Scopolin Attenuates Osteoporotic Bone Loss in Ovariectomized Mice
Abstract
:1. Introduction
2. Materials and Methods
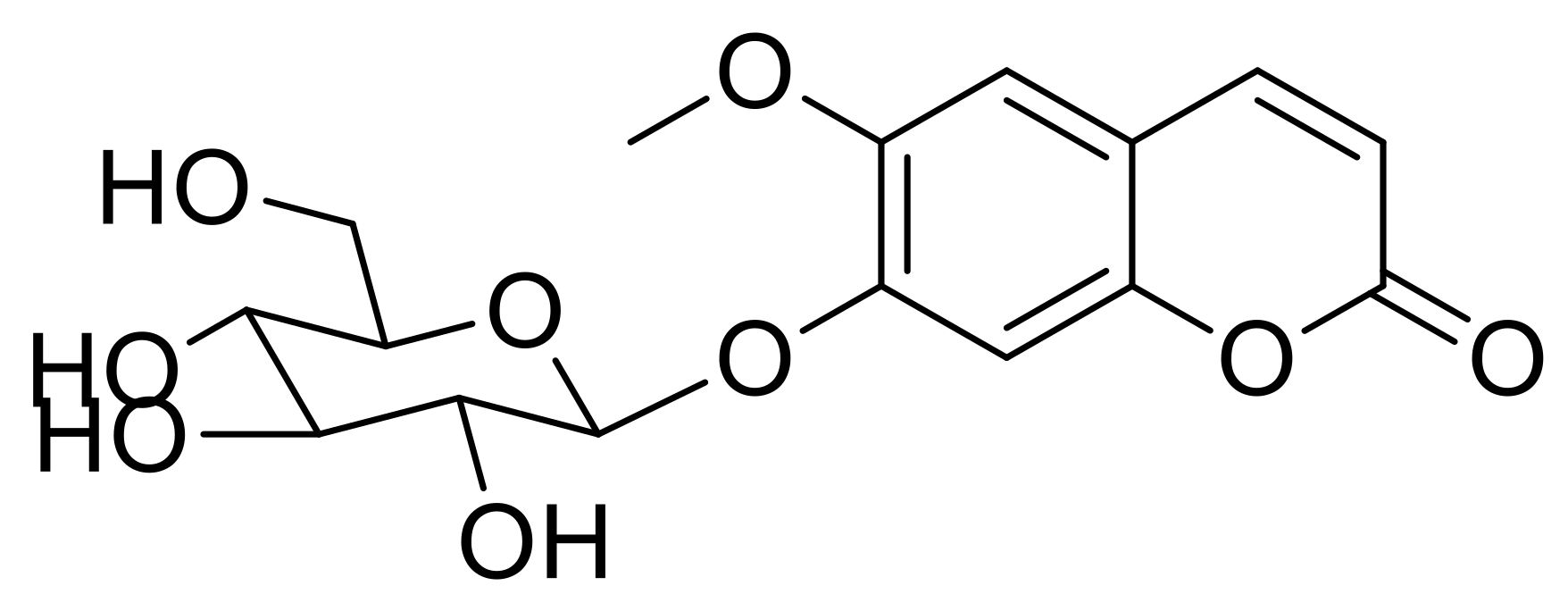
2.1. Fractionation, Isolation, and Structure Elucidation
2.2. Reagents and Cell Culture
2.3. Water-Soluble Tetrazolium Salt (WST) Assay
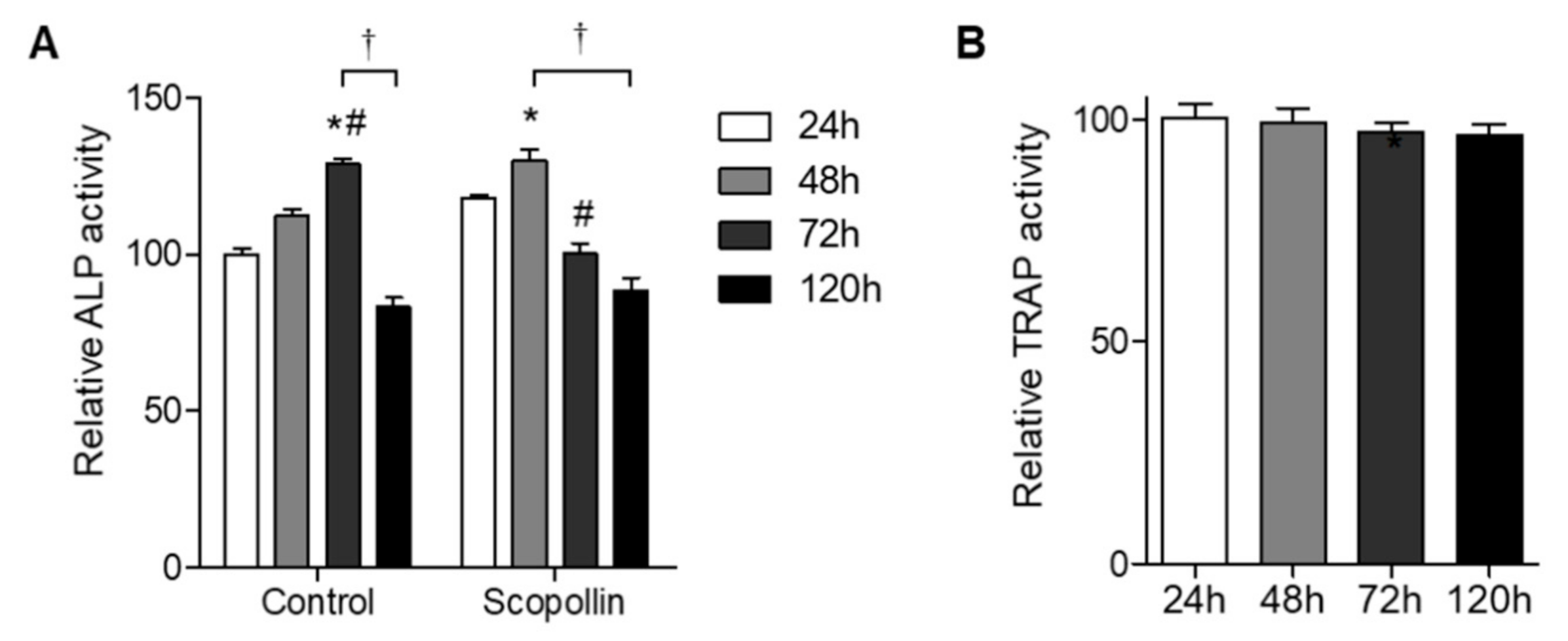
2.4. ALP Activity and Staining
2.5. Analysis of Mineralized Nodule Formation in Osteoblast Cells
2.6. In Vitro Generation of Osteoclasts and the Co-Culture System
2.7. TRAP Staining and Activity Assay
2.8. Quantitative Reverse-Transcription PCR (qRT-PCR)
2.9. Ovariectomized Mouse Model
2.10. Measurement of Bone Mineral Density (BMD)
2.11. Microcomputed Tomography (Micro-CT) and Single-Photon Emission Computerized Tomography Scan
2.12. Blood Sampling and Serum Level Measurements
2.13. Statistical Analysis
3. Results
3.1. Screening for Cellular Proliferation and Differentiation in Osteoblastic MC3T3-E1 Cell Lines
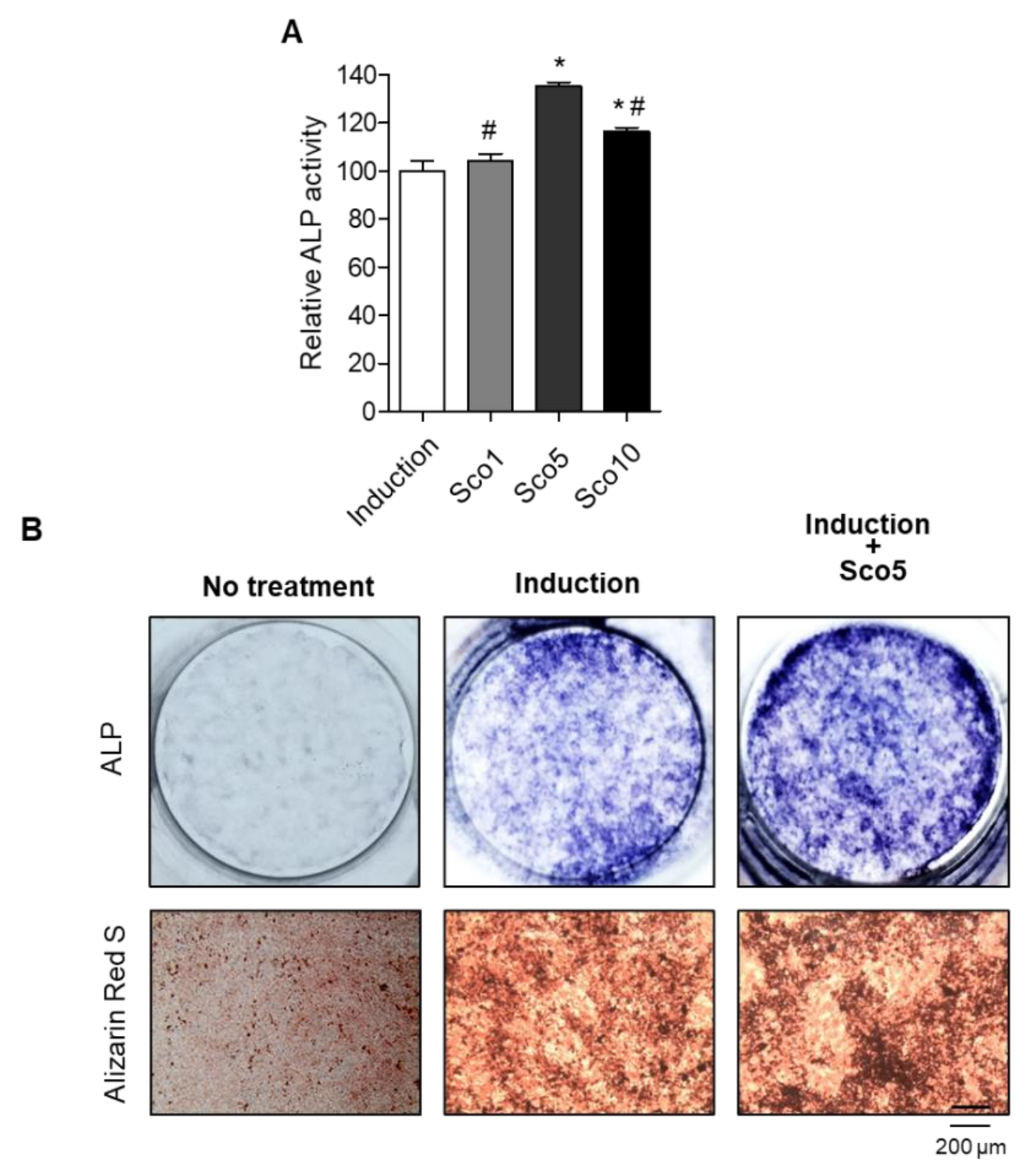
3.2. Scopolin Treatment Increased Osteoblast Differentiation and Mineralized Nodule Formation
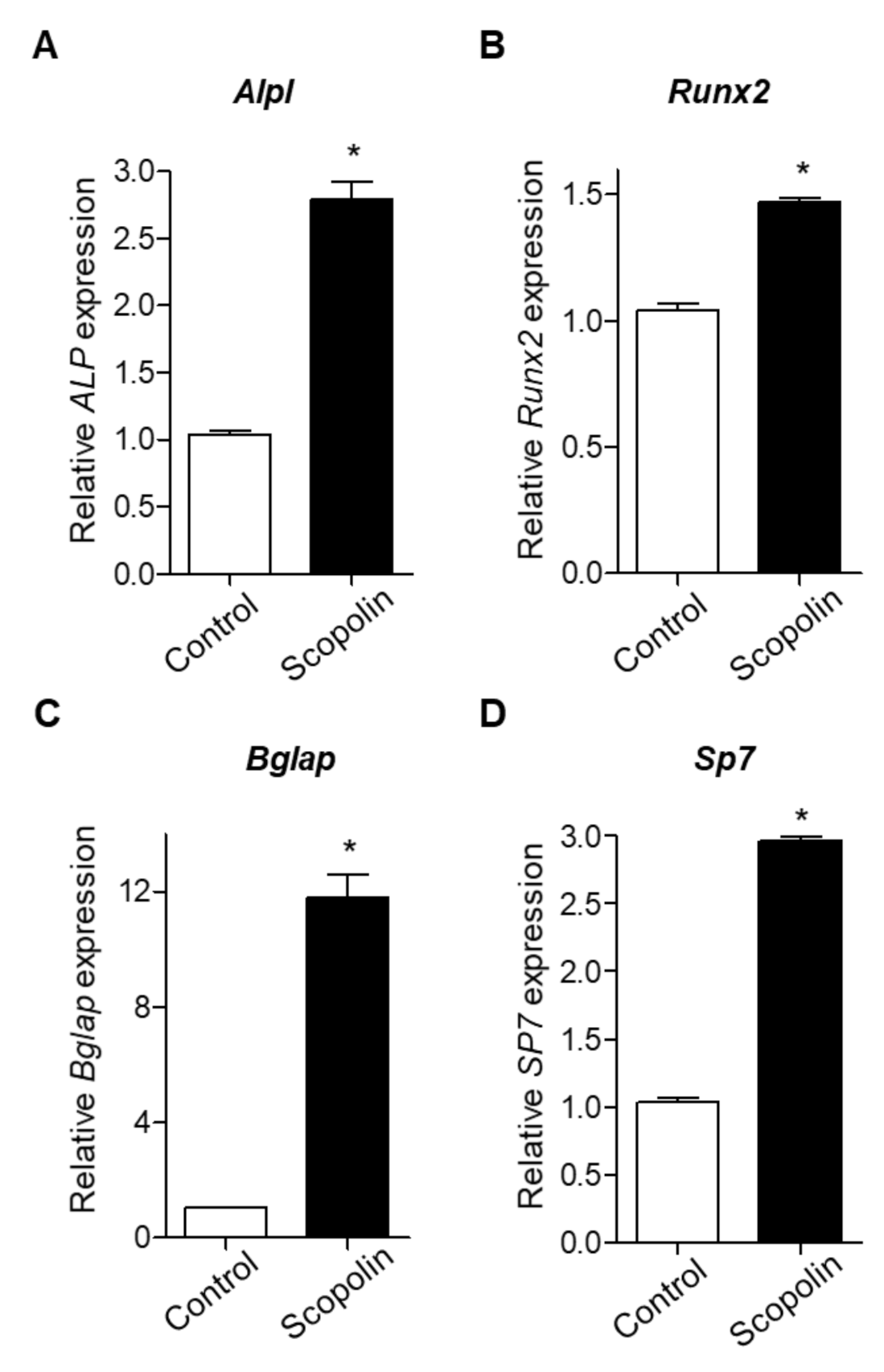
3.3. Scopolin Treatment Increased the mRNA Expression of Osteoblastic Makers
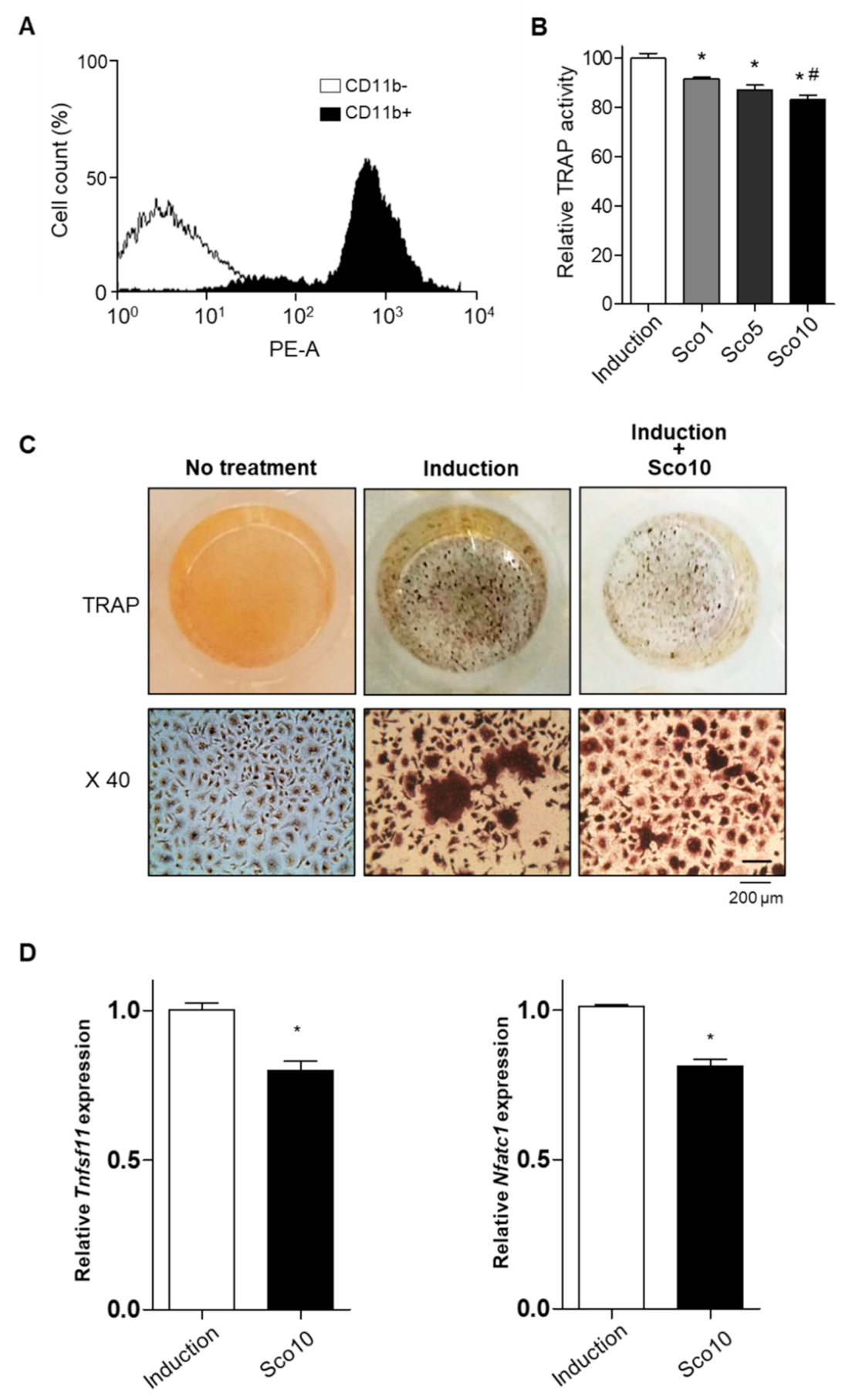
3.4. Scopolin Treatment Decreased Osteoclast Cellular differentiation
3.5. Scopolin Treatment Increased Osteoblast Differentiation in an Isolated Monocyte/MC3T3-E1 Co-Culture System
