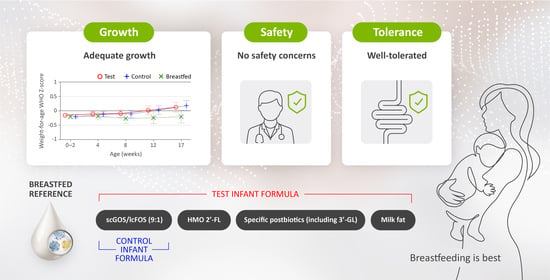
A Partly Fermented Infant Formula with Postbiotics Including 3′-GL, Specific Oligosaccharides, 2′-FL, and Milk Fat Supports Adequate Growth, Is Safe and Well-Tolerated in Healthy Term Infants: A Double-Blind, Randomised, Controlled, Multi-Country Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Study Products
2.4. Measurements
2.5. Statistical Analysis
3. Results
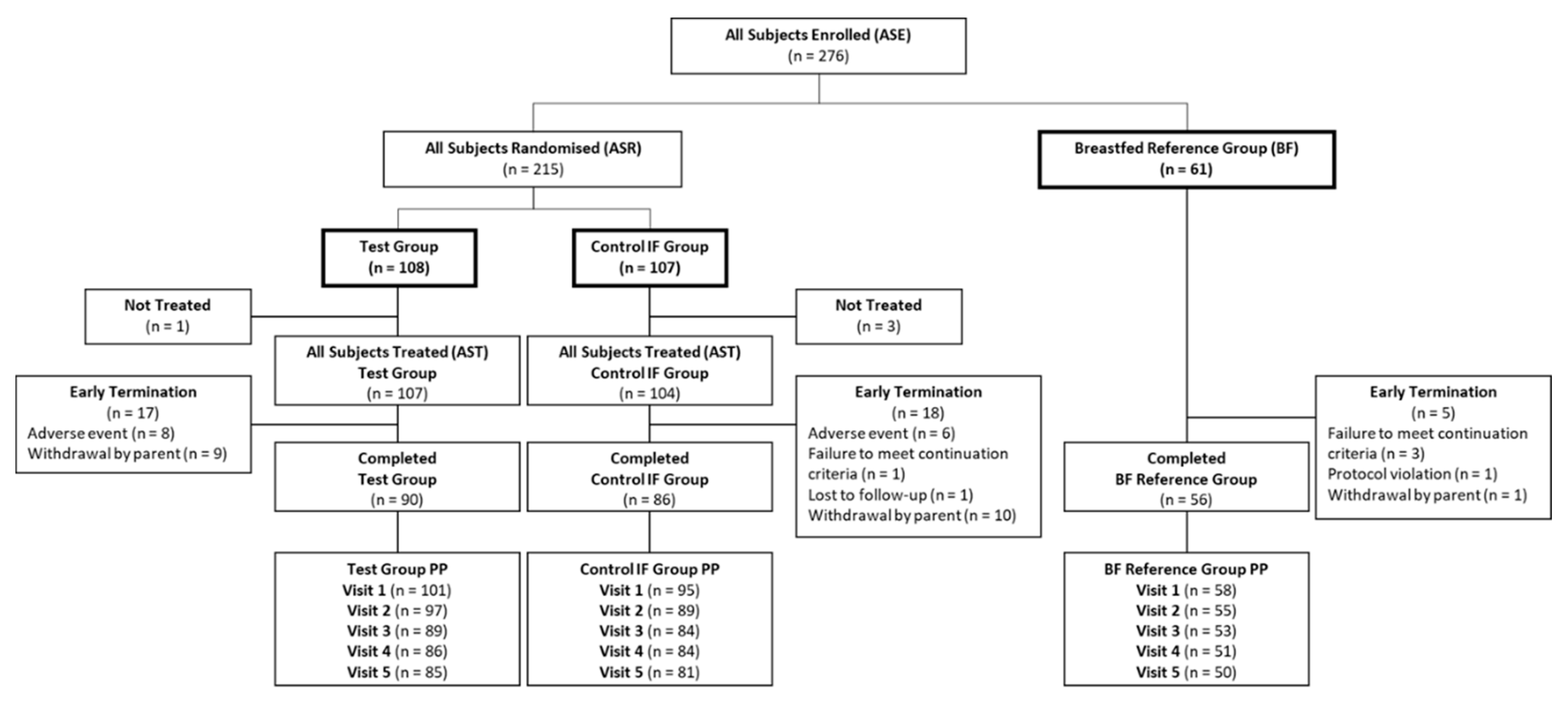
3.1. Subject Characteristics
3.2. Study Product Intake
3.3. Growth Outcomes
3.4. Parent-Reported GI Tolerance
3.5. Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, C.E.; Whaley, S.E.; Crespi, C.M.; Wang, M.C.; Chaparro, M.P. Every month matters: Longitudinal associations between exclusive breastfeeding duration, child growth and obesity among WIC-participating children. J. Epidemiol. Community Health 2020. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; Franca, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Horta, B.L.; de Sousa, B.A.; de Mola, C.L. Breastfeeding and neurodevelopmental outcomes. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 174–178. [Google Scholar] [CrossRef]
- Ninonuevo, M.R.; Park, Y.; Yin, H.; Zhang, J.; Ward, R.E.; Clowers, B.H.; German, J.B.; Freeman, S.L.; Killeen, K.; Grimm, R.; et al. A strategy for annotating the human milk glycome. J. Agric. Food Chem. 2006, 54, 7471–7480. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef]
- Bode, L. The functional biology of human milk oligosaccharides. Early Hum. Dev. 2015, 91, 619–622. [Google Scholar] [CrossRef]
- Jantscher-Krenn, E.; Bode, L. Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Minerva Pediatr. 2012, 64, 83–99. [Google Scholar]
- Hegar, B.; Wibowo, Y.; Basrowi, R.W.; Ranuh, R.G.; Sudarmo, S.M.; Munasir, Z.; Atthiyah, A.F.; Widodo, A.D.; Supriatmo; Kadim, M.; et al. The role of two human milk oligosaccharides, 2’-fucosyllactose and cacto-n-neotetraose, in infant nutrition. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Lewis, Z.T.; Totten, S.M.; Smilowitz, J.T.; Popovic, M.; Parker, E.; Lemay, D.G.; Van Tassell, M.L.; Miller, M.J.; Jin, Y.S.; German, J.B.; et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 2015, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Altaye, M.; Jiang, X.; Guerrero, M.L.; Meinzen-Derr, J.K.; Farkas, T.; Chaturvedi, P.; Pickering, L.K.; Newburg, D.S. Human milk oligosaccharide blood group epitopes and innate immune protection against campylobacter and calicivirus diarrhea in breastfed infants. Adv. Exp. Med. Biol. 2004, 554, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Muller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Castanys-Munoz, E.; Martin, M.J.; Prieto, P.A. 