Sasa quelpaertensis Leaf Extract Ameliorates Dyslipidemia, Insulin Resistance, and Hepatic Lipid Accumulation in High-Fructose-Diet-Fed Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of SQE
2.2. Animals and Diet
2.3. Biochemical Parameter Assays
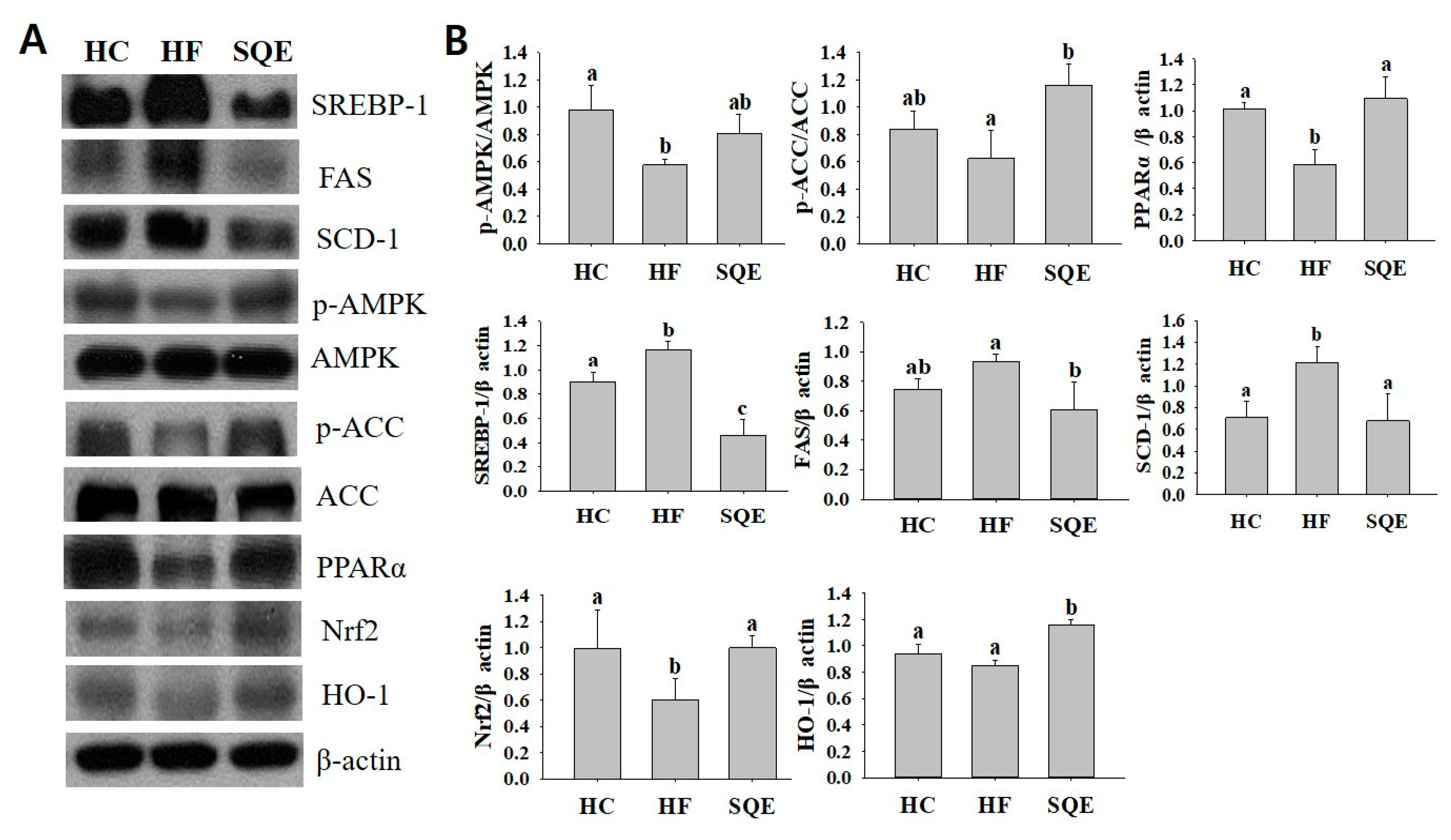
2.4. Western Blot Analysis
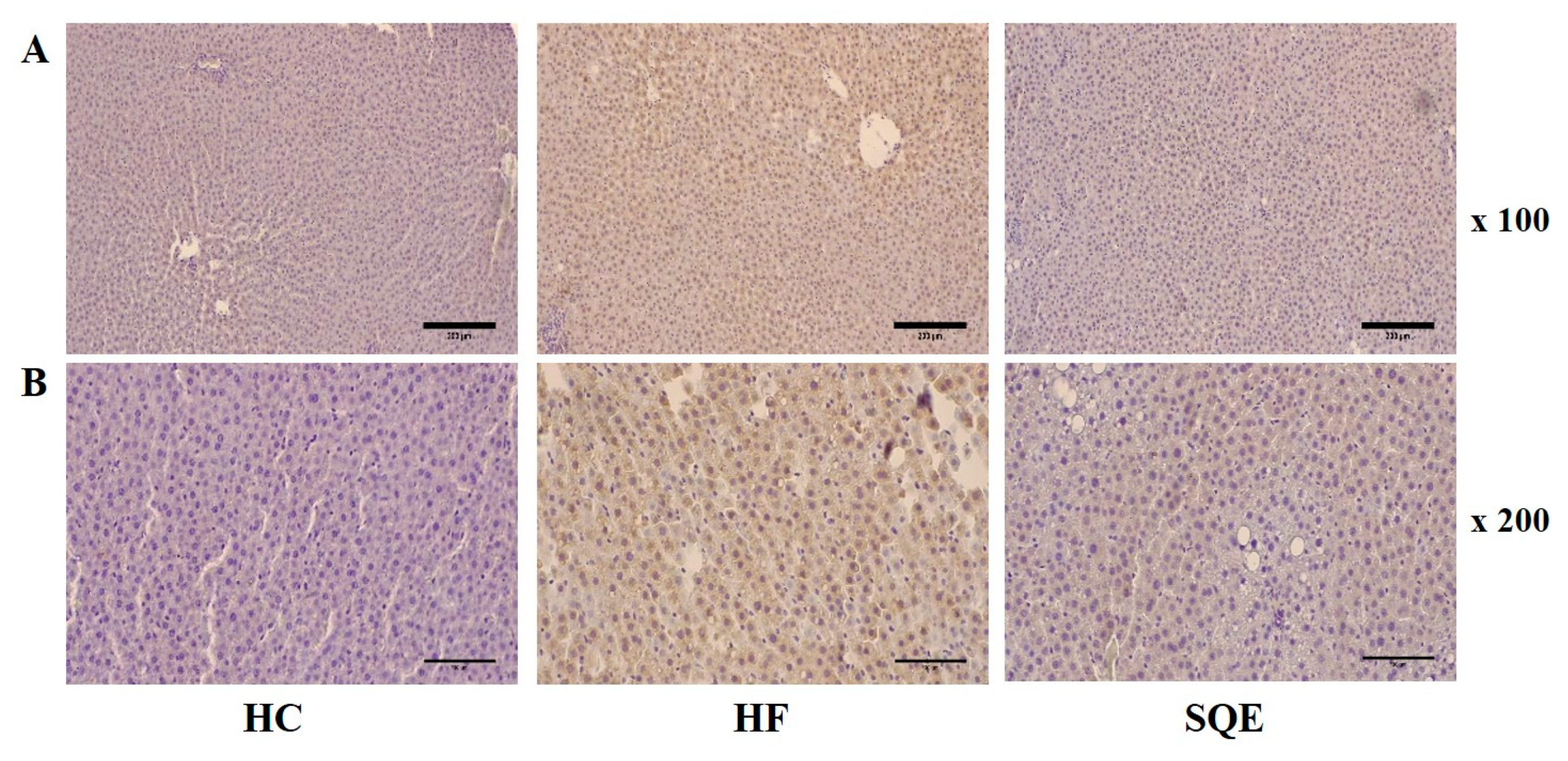
2.5. Histological Analysis
2.6. RNA Extraction, Library, and Sequencing
2.7. mRNA-Seq Data and Gene Expression Level
2.8. Statistical Analysis
3. Results
3.1. Effect of SQE on Changes in Body and Organ Weight
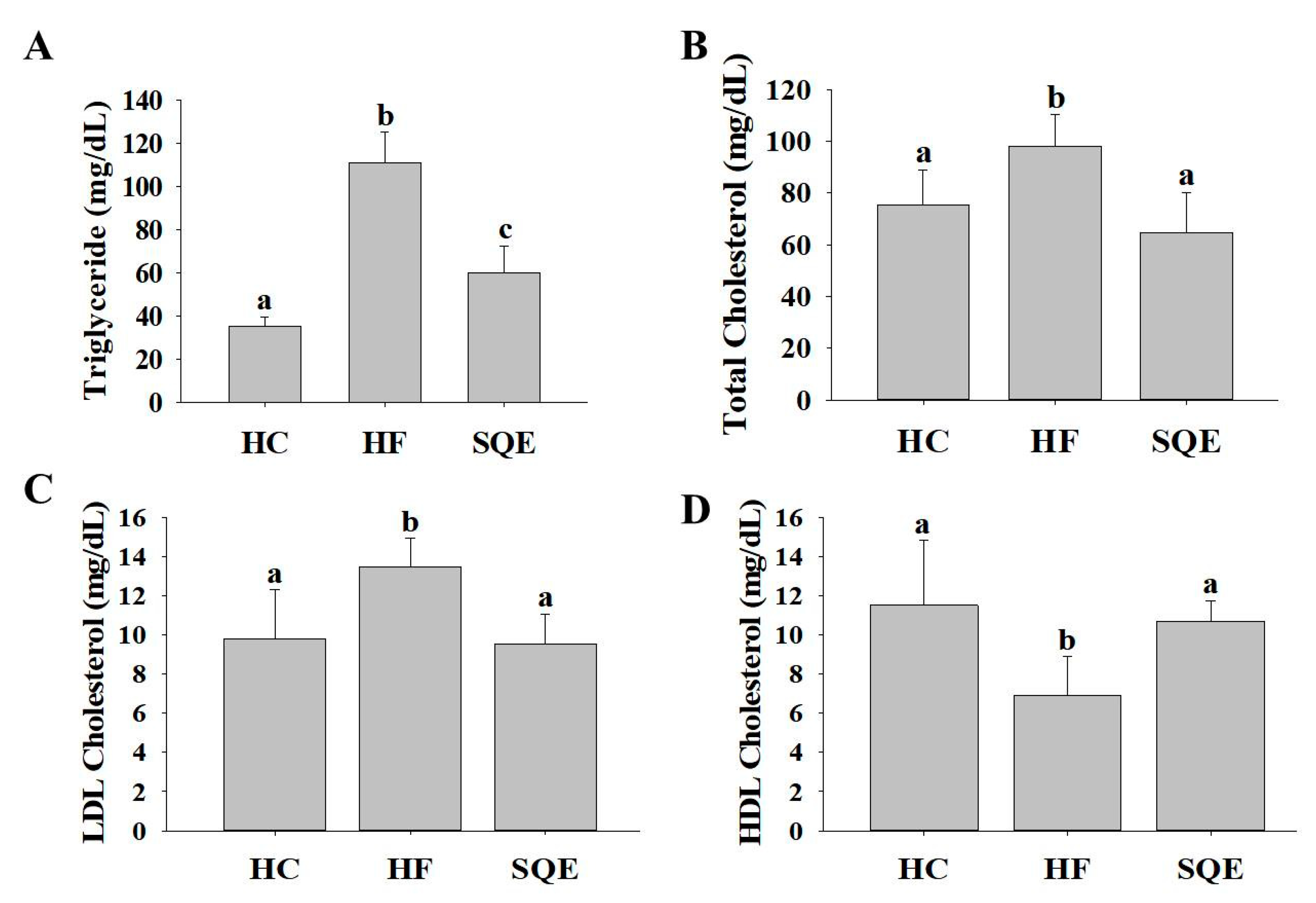
3.2. Effect of SQE on Serum Lipid Profiles
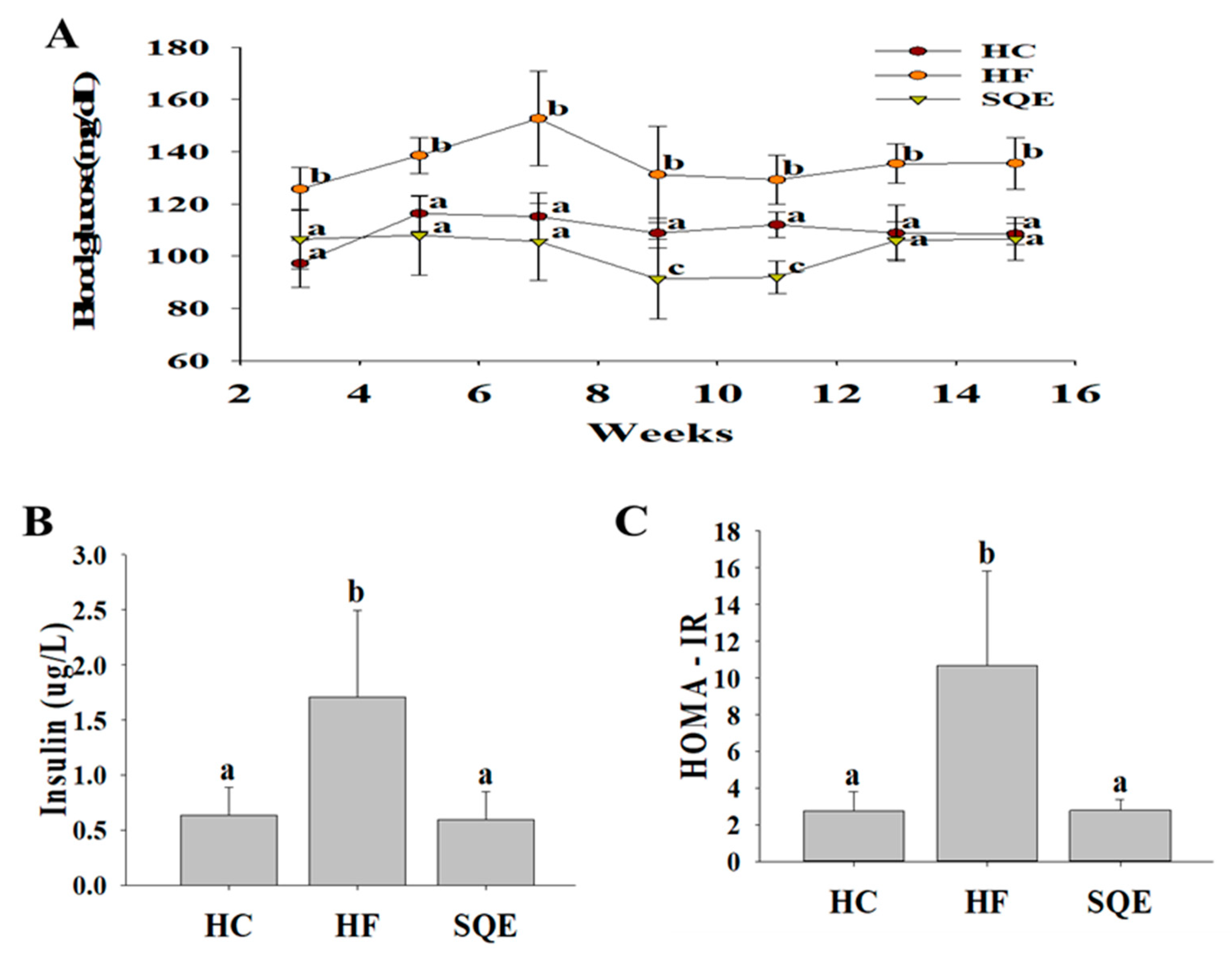
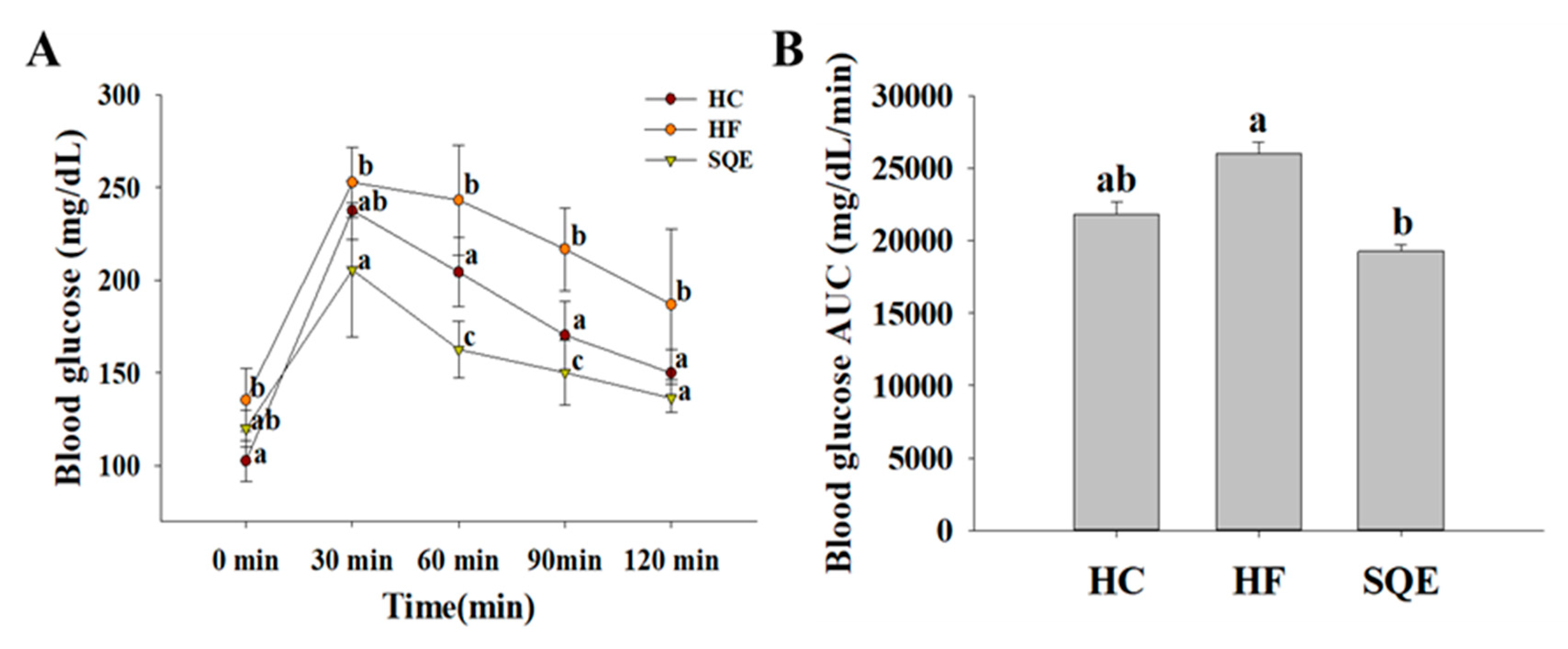
3.3. Effects of SQE on Glucose Homeostasis
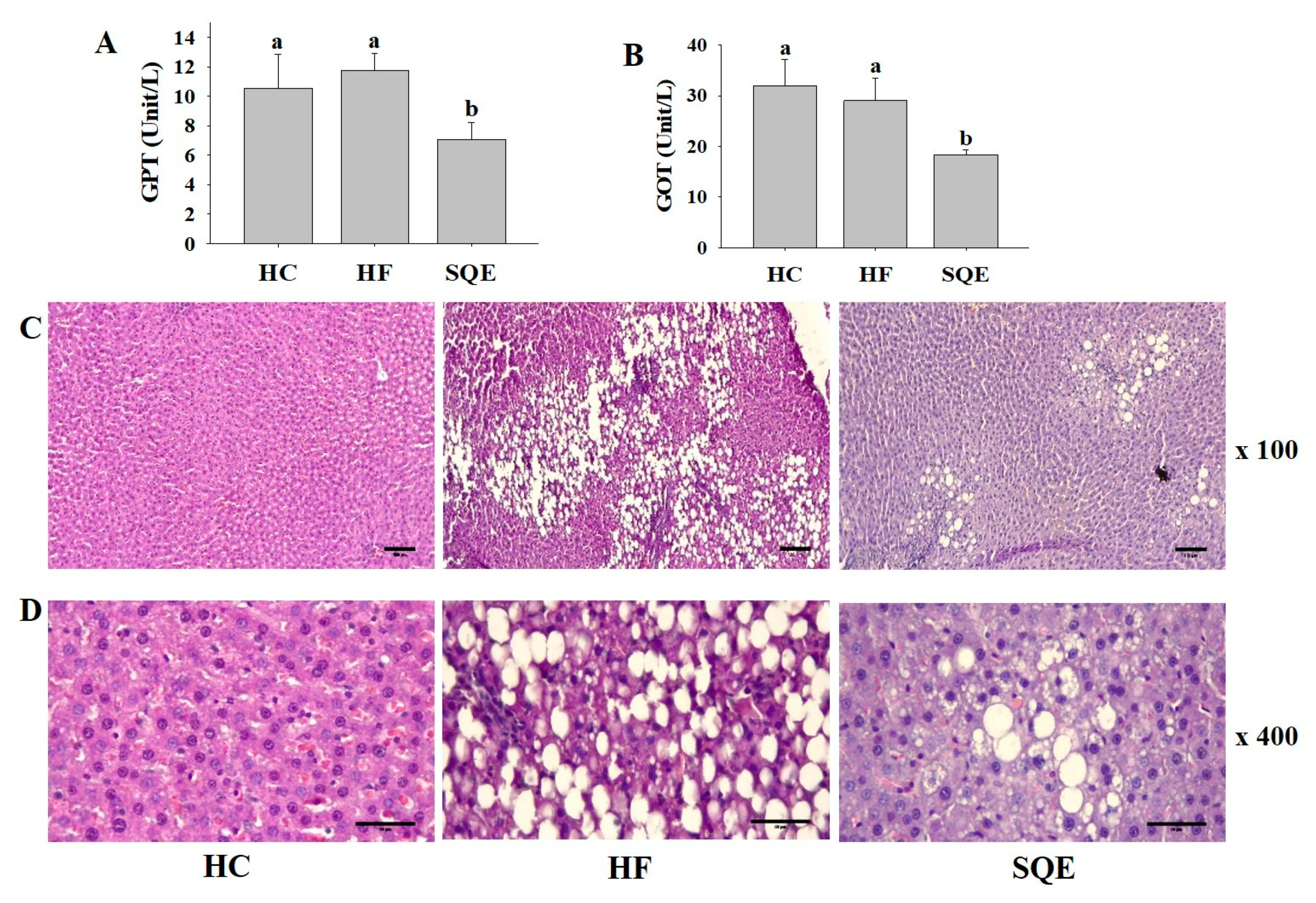
3.4. Effects of SQE on Liver Function
3.5. Effects of SQE on Protein Expression in Liver Tissue
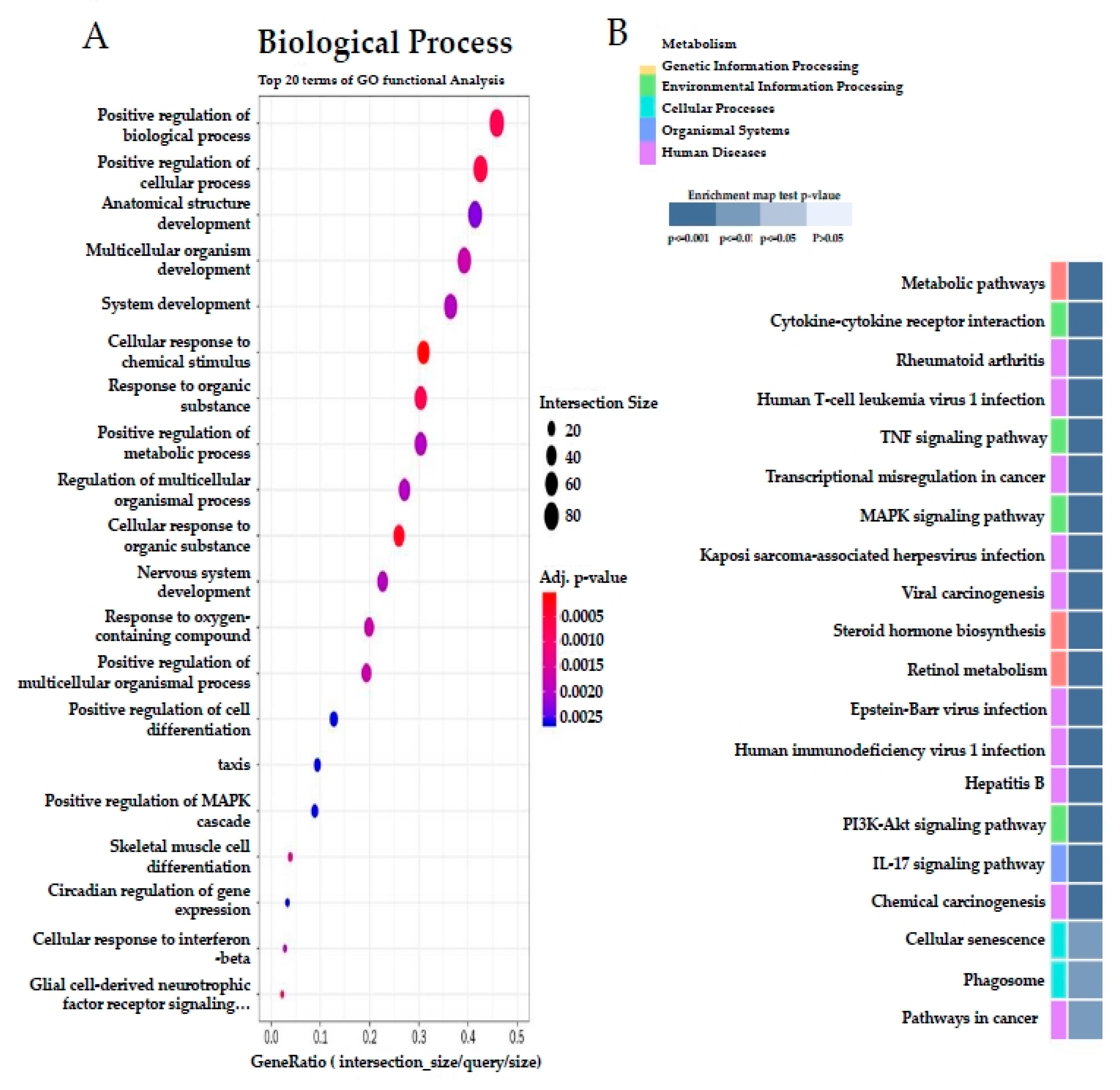
3.6. Effect of SQE on Liver Transcriptome Profiles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zivkovic, A.M.; German, J.B.; Sanyal, A.J. Comparative review of diets for the metabolic syndrome: Implications for nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2007, 86, 285–300. [Google Scholar] [CrossRef]
- Takinen, M.-R.; Packard, C.J.; Boren, J. Dietary fructose and the metabolic syndrome. Neutrients 2019, 12, 1987. [Google Scholar] [CrossRef] [Green Version]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [Green Version]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silbernagel, G.; Machann, J.; Unmuth, S.; Schick, F.; Stefan, N.; Haring, H.U.; Fritsche, A. