNγ and IL10 Expressions in Active Crohn’s Disease Patients Treated with High-Dose Vitamin D Alone or Combined with Infliximab" Nutrients 12, no. 12: 3699. https://doi.org/10.3390/nu12123699
APA StyleBendix, M., Dige, A., Jørgensen, S. P., Dahlerup, J. F., Bibby, B. M., Deleuran, B., & Agnholt, J. (2020). Decrease in Mucosal IL17A, IFNγ and IL10 Expressions in Active Crohn’s Disease Patients Treated with High-Dose Vitamin D Alone or Combined with Infliximab. Nutrients, 12(12), 3699. https://doi.org/10.3390/nu12123699