3.6. Scopolin Treatment Prevented BMD Loss in OVX Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Lee, J.; Jang, J.H.; Sakchaisri, K.; Hwang, J.; Cha-Molstad, H.J.; Kim, K.A.; Ryoo, I.J.; Lee, H.G.; Kim, S.O.; et al. Osteoporosis regulation by salubrinal through eIF2alpha mediated differentiation of osteoclast and osteoblast. Cell. Signal. 2013, 25, 552–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marom, R.; Shur, I.; Solomon, R.; Benayahu, D. Characterization of adhesion and differentiation markers of osteogenic marrow stromal cells. J. Cell. Physiol. 2005, 202, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, H.; Lee, S.H.; Gu, D.R.; Lee, S.Y.; Lee, K.; Jeong, D. ADP-Ribosylation Factor 1 Regulates Proliferation, Migration, and Fusion in Early Stage of Osteoclast Differentiation. Int. J. Mol. Sci. 2015, 16, 29305–29314. [Google Scholar] [CrossRef] [Green Version]
- Sieberath, A.; Della Bella, E.; Ferreira, A.M.; Gentile, P.; Eglin, D.; Dalgarno, K. A Comparison of Osteoblast and Osteoclast In Vitro Co-Culture Models and Their Translation for Preclinical Drug Testing Applications. Int. J. Mol. Sci. 2020, 21, 912. [Google Scholar] [CrossRef] [Green Version]
- Willett, T.L.; Pasquale, J.; Grynpas, M.D. Collagen modifications in postmenopausal osteoporosis: Advanced glycation endproducts may affect bone volume, structure and quality. Curr. Osteoporos. Rep. 2014, 12, 329–337. [Google Scholar] [CrossRef]