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: An exploratory trial. Br. J. Nutr. 2011, 106, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, C.L.; Stanhop, K.L.; Schwarz, J.M.; Graham, J.L.; Hatcher, B.; Griffen, S.C.; Bremer, A.A.; Berglund, L.; McGahan, J.P.; Keim, N.L.; et al. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr. Metab. 2012, 9, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solga, S.; Alkhuraishe, A.R.; Clark, J.M.; Torbenson, M.; Greenwald, A.; Diehl, A.M.; Magnuson, T. Dietary composition and nonalcoholic fatty liver disease. Dig. Dis. Sci. 2004, 49, 1578–1583. [Google Scholar] [CrossRef]
- Samuel, V.T. Fructose induced lipogenesis: From sugar to fat to insulin resistance. Trend. Endocrinol. Metab. 2011, 22, 60–65. [Google Scholar] [CrossRef]
- Miller, A.; Adeli, K. Dietary fructose and the metabolic syndrome. Curr. Opin. Gastroentrol. 2008, 24, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Rutledge, A.C.; Adeli, K. Fructose and the metabolic syndrome: Pathophysiology and molecular mechanisms. Nutr. Rev. 2007, 65, S13–S23. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S.; Takeuchi, K.; Takagi, K.; Shibata, M. Stimulatory effect of the water extract of bamboo grass (Folin solution) on gastric acid secretion in pylorus-ligated rats. Jpn. J. Pharmaco. 1975, 25, 608–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, K. The Medicinal Plants of Korea; Kyo-Hak Publising Co. Ltd.: Seoul, Korea, 2000; p. 567. [Google Scholar]
