Se Status Prediction by Food Intake as Compared to Circulating Biomarkers in a West Algerian Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Samples
2.2. Dietary Intake Assessment
2.3. Selenium Status Assessment
2.4. Statistical Analysis
3. Results
3.1. Characterisation of the Study Cohort
3.2. Assessment of Se Intake via the Food Categories Using Reference Composition Data
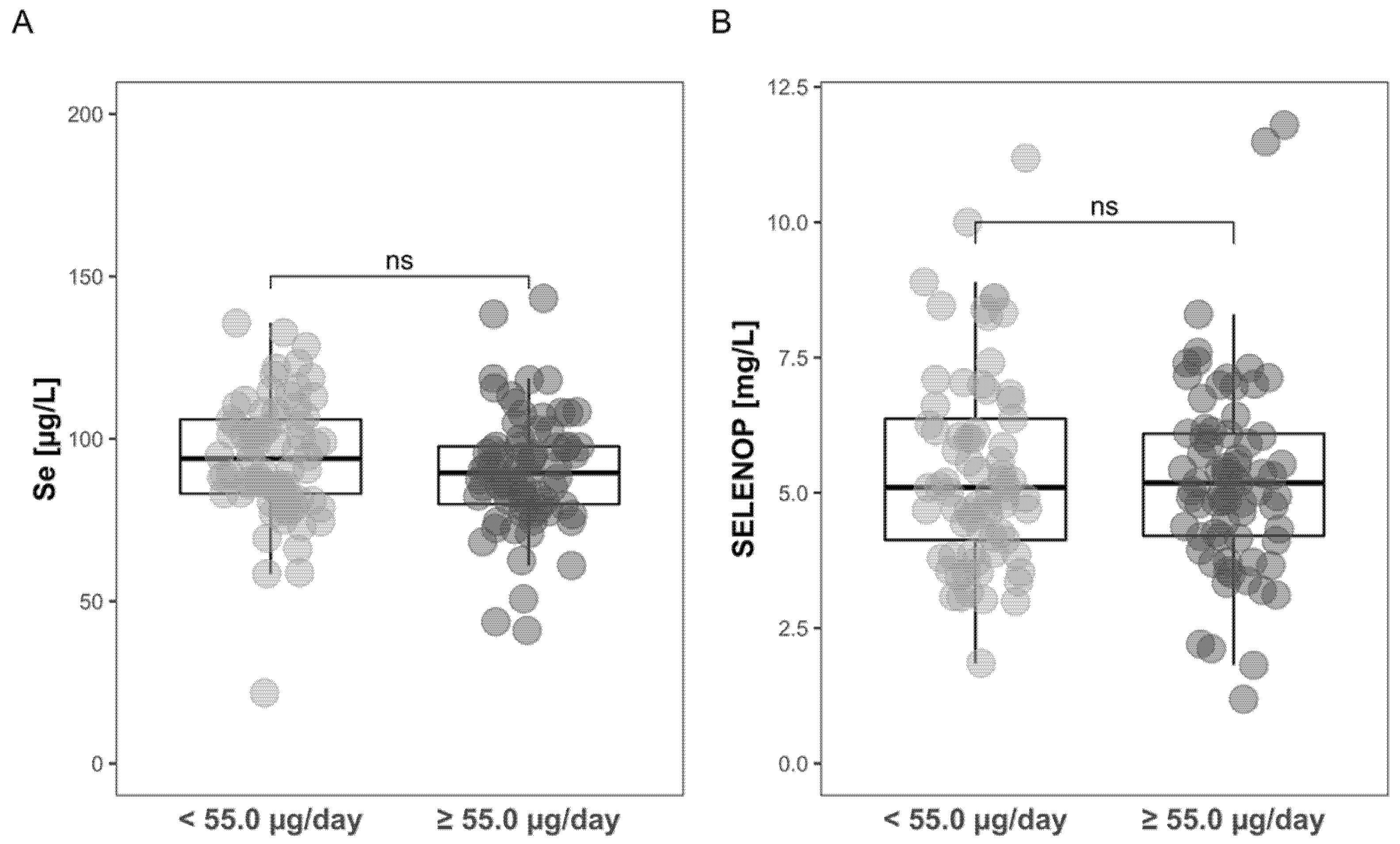
3.3. Comparison of Intake-Deduced Se Status with Plasma Se Status Biomarkers
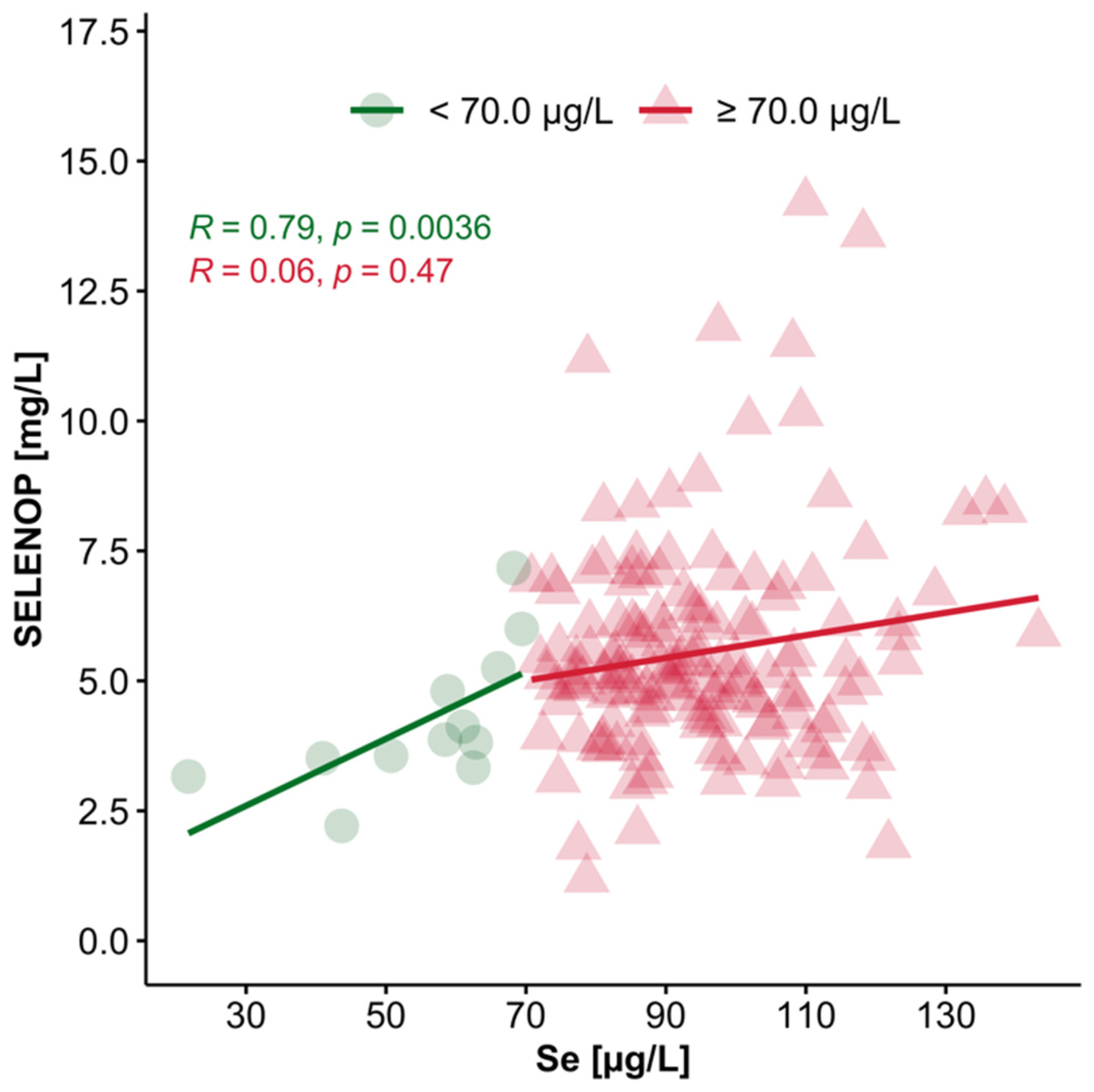
3.4. Interrelation of Plasma Se and SELENOP Concentrations in Se-Deficient vs. Se-Replete Subjects
3.5. Interrelation of GPX3 Activity with Se Intake, Plasma Se and SELENOP
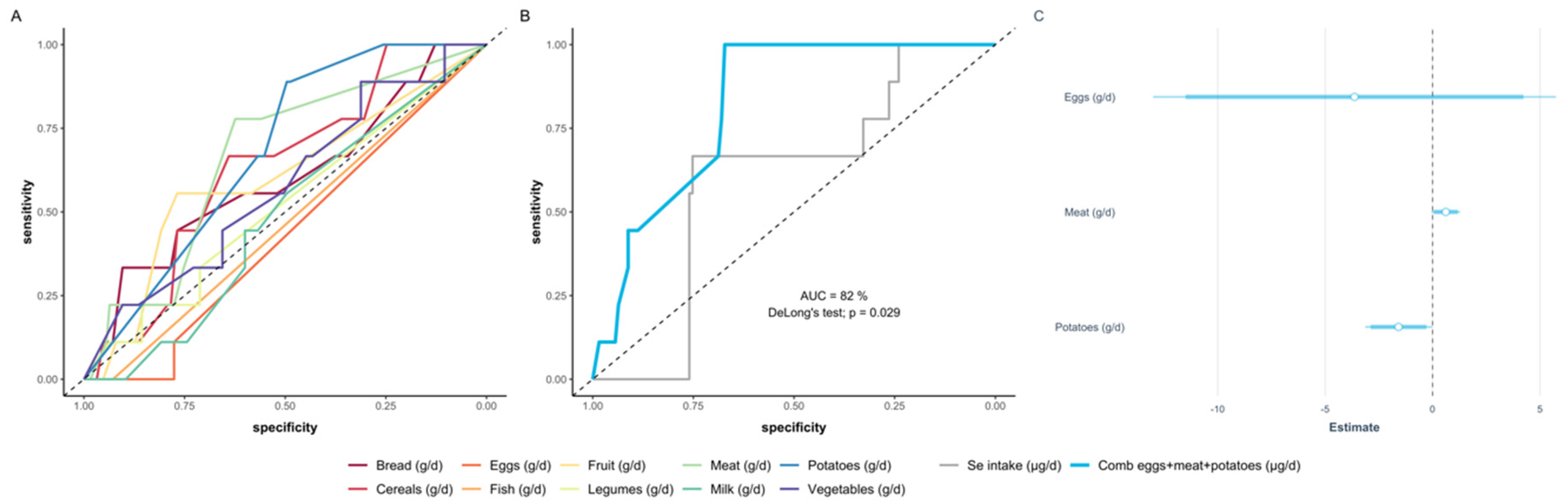
3.6. Deducing a Model of Food Intake according to Food Categories Predicting Se Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stoffaneller, R.; Morse, N.L. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, A.R.; El-Ramady, H.; Santos, E.F.; Gratao, P.L.; Schomburg, L. Overview of Selenium Deficiency and Toxicity Worldwide: Affected Areas, Selenium-Related Health Issues, and Case Studies. Plant Ecophysiol. 2017, 11, 209–230. [Google Scholar] [CrossRef]
- Kieliszek, M.; Blazejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef]
- Combs, G.F., Jr. Biomarkers of selenium status. Nutrients 2015, 7, 2209–2236. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Sunde, R.A.; Li, J.L.; Taylor, R.M. Insights for Setting of Nutrient Requirements, Gleaned by Comparison of Selenium Status Biomarkers in Turkeys and Chickens versus Rats, Mice, and Lambs. Adv. Nutr. 2016, 7, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L. The other view: The trace element selenium as a micronutrient in thyroid disease, diabetes, and beyond. Hormones 2020, 19, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Schomburg, L.; Schweizer, U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim. Biophys. Acta 2009, 1790, 1453–1462. [Google Scholar] [CrossRef]
