Early Infant Formula Feeding Impacts Urinary Metabolite Profile at 3 Months of Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Infant Diet
2.3. Anthropometric Measures
2.4. Self-Reported Outcomes
2.5. Urinary Samples
2.6. Creatinine Analyses
2.7. Urine Metabolome Analysis
2.8. Statistical Analyses
3. Results
3.1. Maternal and Infant Characteristics
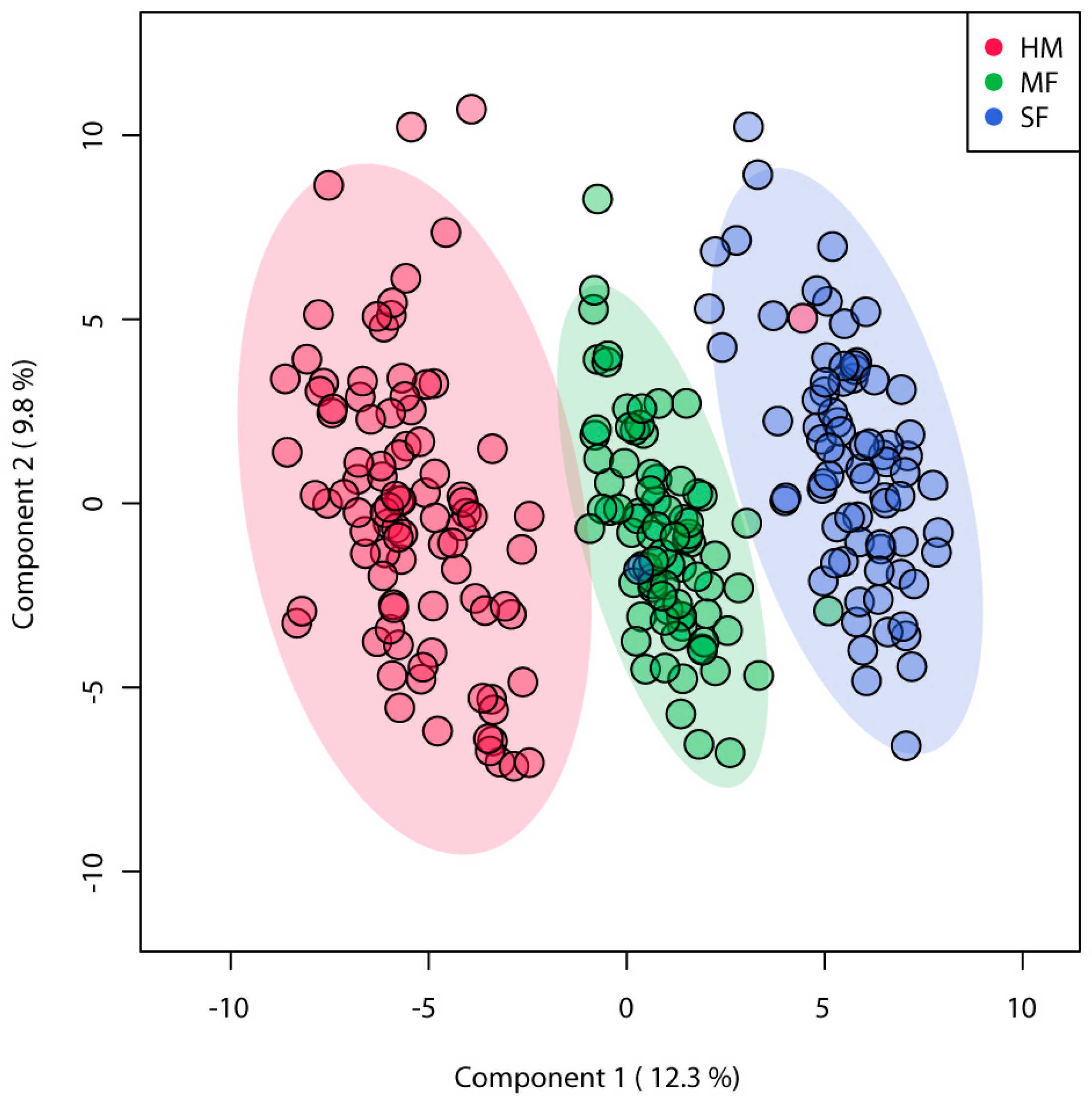
3.2. Urinary Metabolites Profile Was Altered by Neonatal Diet in Infants at 3 Months of Age
3.3. Formula Diet-Fed Infants Have Lower Abundance of Sugar and Sugar Alcohol Metabolites in Urine Relative to HM Infants at 3 Months of Age
3.4. Formula Diet Altered Amino Acid Abundance in the Urine Relative to HM Infants at 3 Months of Age
3.5. Fatty Acid and Dicarboxylic Acid Abundances Were Impacted by Neonatal Diet
3.6. SF Diet Fed Infants Showed Higher Abundance of Polyphenol Metabolites
4. Discussion
5. Conclusions
- (a)
- The main divergence in the metabolic profiling was observed in HM relative to the formula diet groups, while differences in urinary metabolites were also observed between the formula groups.