- Raidaru, G.; Ilomets, T.; Mottus, A.; Maser, M. Isolation of polysaccharides with antitumor activity from Sasa kurilensis (Fr. et Sar.). Exp. Oncol. 1997, 20, 34–39. [Google Scholar]
- Ren, M.; Reilly, R.T.; Sacchi, N. Sasa health exerts a protective effects on Her2/NeuN mammary tumorigenesis. Anticancer Res. 2004, 24, 2879–2884. [Google Scholar] [PubMed]
- Kurokawa, T.; Itagaki, S.; Yamaji, T.; Nakata, C.; Nodo, T.; Hirano, T.; Iseki, K. Antioxidant activity of a novel extract from bamboo grass (AHSS) against ischemia-reperfusion injury in rat small intestine. Biol. Pharm. Bull. 2006, 29, 2301–2303. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, T.; Tanaka, A.; Hosoda, A.; Takano, F.; Ohta, T. Antioxidant C-glycosyl flavones from the leaves of Sasa kurilensis var. gigantea. Phytochemistry 2008, 69, 1419–1424. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lim, H.S.; Choi, J.S.; Shin, S.Y.; Bae, J.Y.; Kang, S.Q.; Kang, I.J.; Kang, Y.H. Blockage of chronic high glucose-induced endothelial apoptosis by Sasa borealis bamboo extract. Exp. Biol. Med. 2008, 233, 580–591. [Google Scholar] [CrossRef]
- Kang, H.; Lee, C. Sasa quelpaertensis Nakai extract suppresses porcine reproductive and respiratory syndrome virus replication and modulates virus-induced cytokine production. Arch. Virol. 2015, 160, 1977–1988. [Google Scholar] [CrossRef]