2′-fucosyllactose: An abundant, genetically determined soluble glycan present in human milk. Nutr. Rev. 2013, 71, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S.; Ko, J.S.; Leone, S.; Nanthakumar, N.N. Human Milk Oligosaccharides and Synthetic Galactosyloligosaccharides Contain 3′-, 4-, and 6′-Galactosyllactose and attenuate inflammation in human T84, NCM-460, and H4 cells and intestinal tissue ex vivo. J. Nutr. 2016, 146, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Stahl, B.; Vinderola, G.; Szajewska, H. Infant Formula Supplemented with Biotics: Current Knowledge and Future Perspectives. Nutrients 2020, 12, 1952. [Google Scholar] [CrossRef] [PubMed]
- Knol, J.; Scholtens, P.; Kafka, C.; Steenbakkers, J.; Gro, S.; Helm, K.; Klarczyk, M.; Schopfer, H.; Bockler, H.M.; Wells, J. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: More like breast-fed infants. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 36–42. [Google Scholar] [CrossRef]
- Scholtens, P.A.; Goossens, D.A.; Staiano, A. Stool characteristics of infants receiving short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides: A review. World J. Gastroenterol. 2014, 20, 13446–13452. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Moro, G.E.; Boehm, G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J. Nutr. 2007, 137, 2420–2424. [Google Scholar] [CrossRef]
- Goehring, K.C.; Marriage, B.J.; Oliver, J.S.; Wilder, J.A.; Barrett, E.G.; Buck, R.H. Similar to those who are breastfed, infants fed a formula containing 2′-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J. Nutr. 2016, 146, 2559–2566. [Google Scholar] [CrossRef]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of infant formula with human milk oligosaccharides on growth and morbidity: A randomized multicenter trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar] [CrossRef]
- Collado, M.C.; Vinderola, G.; Salminen, S. Postbiotics: Facts and open questions. A position paper on the need for a consensus definition. Benef. Microbes 2019, 10, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Thibault, H.; Aubert-Jacquin, C.; Goulet, O. Effects of long-term consumption of a fermented infant formula (with Bifidobacterium breve c50 and Streptococcus thermophilus 065) on acute diarrhea in healthy infants. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Mullie, C.; Yazourh, A.; Thibault, H.; Odou, M.F.; Singer, E.; Kalach, N.; Kremp, O.; Romond, M.B. Increased poliovirus-specific intestinal antibody response coincides with promotion of Bifidobacterium longum-infantis and Bifidobacterium breve in infants: A randomized, double-blind, placebo-controlled trial. Pediatr. Res. 2004, 56, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Herrera, A.; Mulder, K.; Bouritius, H.; Rubio, R.; Munoz, A.; Agosti, M.; Lista, G.; Corvaglia, L.; Ludwig, T.; Abrahamse-Berkeveld, M.; et al. Gastrointestinal tolerance, growth and safety of a partly fermented formula with specific prebiotics in healthy infants: A double-blind, randomized, controlled trial. Nutrients 2019, 11, 1530. [Google Scholar] [CrossRef] [PubMed]
- Huet, F.; Abrahamse-Berkeveld, M.; Tims, S.; Simeoni, U.; Beley, G.; Savagner, C.; Vandenplas, Y.; Hourihane, J.O. Partly fermented infant formulae with specific oligosaccharides support adequate infant growth and are well-tolerated. J. Pediatr. Gastroenterol. Nutr. 2016, 63, e43–e53. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Ludwig, T.; Bouritius, H.; Alliet, P.; Forde, D.; Peeters, S.; Huet, F.; Hourihane, J. Randomised controlled trial demonstrates that fermented infant formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides reduces the incidence of infantile colic. Acta Paediatr. 2017, 106, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Cheikh Ismail, L.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The newborn cross-sectional study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group. WHO Child growth standards based on length/height, weight and age. Acta Paediatr. 2006, 450, 76–85. [Google Scholar]
- Bekkali, N.; Hamers, S.L.; Reitsma, J.B.; Van Toledo, L.; Benninga, M.A. Infant stool form scale: Development and results. J. Pediatr. 2009, 154, 521–526.e521. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Clinical Testing of Infant Formula with Respect to Nutritional Suitability for Term Infants; Report to the FDA; American Academy of Pediatrics, Committee on Nutrition: Elk Grove Village, IL, USA, 1988. [Google Scholar]
- Picaud, J.C.; Pajek, B.; Arciszewska, M.; Tarczon, I.; Escribano, J.; Porcel, R.; Adelt, T.; Hassink, E.; Rijnierse, A.; Abrahamse-Berkeveld, M.; et al. An infant formula with partially hydrolyzed whey protein supports adequate growth and is safe and well-tolerated in healthy, term infants: A randomized, double-blind, equivalence trial. Nutrients 2020, 12, 2072. [Google Scholar] [CrossRef]
- Meli, F.; Puccio, G.; Cajozzo, C.; Ricottone, G.L.; Pecquet, S.; Sprenger, N.; Steenhout, P. Growth and safety evaluation of infant formulae containing oligosaccharides derived from bovine milk: A randomized, double-blind, noninferiority trial. BMC Pediatr. 2014, 14, 306. [Google Scholar] [CrossRef] [PubMed]