- Hoeflich, J.; Hollenbach, B.; Behrends, T.; Hoeg, A.; Stosnach, H.; Schomburg, L. The choice of biomarkers determines the selenium status in young German vegans and vegetarians. Br. J. Nutr. 2010, 104, 1601–1604. [Google Scholar] [CrossRef]
- Xia, Y.M.; Hill, K.E.; Li, P.; Xu, J.Y.; Zhou, D.; Motley, A.K.; Wang, L.; Byrne, D.W.; Burk, R.F. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: A placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am. J. Clin. Nutr. 2010, 92, 525–531. [Google Scholar] [CrossRef]
- Hurst, R.; Armah, C.N.; Dainty, J.R.; Hart, D.J.; Teucher, B.; Goldson, A.J.; Broadley, M.R.; Motley, A.K.; Fairweather-Tait, S.J. Establishing optimal selenium status: Results of a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2010, 91, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohe, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H.; German Nutrition, S. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Ward-Deitrich, C.L.; Whyte, E.; Hopley, C.; Rayman, M.P.; Ogra, Y.; Goenaga-Infante, H. Systematic study of the selenium fractionation in human plasma from a cancer prevention trial using HPLC hyphenated to ICP-MS and ESI-MS/MS. Anal. Bioanal. Chem. 2020, 657, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press (US): Washington, DC, USA, 2000; ISBN 0-309-06949-1. [Google Scholar]
- Longnecker, M.P.; Taylor, P.R.; Levander, O.A.; Howe, M.; Veillon, C.; McAdam, P.A.; Patterson, K.Y.; Holden, J.M.; Stampfer, M.J.; Morris, J.S.; et al. Selenium in diet, blood, and toenails in relation to human health in a seleniferous area. Am. J. Clin. Nutr. 1991, 53, 1288–1294. [Google Scholar] [CrossRef]