- Kang, S.I.; Shin, H.S.; Kim, H.M.; Hong, Y.S.; Yoon, S.A.; Kang, S.W.; Kim, J.H.; Ko, H.C.; Kim, S.J. Anti-obesity properties of a Sasa quelpaertensis extract in high-fat diet-induced obese mice. Biosci. Biotechnol. Biochem. 2012, 76, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kim, Y.S.; Kim, K.M.; Ko, H.C.; Kim, S.J.; Kim, J.H.; Kim, Y. Combination of Sasa quelpaertensis Nakai leaf extract and cisplatin suppresses the cancer stemness and invasion of human lung cancer cells. Integr. Cancer Ther. 2014, 13, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Shaif, N.A.; Cho, D.; Jang, D.; Kim, H.M.; Chung, J.O.; Kim, S.; Seo, D.B.; Kim, K.R.; Shin, J.; Shim, I. Sasa quelpaertensis Nakai induced antidepressant-like effect in ovariectomized rats. Biomed. Res. Int. 2019, 5815604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kim, Y.S.; Lee, H.A.; Lim, J.Y.; Kim, M.; Kwon, O.; Ko, H.C.; Kim, S.J.; Shin, J.H.; Kim, Y. Sasa quelpaertensis Leaf Extract Improves High Fat Diet-Induced Lipid Abnormalities and Regulation of Lipid Metabolism Genes in Rats. J. Med. Food 2014, 17, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Ko, H.C.; Jang, M.G.; Kim, S.J. Preparation and characterization of phytochemical-rich extract from Sasa quelpaertensis leaf. J. Life Sci. 2016, 26, 1330–1335. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Pertea, M.; Kim, D.H.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Yeom, Y.; Kim, B.-S.; Kim, S.J.; Kim, Y. Sasa quelpaertensis leaf extract regulates microbial dysbiosis by modulating the composition and diversity of the microbiota in dextran sulfate sodium induced colitis mice. BMC Complem. Altern. Med. 2016, 16, 481. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.I.; An, S.-M.; Mun, G.-I.; Lee, S.-J.; Park, K.-M.; Park, S.-H.; Boo, Y.-C. Protective effect of Sasa quelpaertensis and p-coumaric acid on ethanol-induced hepatotoxicity in mice. J. Appl. Biol. Chem. 2008, 51, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Oyama, T.; Yasui, Y.; Sugie, S.; Koketsu, M.; Watanabe, K.; Tanaka, T. Dietary tricin suppresses inflammation-related colon carcinogenesis in male Crj: CD-1 mice. Cancer Preven. Res. 2009, 2, 1031–1038. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.C.; Lee, J.Y.; Jang, M.G.; Song, H.; Kim, S.J. Seasonal variations in the phenolic compounds and antioxidant activity of Sasa quelpaertensis. Ind. Crops Prod. 2018, 122, 506–512. [Google Scholar] [CrossRef]
- Nomura, K.; Yamanouchi, T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J. Nutr. Biochem. 2012, 23, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Middela, H.; Matapally, S.; Padiya, R.; Bastia, T.; Madhusudana, K.; Reddy, B.R.; Chakravarty, S.; Banerjee, S.K. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacol. Res. 2012, 66, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, J.; Zhang, Y.; Li, J.; Sun, W. Black sesame seeds ethanol extract ameliorates hepatic lipid accumulation, oxidative stress, and insulin resistance in fructose-induced nonalcoholic fatty live disease. Agric. Food Chem. 2018, 66, 10458–10469. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, A.E.; Werneburg, N.W.; Canbay, A.; Guicciardi, M.E.; Bronk, S.F.; Rydzewski, R.; Burgart, L.J.; Gores, G.J. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology 2004, 40, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Al-Dayyat, H.M.; Rayan, Y.M.; Tayyem, R.F. Non-alcoholic fatty liver disease and associated dietary and lifestyle risk factors. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 569–575. [Google Scholar] [CrossRef]
- Li, S.; Eguchi, N.; Lau, H.; Ichii, H. The role of the Nrf2 signaling in obesity and insulin resistance. Int. J. Mol. Sci. 2020, 21, 6973. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef] [Green Version]
- Todoric, J.; Di Caro, G.; Reibe, S.; Henstridge, D.C.; Green, C.R.; Vrbanac, A.; Ceteci, F.; Conche, C.; McNulty, R.; Shalapour, S.; et al. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat. Metab. 2020, 2, 1034–1045. [Google Scholar] [CrossRef]
- Woods, A.; Williams, J.R.; Muckett, P.J.; Mayer, F.V.; Liljevald, M.; Bohlooly-Y, M.; Carling, D. Liver-specific activation of AMPK prevents steatosis on a high-fructose diet. Cell Rep. 2017, 28, 3043–3051. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Hu, Z.; Cui, A.; Liu, Z.; Ma, F.; Xue, Y.; Zhang, F.; Zhao, Z.; Yu, Y.; Gao, J.; et al. Post-translational regulation of lipogenesis via AMPK-dependent phosphorylation of insulin-induced gene. Nat. Commun. 2019, 10, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Jiang, Z.; Li, C.; Zhu, Y.; Li, Z.; Tang, Y.; Ni, C. Apigenin prevents metabolic syndrome in high-fructose diet-fed mice by Keap1-Nrf2 pathway. Biomed. Pharmacother. 2018, 105, 1283–1290. [Google Scholar] [CrossRef] [PubMed]