- Breij, L.M.; Abrahamse-Berkeveld, M.; Vandenplas, Y.; Jespers, S.N.J.; de Mol, A.C.; Khoo, P.C.; Kalenga, M.; Peeters, S.; van Beek, R.H.T.; Norbruis, O.F.; et al. An infant formula with large, milk phospholipid-coated lipid droplets containing a mixture of dairy and vegetable lipids supports adequate growth and is well tolerated in healthy, term infants. Am. J. Clin. Nutr. 2019, 109, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.E.; Rogers, R.R.; Ziegler, E.E.; Fomon, S.J. Gain in weight and length during early infancy. Early Hum. Dev. 1989, 19, 223–239. [Google Scholar] [CrossRef]
- Dewey, K.G. Growth characteristics of breast-fed compared to formula-fed infants. Biol. Neonate 1998, 74, 94–105. [Google Scholar] [CrossRef]
- Bell, K.A.; Wagner, C.L.; Feldman, H.A.; Shypailo, R.J.; Belfort, M.B. Associations of infant feeding with trajectories of body composition and growth. Am. J. Clin. Nutr. 2017, 106, 491–498. [Google Scholar] [CrossRef]
- Li, R.; Magadia, J.; Fein, S.B.; Grummer-Strawn, L.M. Risk of bottle-feeding for rapid weight gain during the first year of life. Arch. Pediatr. Adolesc. Med. 2012, 166, 431–436. [Google Scholar] [CrossRef]
- Azad, M.B.; Vehling, L.; Chan, D.; Klopp, A.; Nickel, N.C.; McGavock, J.M.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; et al. Infant feeding and weight gain: Separating breast milk from breastfeeding and formula from food. Pediatrics 2018, 142. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Abkari, A.; Bellaiche, M.; Benninga, M.; Chouraqui, J.P.; Cokura, F.; Harb, T.; Hegar, B.; Lifschitz, C.; Ludwig, T.; et al. Prevalence and health outcomes of functional gastrointestinal dymptoms in infants from birth to 12 months of age. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 531–537. [Google Scholar] [CrossRef]
- Quinlan, P.T.; Lockton, S.; Irwin, J.; Lucas, A.L. The relationship between stool hardness and stool composition in breast- and formula-fed infants. J. Pediatr. Gastroenterol. Nutr. 1995, 20, 81–90. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. The influence of the position of palmitate in infant formula triacylglycerols on health outcomes. Nutr. Res. 2017, 44, 1–8. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M.; Cervantes, L.E.; Ramos, P.; Chavez-Munguia, B.; Newburg, D.S. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 2003, 278, 14112–14120. [Google Scholar] [CrossRef] [PubMed]
- Weichert, S.; Koromyslova, A.; Singh, B.K.; Hansman, S.; Jennewein, S.; Schroten, H.; Hansman, G.S. Structural basis for norovirus inhibition by human milk oligosaccharides. J. Virol. 2016, 90, 4843–4848. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, S.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The human milk oligosaccharide 2’-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef]