- (b)
- The dietary-specific pattern of urinary metabolites of amino acids and monosaccharides were found in HM infants aged three months, which might be linked to the microbial catabolism of proteins and carbohydrates. For instance, studies in mouse models [41,42] have shown that human milk oligosaccharides present in high abundance in human milk serve as substrates to the beneficial bacteria in the distal gut lowering the development of gastrointestinal diseases. Thus, we speculate that the sugar excretion in our cohort reflect human milk components interactions with the host-microbiota. Additionally, microbial metabolism was reported as the source for amino acids excretion in feces of breastfed vs formula-fed infants prior to solid food introduction at 24-months of age [43]. Thus, it is possible that the amino acids excretion in the urine of breastfed vs formula-fed infants in this study were driven by the microbial modification of amino acids through specialized microbial populations.
- (c)
- The SF diet enhanced the excretion of metabolites from polyphenols microbial catabolism. Furthermore, early life gut microbiota colonization via maternal milk components rather than shaping the neonate’s gut microbiota [44], can also affect the host-microbial metabolism. Thus, our findings indicate that urinary metabolites may mirror the infant’s metabolism as noninvasive biomarkers and a potential tool to evaluate the impact of infant diets in early life.
- (d)
- We speculate that metabolite changes could affect other organ systems in the body (i.e., liver and brain). Thus, future studies are needed to determine the early diet impact on short and long-term health effects.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, C.R.; Ling, P.-R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaudry, M.; Dufour, R.; Marcoux, S. Relation between infant feeding and infections during the first six months of life. J. Pediatr. 1995, 126, 191–197. [Google Scholar] [CrossRef]
- Donovan, S.M.; Comstock, S.S. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann. Nutr. Metab. 2016, 69, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.Å.; Korotkova, M.; Telemo, E. Breast-feeding, infant formulas, and the immune system. Ann. Allerg. Asthma Immunol. 2003, 90, 59–63. [Google Scholar] [CrossRef]
- Davis, E.C.; Wang, M.; Donovan, S. The role of early life nutrition in the establishment of gastrointestinal microbial composition and function. Gut Microbes 2017, 8, 143–171. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.; McGuire, M.; Rodriguez, J.M.; Geddes, D.T.; Hassiotou, F.; Hartmann, P.E.; McGuire, M.K. It’s Alive: Microbes and Cells in Human Milk and Their Potential Benefits to Mother and Infant. Adv. Nutr. 2014, 5, 571–573. [Google Scholar] [CrossRef]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A.; et al. Randomized Trial of Exclusive Human Milk versus Preterm Formula Diets in Extremely Premature Infants. J. Pediatr. 2013, 163, 1592–1595.e1. [Google Scholar] [CrossRef]
- Henderson, G.; Anthony, M.Y.; McGuire, W.L. Formula milk versus maternal breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2007, CD002972. [Google Scholar] [CrossRef]
- Hanson, L.Å. Session 1: Feeding and infant development Breast-feeding and immune function. Proc. Nutr. Soc. 2007, 66, 384–396. [Google Scholar] [CrossRef] [Green Version]
- Hanson, L.Å. The role of breastfeeding in prevention of neonatal infection. Semin. Neonatol. 2002, 7, 275–281. [Google Scholar] [CrossRef]
- Lönnerdal, B. Infant formula and infant nutrition: Bioactive proteins of human milk and implications for composition of infant formulas. Am. J. Clin. Nutr. 2014, 99, 712S–717S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, M.; Momin, S.R.; Senn, M.K.; Frazier-Wood, A.C. Metabolomic Insights into the Effects of Breast Milk Versus Formula Milk Feeding in Infants. Curr. Nutr. Rep. 2019, 8, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Shoji, H.; Shimizu, T. Effect of human breast milk on biological metabolism in infants. Pediatr. Int. 2018, 61, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardanzellu, F.; Fanos, V.; Strigini, F.A.L.; Artini, P.G.; Peroni, D.G. Human Breast Milk: Exploring the Linking Ring Among Emerging Components. Front. Pediatr. 2018, 6, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brink, L.R.; Mercer, K.E.; Piccolo, B.D.; Chintapalli, S.V.; Elolimy, A.; Bowlin, A.K.; Matazel, K.S.; Pack, L.; Adams, S.H.; Shankar, K.; et al. Neonatal diet alters fecal microbiota and metabolome profiles at different ages in infants fed breast milk or formula. Am. J. Clin. Nutr. 2020, 111, 1190–1202. [Google Scholar] [CrossRef]