- Sambrook, P.; Cooper, C. Osteoporosis. Lancet 2006, 367, 2010–2018. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J. Epidemiology of osteoporotic fractures. Osteoporos. Int. 2005, 16 (Suppl. S2), S3–S7. [Google Scholar] [CrossRef]
- Das, S.; Crockett, J.C. Osteoporosis—A current view of pharmacological prevention and treatment. Drug. Des. Dev. Ther. 2013, 7, 435–448. [Google Scholar]
- Bonura, F. Prevention, screening, and management of osteoporosis: An overview of the current strategies. Postgrad. Med. 2009, 121, 5–17. [Google Scholar] [CrossRef]
- Derman, R.; Kohles, J.D.; Babbitt, A. Gastrointestinal tolerability with ibandronate after previous weekly bisphosphonate treatment. Clin. Interv. Aging 2009, 4, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Baik, O.D.; Choi, Y.J.; Kim, S.M. Pretreatments for the efficient extraction of bioactive compounds from plant-based biomaterials. Crit. Rev. Food. Sci. Nutr. 2014, 54, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.C.; Kim, S.J.; Kim, Y.K.; Kwon, C.H.; Kim, K.H. Lycii cortex radicis extract inhibits glioma tumor growth in vitro and in vivo through downregulation of the Akt/ERK pathway. Oncol. Rep. 2012, 27, 1467–1474. [Google Scholar]