- Marriage, B.J.; Buck, R.H.; Goehring, K.C.; Oliver, J.S.; Williams, J.A. Infants Fed a Lower Calorie Formula With 2’FL Show Growth and 2’FL Uptake Like Breast-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 649–658. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Moro, G.E.; Schmitt, J.; Tandoi, L.; Rizzardi, S.; Boehm, G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J. Nutr. 2008, 138, 1091–1095. [Google Scholar] [CrossRef] [PubMed]


| Test IF Per 100 mL | Control IF Per 100 mL | ||
|---|---|---|---|
| Energy | Kcal | 66 | 66 |
| Carbohydrates | g | 7.3 | 7.3 |
| scGOS/lcFOS | g | 0.8 | 0.8 |
| 2′-FL | g | 0.1 | 0 |
| 3′-GL | mg | 15 | 0 |
| Protein | g | 1.3 | 1.3 |
| Whey | g | 0.7 | 0.8 |
| Casein | g | 0.7 | 0.5 |
| Fat | g | 3.4 | 3.4 |
| Vegetable oil | g | 1.6 | 3.3 |
| Dairy lipids | g | 1.6 | 0.1 |
| Saturates | g | 1.7 | 1.5 |
| Palmitic acid | mg | 593 | 581 |
| sn-2 Palmitic acid | mg | 202 | 66.9 |
| Monounsaturates | g | 1.1 | 1.3 |
| Polyunsaturates | g | 0.6 | 0.6 |
| Linoleic acid (LA) | mg | 448 | 445 |
| α-Linolenic acid (ALA) | mg | 54.9 | 82 |
| LA:ALA | ratio | 8.15 | 5.40 |
| Arachidonic acid | mg | 16.5 | 11 |
| Docosahexaenoic acid | mg | 16.5 | 10 |
| Statistic | Test (N = 101) | Control (N = 95) | Total IF (N = 196) | Breastfed (N = 58) | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | n (%) | 54 (53.5) | 49 (51.6) | 103 (52.6) | 31 (53.4) |
| Male | n (%) | 47 (46.5) | 46 (48.4) | 93 (47.4) | 27 (46.6) |
| Country | |||||
| Belgium | n (%) | 11 (10.9) | 10 (10.5) | 21 (10.7) | 4 (6.90) |
| Hungary | n (%) | 4 (3.96) | 4 (4.21) | 8 (4.1) | 9 (15.5) |
| Poland | n (%) | 54 (53.5) | 53 (55.8) | 107 (54.6) | 28 (48.3) |
| Spain | n (%) | 20 (19.8) | 12 (12.6) | 32 (16.3) | 10 (17.2) |
| Ukraine | n (%) | 12 (11.9) | 16 (16.8) | 28 (14.3) | 7 (12.1) |
| Age at Baseline (days) 3 | Median (Q1–Q3) | 10 (5–12) | 10 (6–12) | 10 (5–12) | 11 (8–13) |
| Gestational Age (days) 4 | Mean (SD) | 39.4 (1.1) | 39.3 (1.2) | 39.3 (1.2) | 39.3 (1.2) |
| Mode of Delivery | |||||
| Caesarean Section | n (%) | 49 (48.5) | 50 (52.6) | 99 (50.5) | 20 (34.5) |
| Vaginal | n (%) | 52 (51.5) | 45 (47.4) | 97 (49.5) | 38 (65.5) |
| Birth Weight (g) | Mean (SD) | 3390 (368) | 3317 (334) | 3354 (353) | 3346 (329) |
| Birth Length (cm) | Mean (SD) | 53.1 (3.1) | 52.7 (2.9) | 52.9 (3.0) | 52.8 (2.7) |
| Birth Head Circumference (cm) | Mean (SD) | 34.7 (1.1) | 34.4 (1.1) | 34.5 (1.1) | 34.5 (1.1) |
| Maternal Age (years) | Mean (SD) | 30.7 (5.5) | 30.5 (6.0) | 30.6 (5.7) | 32.3 (4.9) |
| Maternal Education5 | |||||
| Primary or Less | n (%) | 10 (9.9) | 11 (11.6) | 21 (10.7) | 2 (3.4) |