- Yang, G.Q.; Wang, S.Z.; Zhou, R.H.; Sun, S.Z. Endemic selenium intoxication of humans in China. Am. J. Clin. Nutr. 1983, 37, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.S.; Crane, S.B. Selenium toxicity from a misformulated dietary supplement, adverse health effects, and the temporal response in the nail biologic monitor. Nutrients 2013, 5, 1024–1057. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef]
- Schomburg, L.; Orho-Melander, M.; Struck, J.; Bergmann, A.; Melander, O. Selenoprotein-P Deficiency Predicts Cardiovascular Disease and Death. Nutrients 2019, 11, 1852. [Google Scholar] [CrossRef]
- Beck, M.A.; Levander, O.A.; Handy, J. Selenium deficiency and viral infection. J. Nutr. 2003, 133, 1463S–1467S. [Google Scholar] [CrossRef]
- Meyer, H.A.; Endermann, T.; Stephan, C.; Stoedter, M.; Behrends, T.; Wolff, I.; Jung, K.; Schomburg, L. Selenoprotein P status correlates to cancer-specific mortality in renal cancer patients. PLoS ONE 2012, 7, e46644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Forceville, X.; Vitoux, D.; Gauzit, R.; Combes, A.; Lahilaire, P.; Chappuis, P. Selenium, systemic immune response syndrome, sepsis, and outcome in critically ill patients. Crit. Care Med. 1998, 26, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, M.; Kusmenkov, T.; Zuck, C.; Angstwurm, M.; Becker, N.P.; Bocker, W.; Schomburg, L.; Bogner-Flatz, V. Selenium and Selenoprotein P Deficiency Correlates With Complications and Adverse Outcome After Major Trauma. Shock 2020, 53, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Pfrimer, K.; Doreste-Alonso, J.; Ribas-Barba, L.; Sanchez-Villegas, A.; Ortiz-Andrellucchi, A.; Henriquez-Sanchez, P. Dietary assessment methods for intakes of iron, calcium, selenium, zinc and iodine. Br. J. Nutr. 2009, 102, S38–S55. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef]