- Ye, Z.; Huang, Q.; Ni, H.X.; Wang, D. Cortex Lycii Radicis extracts improve insulin resistance and lipid metabolism in obese-diabetic rats. Phytother. Res. 2008, 22, 1665–1670. [Google Scholar] [CrossRef]
- Xiaofeng, Y.; Shenggang, L.; Dezhi., Y.; Wang, S. Effects of Cortex Lucii Granules CO. on serum glucose, lipid and immunologic function. Med. J. Qilu 2000, 5, 84–86. [Google Scholar]
- Park, E.; Jin, H.S.; Cho, D.Y.; Kim, J.; Kim, M.C.; Chio, C.W.; Lee, J.W.; Park, J.H.; Chung, Y.S.; Huh, D.; et al. The effect of Lycii Radicis Cortex extract on bone formation in vitro and in vivo. Molecules 2014, 19, 19594–19609. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Shiotsu, M.; Ebihara, T.; Kawai, H.; Ueno, A.; Fukushima, S. Chemical Studies on Viburnum awabuki K.KOCH. Chem. Pharm. Bull. 1986, 34, 4012–4017. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Peng, Y.; Sharrow, A.C.; Iqbal, J.; Zhang, Z.; Papachristou, D.J.; Zaidi, S.; Zhu, L.L.; Yaroslavskiy, B.B.; Zhou, H.; et al. FSH directly regulates bone mass. Cell 2006, 125, 247–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.H.M.; Lim, S.S.; Tiong, T.J.; Show, P.L.; Zaid, H.F.M.; Loh, H.S. Preliminary In Vitro Evaluation of Chitosan-Graphene Oxide Scaffolds on Osteoblastic Adhesion, Proliferation, and Early Differentiation. Int. J. Mol. Sci. 2020, 21, 5202. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Harruff, R.; Ambrosius, W.; Burr, D.B. Trabecular bone volume and microdamage accumulation in the femoral heads of women with and without femoral neck fractures. Bone 1997, 21, 521–526. [Google Scholar] [CrossRef]