| Groups | HC | HF | SQE |
|---|---|---|---|
| Initial body weight (g) | 322.50 ± 17.77 | 322.87 ± 22.56 | 309.79 ± 12.87 |
| Final body weight (g) | 699.63 ± 70.02 a | 703.13 ± 42.48 a | 638.75 ± 35.75 b |
| Weight gain (g) | 377.12 ± 57.08 a | 380.25 ± 28.17 a | 329.00 ± 27.17 b |
| Total food intake for 16 weeks (kcal/g) | 11,600.82 ± 79.22 a | 16,520.70 ± 148.46 b | 16,030.99 ± 113.54 b |
| Total water intake for 16 weeks (mL) | 5197.25 ± 27.30 a | 6597.06 ± 37.49 b | 7476.96 ± 95.24 c |
| Food efficiency ratio (%) | 9.31 ± 1.29 a | 8.43 ± 0.49 a | 7.28 ± 0.57 b |
| Liver weight (g) | 14.93 ± 2.63 a | 20.15 ± 4.97 b | 15.11 ± 1.78 a |
| Spleen weight (g) | 1.01 ± 0.19 a | 1.00 ± 0.14 a | 0.78 ± 0.09 b |
| Kidney weight (g) | 1.69 ± 0.17 a | 2.08 ± 0.20 b | 1.87 ± 0.20 ab |
| Testis weight (g) | 1.70 ± 0.11 | 1.82 ± 0.11 | 1.61 ± 0.38 |
| Epididymal fat (g) | 0.43 ± 0.11 a | 0.57 ± 0.11 b | 0.42 ± 0.06 a |
| Sample | Total Reads | GC Contents (%) | Q30 (%) | Processed Reads | Mapped Reads | Unmapped Reads |
|---|---|---|---|---|---|---|
| HF-1 | 63,992,692 | 49.00 | 95.58 | 63,992,692 | 62,592,420 (97.81%) | 1,400,273 (2.19%) |
| HF-2 | 63,132,988 | 49.18 | 95.80 | 63,132,988 | 61,846,383 (97.96%) | 1,286,605 (2.04%) |
| HF-3 | 75,518,180 | 49.50 | 95.59 | 75,518,180 | 73,953,123 (97.93%) | 1,565,057 (2.07%) |