- Voici Quelques Photos Pour Aider vos Patients à Estimer les Quantités. Available online: https://nutrition.pasteur-lille.fr/fileadmin/user_upload/nutrition/nutrigold_outil_de_calcul_pour_les_medecins.pdf (accessed on 29 October 2020).
- ANSES French Food Composition Table Ciqual 2017. Available online: https://ciqual.anses.fr/# (accessed on 29 October 2020).
- Hughes, D.J.; Fedirko, V.; Jenab, M.; Schomburg, L.; Meplan, C.; Freisling, H.; Bueno-de-Mesquita, H.B.; Hybsier, S.; Becker, N.P.; Czuban, M.; et al. Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. Int. J. Cancer 2015, 136, 1149–1161. [Google Scholar] [CrossRef]
- Combs, G.F.; Watts, J.C.; Jackson, M.I.; Johnson, L.K.; Zeng, H.; Scheett, A.J.; Uthus, E.O.; Schomburg, L.; Hoeg, A.; Hoefig, C.S.; et al. Determinants of selenium status in healthy adults. Nutr. J. 2011, 10, 75. [Google Scholar] [CrossRef]
- Hybsier, S.; Schulz, T.; Wu, Z.; Demuth, I.; Minich, W.B.; Renko, K.; Rijntjes, E.; Kohrle, J.; Strasburger, C.J.; Steinhagen-Thiessen, E.; et al. Sex-specific and inter-individual differences in biomarkers of selenium status identified by a calibrated ELISA for selenoprotein P. Redox Biol. 2017, 11, 403–414. [Google Scholar] [CrossRef]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Variable selection with stepwise and best subset approaches. Ann. Transl. Med. 2016, 4, 136. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. Likelihood of a model and information criteria. J. Econ. 1981, 16, 3–14. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Wickham, H. Tidyverse: Easily Install and Load the ‘Tidyverse’. Available online: https://CRAN.R-project.org/package=tidyverse (accessed on 29 October 2020).