- Katarivas Levy, G.; Birch, M.A.; Brooks, R.A.; Neelakantan, S.; Markaki, A.E. Stimulation of Human Osteoblast Differentiation in Magneto-Mechanically Actuated Ferromagnetic Fiber Networks. J. Clin. Med. 2019, 8, 1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, N.B. Clinical utility of biochemical markers of bone remodeling. Clin. Chem. 1999, 8 Pt 2, 1359–1368. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Oida, H.; Kobayashi, T.; Maruyama, T.; Tanaka, M.; Katayama, T.; Yamaguchi, K.; Segi, E.; Tsuboyama, T.; Matsushita, M.; et al. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc. Natl. Acad. Sci. USA 2002, 99, 4580–4585. [Google Scholar] [CrossRef] [Green Version]
- Ekici, H.; Sontas, B.H.; Toydemir, T.S.; Senmevsim, O.; Kabasakal, L.; Imre, Y. Effect of prepubertal ovariohysterectomy on bone mineral density and bone mineral content in puppies. Acta. Vet. Hung. 2005, 53, 469–478. [Google Scholar] [CrossRef]
- Park, E.; Kim, J.; Kim, M.C.; Yeo, S.; Kim, J.; Park, S.; Jo, M.; Choi, C.W.; Jin, H.S.; Lee, S.W.; et al. Anti-Osteoporotic Effects of Kukoamine B Isolated from Lycii Radicis Cortex Extract on Osteoblast and Osteoclast Cells and Ovariectomized Osteoporosis Model Mice. Int. J. Mol. Sci. 2019, 20, 2784. [Google Scholar] [CrossRef] [Green Version]