| Secondary | n (%) | 57 (56.4) | 46 (48.4) | 103 (52.6) | 10 (17.2) |
| Tertiary | n (%) | 34 (33.7) | 38 (40.0) | 72 (36.7) | 46 (79.3) |
| Maternal pre-pregnancy BMI (kg/m2) | Mean (SD) | 23.8 (4.9) | 24.4 (5.8) | 24.1 (5.4) | 22.4 (2.8) |
| Paternal BMI (kg/m2) | Mean (SD) | 27.4 (4.0) | 27.1 (3.8) | 27.2 (3.9) | 26.9 (3.8) |
| mL/day | mL/kg/day | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test | Control | Test | Control | |||||||
| n (Missing) | Mean (SD) | n (Missing) | Mean (SD) | p-Value 3 | n (Missing) | Mean (SD) | N (Missing) | Mean (SD) | p-Value 3 | |
| All Subjects Randomised population | ||||||||||
| Visit 1 | 98 (10) | 598 (130) | 98 (9) | 626 (147) | 0.237 | 98 (10) | 176 (38.5) | 98 (9) | 187 (45.3) | 0.091 |
| Visit 2 | 96 (9) | 758 (147) | 95 (6) | 774 (161) | 0.981 | 95 (10) | 178 (31.4) | 92 (9) | 186 (37.0) | 0.326 |
| Visit 3 | 91 (3) | 851 (184) | 88 (4) | 881 (183) | 0.412 | 91 (3) | 164 (33.4) | 88 (4) | 172 (35.4) | 0.236 |
| Visit 4 | 90 (3) | 939 (176) | 85 (4) | 950 (207) | 0.993 | 89 (4) | 157 (28.9) | 85 (4) | 159 (37.0) | 0.878 |
| Visit 5 | 89 (1) | 1007 (225) | 85 (2) | 1022 (220) | 0.533 | 89 (1) | 148 (31.6) | 85 (2) | 150 (35.8) | 0.718 |
| Per Protocol population | ||||||||||
| Visit 1 | 93 (8) | 602 (125) | 94 (1) | 624 (141) | 0.275 | 93 (8) | 176 (37.8) | 94 (1) | 187 (44.1) | 0.084 |
| Visit 2 | 92 (5) | 762 (147) | 87 (2) | 767 (162) | 0.557 | 92 (5) | 179 (31.7) | 87 (2) | 184 (36.7) | 0.559 |
| Visit 3 | 87 (2) | 861 (173) | 83 (1) | 878 (180) | 0.596 | 87 (2) | 165 (31.8) | 83 (1) | 171 (34.4) | 0.318 |
| Visit 4 | 85 (1) | 941 (178) | 80 (4) | 944 (207) | 0.818 | 85 (1) | 155 (28.7) | 80 (4) | 158 (36.8) | 0.939 |
| Visit 5 | 84 (1) | 1009 (227) | 79 (2) | 1025 (224) | 0.518 | 84 (1) | 147 (31.9) | 79 (2) | 151 (36.3) | 0.425 |
| Test (n = 101) | Control (N = 95) | Breastfed (n = 58) | ||||
|---|---|---|---|---|---|---|
| Mean Estimate (SE) | 95% CI | Mean Estimate (SE) | 95% CI | Mean Estimate (SE) | 95% CI | |
| Weight gain | ||||||
| g | 3416 (64.5) | 3289, 3543 | 3425 (66.6) | 3293,3556 | 3109 (87.1) | 2938, 3281 |
| g/day | 31.0 (0.59) | 29.8, 32.2 | 31.1 (0.60) | 29.9, 32.3 | 28.3 (0.79) | 26.7, 29.9 |
| Length gain | ||||||
| cm | 11.0 (0.2) | 10.6, 11.4 | 10.8 (0.2) | 10.4, 11.2 | 11.1 (0.3) | 10.6, 11.6 |
| cm/day | 0.10 (0.0) | 0.10, 0.10 | 0.10 (0.0) | 0.09, 0.10 | 0.1 (0.0) | 0.10, 0.11 |
| Head circumference gain | ||||||
| cm | 6.54 (0.1) | 6.31, 6.77 | 6.64 (0.12) | 6.41, 6.88 | 6.41 (0.15) | 6.12, 6.71 |
| cm/day | 0.06 (0.0) | 0.06, 0.06 | 0.06 (0.0) | 0.06, 0.06 | 0.06 (0.0) | 0.06, 0.06 |