- Wickham, H.; Francois, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation; R Package Version 0.4. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 29 October 2020).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Muller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Thomson, C.D. Assessment of requirements for selenium and adequacy of selenium status: A review. Eur. J. Clin. Nutr. 2004, 58, 391–402. [Google Scholar] [CrossRef]
- Al-Mubarak, A.A.; Grote Beverborg, N.; Anker, S.D.; Samani, N.J.; Dickstein, K.; Filippatos, G.; van Veldhuisen, D.J.; Voors, A.A.; Bomer, N.; van der Meer, P. A Clinical Tool to Predict Low Serum Selenium in Patients with Worsening Heart Failure. Nutrients 2020, 12, 2541. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Selenoprotein P-expression, functions, and roles in mammals. Biochim. Biophys. Acta 2009, 1790, 1441–1447. [Google Scholar] [CrossRef]
- Renner, R. EPA’s selenium revision heads into murky waters. J. Environ. Monit. 1999, 1, 67N–68N. [Google Scholar] [CrossRef]
- Flohé, L. The labour pains of biochemical selenology: The history of selenoprotein biosynthesis. Biochim. Biophys. Acta 2009, 1790, 1389–1403. [Google Scholar] [CrossRef]
- Winkel, L.H.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Banuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef] [PubMed]
- Dennouni-Medjati, N.; Harek, Y.; Tarik, A.; Lahcene, L. Whole blood selenium levels in healthy adults from the west of Algeria. Biol. Trace Elem. Res. 2012, 147, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Lachili, B.; Faure, H.; Arnaud, J.; Richard, M.J.; Benlatreche, C.; Favier, A.; Roussel, A.M. Blood micronutrients in Algeria, relationships with sex and age. Int. J. Vitam. Nutr. Res. 2001, 71, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Niskar, A.S.; Paschal, D.C.; Kieszak, S.M.; Flegal, K.M.; Bowman, B.; Gunter, E.W.; Pirkle, J.L.; Rubin, C.; Sampson, E.J.; McGeehin, M. Serum Selenium Levels in the US Population: Third National Health and Nutrition Examination Survey, 1988–1994. Biol. Trace Elem. Res. 2003, 91, 1–10. [Google Scholar] [CrossRef]
- Wu, Q.; Rayman, M.P.; Lv, H.; Schomburg, L.; Cui, B.; Gao, C.; Chen, P.; Zhuang, G.; Zhang, Z.; Peng, X.; et al. Low Population Selenium Status Is Associated with Increased Prevalence of Thyroid Disease. J. Clin. Endocrinol. Metab. 2015, 100, 4037–4047. [Google Scholar] [CrossRef]
- González-Estecha, M.; Palazón-Bru, I.; Bodas-Pinedo, A.; Trasobares, E.; Palazón-Bru, A.; Fuentes, M.; Cuadrado-Cenzual, M.A.; Calvo-Manuel, E. Relationship between serum selenium, sociodemographic variables, other trace elements and lipid profile in an adult Spanish population. J. Trace Elem. Med. Biol. 2017, 43, 93–105. [Google Scholar] [CrossRef]
- Mannisto, S.; Virtanen, M.; Mikkonen, T.; Pietinen, P. Reproducibility and validity of a food frequency questionnaire in a case-control study on breast cancer. J. Clin. Epidemiol. 1996, 49, 401–409. [Google Scholar] [CrossRef]
- Combs, G.F. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef]
- Finley, J.W. Selenium accumulation in plant foods. Nutr. Rev. 2005, 63, 196–202. [Google Scholar] [CrossRef]
- Bessaoud, O.; Pellissier, J.P.; Rolland, J.P.; Khechimi, W. Rapport de Synthèse sur L’agriculture en Algérie; CIHEAM-IAMM: Montpellier, France, 2019; p. 82. [Google Scholar]
- Statistiques du Commerce Exterieur de L’algerie Période: Premier Trimestre de L’année. 2020. Available online: https://www.douane.gov.dz/IMG/pdf/rapport_comext_1er_trimestre_20_vf.pdf (accessed on 30 June 2020).