- Ducy, P.; Schinke, T.; Karsenty, G. The osteoblast: A sophisticated fibroblast under central surveillance. Science 2000, 289, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Gough, J.E.; Jones, J.R.; Hench, L.L. Nodule formation and mineralisation of human primary osteoblasts cultured on a porous bioactive glass scaffold. Biomaterials 2004, 25, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Isotupa, K. Staining properties of alizarin red S for growing bone in vitro. Acta Anat. 1980, 108, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ding, Y.; Yan, X.T.; Kim, Y.H.; Jang, H.D. Scopoletin and scopolin isolated from Artemisia iwayomogi suppress differentiation of osteoclastic macrophage RAW 264.7 cells by scavenging reactive oxygen species. J. Nat. Prod. 2013, 76, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Bain, S.D.; Jerome, C.; Shen, V.; Dupin-Roger, I.; Ammann, P. Strontium ranelate improves bone strength in ovariectomized rat by positively influencing bone resistance determinants. Osteoporos. Int. 2009, 20, 1417–1428. [Google Scholar] [CrossRef] [Green Version]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. Biomed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [Green Version]
- Ominsky, M.S.; Li, X.; Asuncion, F.J.; Barrero, M.; Warmington, K.S.; Dwyer, D.; Stolina, M.; Geng, Z.; Grisanti, M.; Tan, H.L.; et al. RANKL inhibition with osteoprotegerin increases bone strength by improving cortical and trabecular bone architecture in ovariectomized rats. J. Bone. Miner. Res. 2008, 23, 672–682. [Google Scholar] [CrossRef]
- Kapasa, E.R.; Giannoudis, P.V.; Jia, X.; Hatton, P.V.; Yang, X.B. The Effect of RANKL/OPG Balance on Reducing Implant Complications. J. Funct. Biomater. 2017, 8, 42. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.; Kim, J.; Jin, H.-S.; Choi, C.W.; Choi, T.H.; Choi, S.; Huh, D.; Jeong, S.-Y. Scopolin Attenuates Osteoporotic Bone Loss in Ovariectomized Mice. Nutrients 2020, 12, 3565. https://doi.org/10.3390/nu12113565
Park E, Kim J, Jin H-S, Choi CW, Choi TH, Choi S, Huh D, Jeong S-Y. Scopolin Attenuates Osteoporotic Bone Loss in Ovariectomized Mice. Nutrients. 2020; 12(11):3565. https://doi.org/10.3390/nu12113565
Chicago/Turabian StylePark, Eunkuk, Jeonghyun Kim, Hyun-Seok Jin, Chun Whan Choi, Tae Hyun Choi, Sangho Choi, Dam Huh, and Seon-Yong Jeong. 2020. "Scopolin Attenuates Osteoporotic Bone Loss in Ovariectomized Mice" Nutrients 12, no. 11: 3565. https://doi.org/10.3390/nu12113565
APA StylePark, E., Kim, J., Jin, H.-S., Choi, C. W., Choi, T. H., Choi, S., Huh, D., & Jeong, S.-Y. (2020). Scopolin Attenuates Osteoporotic Bone Loss in Ovariectomized Mice. Nutrients, 12(11), 3565. https://doi.org/10.3390/nu12113565