| SQE-1 | 83,369,682 | 49.13 | 95.71 | 83,396,682 | 81,437,777 (97.65%) | 1,958,905 (2.35%) |
| SQE-2 | 64,484,030 | 49.11 | 95.83 | 64,484,030 | 63,011,809 (97.72%) | 1,472,221 (2.28%) |
| SQE-3 | 87,240,986 | 49.30 | 95.74 | 87,240,986 | 85,412,156 (97.90%) | 1,828,830 (2.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.Y.; Jang, M.G.; Oh, J.M.; Ko, H.C.; Hur, S.-P.; Kim, J.-W.; Baek, S.; Kim, S.-J. Sasa quelpaertensis Leaf Extract Ameliorates Dyslipidemia, Insulin Resistance, and Hepatic Lipid Accumulation in High-Fructose-Diet-Fed Rats. Nutrients 2020, 12, 3762. https://doi.org/10.3390/nu12123762
Park JY, Jang MG, Oh JM, Ko HC, Hur S-P, Kim J-W, Baek S, Kim S-J. Sasa quelpaertensis Leaf Extract Ameliorates Dyslipidemia, Insulin Resistance, and Hepatic Lipid Accumulation in High-Fructose-Diet-Fed Rats. Nutrients. 2020; 12(12):3762. https://doi.org/10.3390/nu12123762
Chicago/Turabian StylePark, Jeong Yong, Mi Gyeong Jang, Jung Min Oh, Hee Chul Ko, Sung-Pyo Hur, Jae-Won Kim, Songyee Baek, and Se-Jae Kim. 2020. "Sasa quelpaertensis Leaf Extract Ameliorates Dyslipidemia, Insulin Resistance, and Hepatic Lipid Accumulation in High-Fructose-Diet-Fed Rats" Nutrients 12, no. 12: 3762. https://doi.org/10.3390/nu12123762
APA StylePark, J. Y., Jang, M. G., Oh, J. M., Ko, H. C., Hur, S.-P., Kim, J.-W., Baek, S., & Kim, S.-J. (2020). Sasa quelpaertensis Leaf Extract Ameliorates Dyslipidemia, Insulin Resistance, and Hepatic Lipid Accumulation in High-Fructose-Diet-Fed Rats. Nutrients, 12(12), 3762. https://doi.org/10.3390/nu12123762