- Hartikainen, H. Biogeochemistry of selenium and its impact on food chain quality and human health. J. Trace Elem. Med. Biol. 2005, 18, 309–318, r 2005 Elsevier GmbH. All rights reserved. [Google Scholar] [CrossRef]
- Beladel, B.; Nedjimi, B.; Mansouri, A.; Tahtat, D.; Belamri, M.; Tchanchane, A.; Khelfaoui, F.; Benamar, M.E. Selenium content in wheat and estimation of the selenium daily intake in different regions of Algeria. Appl. Radiat. Isot. 2013, 71, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Office Algerien Interprofessionnel des Cereales (OAIC), Algeria-Company Information. Available online: https://www.zawya.com/mena/en/company/Office_Algerien_Interprofessionnel_des_Cereales-705737/ (accessed on 27 July 2020).
- Adriano, D.C. Biogeochemistry of Trace Metals; Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 22–45. [Google Scholar]
- Shen, J.; Jiang, C.; Yan, Y.; Zu, C. Selenium Distribution and Translocation in Rice (Oryza sativa L.) under Different Naturally Seleniferous Soils. Sustainability 2019, 11, 520. [Google Scholar] [CrossRef]
- Sun, H.B. Associations of Spatial Disparities of Alzheimer’s Disease Mortality Rates with Soil Selenium and Sulfur Concentrations and Four Common Risk Factors in the United States. J. Alzheimers Dis. 2017, 58, 897–907. [Google Scholar] [CrossRef] [PubMed]
- D’Odorico, P.; Carr, J.; Dalin, J.; Dell’Angello, J.; Konar, M.; Laio, F.; Ridolfi, L.; Rosa, L.; Suweis, S.; Tamea, S.; et al. Global virtual water trade and the hydrogical cycle: Patterns, drivers, and socio-environmental impacts. Environ. Res. Lett. 2019, 14, 053001. [Google Scholar] [CrossRef]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef] [PubMed]
| Se-Deficient | Se-Replete | Total | p Value | |
|---|---|---|---|---|
| Total number (n) | 12 | 146 | 158 | |
| Age | 0.728 | |||
| median (IQR) | 45 (30, 69) | 46 (19, 90) | 46 (19, 90) | |
| mean (95% CI) | 47 (38, 56) | 49 (46, 51) | 49 (46, 51) | |
| BMI | 0.208 | |||
| median (IQR) | 25.4 (19.5, 32.5) | 27.2 (16.0, 43.2) | 26.8 (16.0, 43.2) | |
| mean (95% CI) | 25.8 (23.0, 28.5) | 27.8 (27.0, 28.7) | 27.7 (26.9, 28.5) | |
| Educational level | 0.151 | |||
| <high school | 6 (54.5%) | 68 (50.0%) | 74 (50.3%) | |
| >high school | 1 (9.1%) | 43 (31.6%) | 44 (29.9%) | |
| high school | 4 (36.4%) | 25 (18.4%) | 29 (19.7%) | |
| Family income (k DZD) * | 0.130 | |||
| median (IQR) | 28.0 (6.0, 200.0) | 50.0 (8.0, 200.0) | 47.5 (6.0, 200.0) | |
| mean (95% CI) | 48.9 (13.7, 111.4) | 54.7 (46.1, 63.4) | 54.3 (45.6, 63.0) | |
| Gender | 0.416 | |||
| Female | 9 (75.0%) | 119 (84.4%) | 128 (83.7%) | |
| Male | 3 (25.0%) | 22 (15.6%) | 25 (16.3%) | |
| Hypertension | 0.469 | |||
| No | 7 (63.6%) | 103 (75.7%) | 110 (74.8%) | |
| Yes | 4 (36.4%) | 33 (24.3%) | 37 (25.2%) | |
| Marital status | 0.626 | |||
| Married | 9 (81.8%) | 120 (88.2%) | 129 (87.8%) | |
| Single | 2 (18.2%) | 16 (11.8%) | 18 (12.2%) | |
| Smoking | 0.510 | |||
| No | 9 (81.8%) | 95 (69.9%) | 104 (70.7%) | |