| Adverse Event | Test (n = 107) | Control (n = 104) | p-Value 3 | Breastfed (n = 61) |
|---|---|---|---|---|
| Any Event | 42 (39.3) | 33 (31.7) | 15 (24.6) | |
| Gastrointestinal disorders | 22 (20.6) | 17 (16.3) | 0.431 | 6 (9.8) |
| Abdominal pain | 2 (1.9) | 3 (2,9) | 0 (0.0) | |
| Constipation | 4 (3.7) | 3 (2.9) | 0.730 | 1 (1.6) |
| Diarrhoea | 1 (0.9) | 2 (1.9) | 1 (1.6) | |
| Flatulence | 1 (0.9) | 2 (1.9) | 2 (3.3) | |
| Infantile colic | 8 (7.5) | 4 (3.8) | 0.256 | 1 (1.6) |
| Infantile vomiting | 3 (2.8) | 2 (1.9) | 0 (0.0) | |
| Regurgitation | 6 (5.6) | 1 (1.0) | 0.060 | 1 (1.6) |
| Other 2 | 6 (5.6) | 4 (3.8) | 1 (1.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandenplas, Y.; de Halleux, V.; Arciszewska, M.; Lach, P.; Pokhylko, V.; Klymenko, V.; Schoen, S.; Abrahamse-Berkeveld, M.; Mulder, K.A.; Porcel Rubio, R.; et al. A Partly Fermented Infant Formula with Postbiotics Including 3′-GL, Specific Oligosaccharides, 2′-FL, and Milk Fat Supports Adequate Growth, Is Safe and Well-Tolerated in Healthy Term Infants: A Double-Blind, Randomised, Controlled, Multi-Country Trial. Nutrients 2020, 12, 3560. https://doi.org/10.3390/nu12113560
Vandenplas Y, de Halleux V, Arciszewska M, Lach P, Pokhylko V, Klymenko V, Schoen S, Abrahamse-Berkeveld M, Mulder KA, Porcel Rubio R, et al. A Partly Fermented Infant Formula with Postbiotics Including 3′-GL, Specific Oligosaccharides, 2′-FL, and Milk Fat Supports Adequate Growth, Is Safe and Well-Tolerated in Healthy Term Infants: A Double-Blind, Randomised, Controlled, Multi-Country Trial. Nutrients. 2020; 12(11):3560. https://doi.org/10.3390/nu12113560
Chicago/Turabian StyleVandenplas, Yvan, Virginie de Halleux, Małgorzata Arciszewska, Piotr Lach, Valeriy Pokhylko, Viktoriia Klymenko, Stefanie Schoen, Marieke Abrahamse-Berkeveld, Kelly A Mulder, Rocio Porcel Rubio, and et al. 2020. "A Partly Fermented Infant Formula with Postbiotics Including 3′-GL, Specific Oligosaccharides, 2′-FL, and Milk Fat Supports Adequate Growth, Is Safe and Well-Tolerated in Healthy Term Infants: A Double-Blind, Randomised, Controlled, Multi-Country Trial" Nutrients 12, no. 11: 3560. https://doi.org/10.3390/nu12113560
APA StyleVandenplas, Y., de Halleux, V., Arciszewska, M., Lach, P., Pokhylko, V., Klymenko, V., Schoen, S., Abrahamse-Berkeveld, M., Mulder, K. A., Porcel Rubio, R., & on behalf of the VOYAGE Study Group. (2020). A Partly Fermented Infant Formula with Postbiotics Including 3′-GL, Specific Oligosaccharides, 2′-FL, and Milk Fat Supports Adequate Growth, Is Safe and Well-Tolerated in Healthy Term Infants: A Double-Blind, Randomised, Controlled, Multi-Country Trial. Nutrients, 12(11), 3560. https://doi.org/10.3390/nu12113560