| Yes | 2 (18.2%) | 41 (30.1%) | 43 (29.3%) | |
| SELENOP (mg/L) | 0.010 | |||
| median (IQR) | 3.84 (2.21, 7.17) | 5.20 (1.19, 14.21) | 5.11 (1.19, 14.21) | |
| mean (95% CI) | 4.23 (3.37, 5.10) | 5.56 (5.22, 5.89) | 5.46 (5.14, 5.78) |
| Se-Deficient | Se-Replete | Total | p-Value | |
|---|---|---|---|---|
| Bread (g/day) | 0.307 | |||
| mean (95% CI) | 114 (45, 184) | 154 (136, 171) | 151 (134, 168) | |
| Cereals (g/day) | 0.176 | |||
| mean (95% CI) | 152 (96, 208) | 204 (184, 224) | 201 (181, 220) | |
| Eggs (g/day) | 0.344 | |||
| mean (95% CI) | 0.3 (−0.4, 1.1) | 23.0 (13.4, 32.6) | 21.5 (12.5, 30.5) | |
| Fish & Seafood (g/day) | 0.382 | |||
| mean (95% CI) | 0.0 (0.0, 0.0) | 9.1 (2.8, 15.4) | 8.4 (2.6, 14.3) | |
| Fresh fruits (g/day) | 0.263 | |||
| mean (95% CI) | 122.2 (24.0, 220.4) | 80.4 (58.0, 102.8) | 83.2 (61.5, 104.8) | |
| Legumes (g/day) | 0.800 | |||
| mean (95% CI) | 53.4 (48.6, 155.3) | 41.9 (24.1, 59.7) | 42.6 (25.2, 60.1) | |
| Meat (g/day) | 0.073 | |||
| mean (95% CI) | 67.8 (17.1, 118.4) | 39.1 (28.3, 49.9) | 41.0 (30.5, 51.6) | |
| Milk (g/day) | 0.814 | |||
| mean (95% CI) | 56 (1.9, 109) | 85 (57, 113) | 83 (57, 110) | |
| Potatoes (g/day) | 0.054 | |||
| mean (95% CI) | 22.2 (−5.7, 50.1) | 85.9 (67.5, 104.4) | 81.7 (64.2, 99.1) | |
| Vegetables (g/day) | 0.443 | |||
| mean (95% CI) | 201.1 (84.3, 318.0) | 253.4 (222.8, 284.0) | 249.9 (220.6, 279.3) | |
| Calculated Se Intake (µg/day) | 0.669 | |||
| median (IQR) | 77.0 (36.6, 84.8) | 54.5 (17.8, 247.3) | 55.2 (17.8, 247.3) | |
| mean (95% CI) | 62.5 (48.6, 76.4) | 62.7 (57.0, 68.3) | 62.7 (57.4, 68.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belhadj, M.; Kazi Tani, L.S.; Dennouni Medjati, N.; Harek, Y.; Dali Sahi, M.; Sun, Q.; Heller, R.; Behar, A.; Charlet, L.; Schomburg, L. Se Status Prediction by Food Intake as Compared to Circulating Biomarkers in a West Algerian Population. Nutrients 2020, 12, 3599. https://doi.org/10.3390/nu12123599
Belhadj M, Kazi Tani LS, Dennouni Medjati N, Harek Y, Dali Sahi M, Sun Q, Heller R, Behar A, Charlet L, Schomburg L. Se Status Prediction by Food Intake as Compared to Circulating Biomarkers in a West Algerian Population. Nutrients. 2020; 12(12):3599. https://doi.org/10.3390/nu12123599
Chicago/Turabian StyleBelhadj, Moussa, Latifa Sarra Kazi Tani, Nouria Dennouni Medjati, Yahia Harek, Majda Dali Sahi, Qian Sun, Raban Heller, Ammaria Behar, Laurent Charlet, and Lutz Schomburg. 2020. "Se Status Prediction by Food Intake as Compared to Circulating Biomarkers in a West Algerian Population" Nutrients 12, no. 12: 3599. https://doi.org/10.3390/nu12123599
APA StyleBelhadj, M., Kazi Tani, L. S., Dennouni Medjati, N., Harek, Y., Dali Sahi, M., Sun, Q., Heller, R., Behar, A., Charlet, L., & Schomburg, L. (2020). Se Status Prediction by Food Intake as Compared to Circulating Biomarkers in a West Algerian Population. Nutrients, 12(12), 3599. https://doi.org/10.3390/nu12123599