- Hascoet, J.-M.; Hubert, C.; Rochat, F.; Legagneur, H.; Gaga, S.; Emady-Azar, S.; Steenhout, P.G. Effect of Formula Composition on the Development of Infant Gut Microbiota. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 756–762. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [Green Version]
- Uhl, O.; Fleddermann, M.; Hellmuth, C.; Demmelmair, H.; Koletzko, B. Phospholipid Species in Newborn and 4 Month Old Infants after Consumption of Different Formulas or Breast Milk. PLoS ONE 2016, 11, e0162040. [Google Scholar] [CrossRef]
- Anderson, M.; Eliot, K.; Kelly, P.; Shoemaker, J. Dicarboxylic Acid Excretion in Normal Formula-Fed and Breastfed Infants. Nutr. Clin. Pract. 2016, 31, 819–823. [Google Scholar] [CrossRef]
- Wahlén, E.; Strandvik, B. Effects of Different Formula Feeds on the Developmental Pattern of Urinary Bile Acid Excretion in Infants. J. Pediatr. Gastroenterol. Nutr. 1994, 18, 9–19. [Google Scholar] [CrossRef]
- Andres, A.; Cleves, M.A.; Bellando, J.B.; Pivik, R.T.; Casey, P.H.; Badger, T. Developmental Status of 1-Year-Old Infants Fed Breast Milk, Cow’s Milk Formula, or Soy Formula. Pediatrics 2012, 129, 1134–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andres, A.; Casey, P.H.; Cleves, M.A.; Badger, T.M. Body Fat and Bone Mineral Content of Infants Fed Breast Milk, Cow’s Milk Formula, or Soy Formula during the First Year of Life. J. Pediatr. 2013, 163, 49–54. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height, and Body Mass Index-for Age: Methods and Development, 1st ed.; World Health Organization: Geneva, Switzerland, 2006; ISBN 9294154693X. [Google Scholar]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant. J. 2008, 53, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinforma. 2019, 68, e86. [Google Scholar] [CrossRef]
- He, X.; Sotelo-Orozco, J.; Rudolph, C.; Lönnerdal, B.; Slupsky, C.M. The Role of Protein and Free Amino Acids on Intake, Metabolism, and Gut Microbiome: A Comparison between Breast-Fed and Formula-Fed Rhesus Monkey Infants. Front. Pediatr. 2020, 7, 563. [Google Scholar] [CrossRef]
- He, X.; Parenti, M.; Grip, T.; Lönnerdal, B.; Timby, N.; Domellöf, M.; Hernell, O.; Slupsky, C.M. Fecal microbiome and metabolome of infants fed bovine MFGM supplemented formula or standard formula with breast-fed infants as reference: A randomized controlled trial. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Slupsky, C.M.; He, X.; Hernell, O.; Andersson, Y.; Rudolph, C.; Lönnerdal, B.; West, C.E. Postprandial metabolic response of breast-fed infants and infants fed lactose-free vs regular infant formula: A randomized controlled trial. Sci. Rep. 2017, 7, 3640. [Google Scholar] [CrossRef]
- Butte, N.F.; Wong, W.W.; Hopkinson, J.M.; Smith, E.O.; Ellis, K.J. Infant feeding mode affects early growth and body composition. Pediatrics 2000, 106, 1355–1366. [Google Scholar] [CrossRef]
- Koletzko, B. Glutamate Supply and Metabolism in Infants. Ann. Nutr. Metab. 2018, 73 (Suppl. 5), 29–35. [Google Scholar] [CrossRef]
- Chuang, C.-K.; Lin, S.-P.; Lee, H.-C.; Wang, T.-J.; Shih, Y.-S.; Huang, F.-Y.; Yeung, C.-Y. Free Amino Acids in Full-Term and Pre-Term Human Milk and Infant Formula. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 496–500. [Google Scholar] [CrossRef]
- Agostoni, C.; Carratù, B.; Boniglia, C.; Riva, E.; Sanzini, E. Free Amino Acid Content in Standard Infant Formulas: Comparison with Human Milk. J. Am. Coll. Nutr. 2000, 19, 434–438. [Google Scholar] [CrossRef]
- Kirchberg, F.F.; Harder, U.U.; Weber, M.; Grote, V.; Demmelmair, H.; Peissner, W.W.; Rzehak, P.; Xhonneux, A.; Carlier, C.C.; Ferre, N.N.; et al. Dietary Protein Intake Affects Amino Acid and Acylcarnitine Metabolism in Infants Aged 6 Months. J. Clin. Endocrinol. Metab. 2015, 100, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Childhood Obesity Trial Study Group; Socha, P.; Grote, V.; Gruszfeld, D.; Janas, R.M.; Demmelmair, H.; Closa-Monasterolo, R.; Subías, J.E.; Scaglioni, S.; Verduci, E.; et al. Milk protein intake, the metabolic-endocrine response, and growth in infancy: Data from a randomized clinical trial. Am. J. Clin. Nutr. 2011, 94, 1776S–1784S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Xiu, H.; Zhou, Q.; Sun, L.; Mu, H.; Li, H.; Wang, S.; Li, X.; Chen, W.; Dong, J. Application of Urinary Polyphenol Biomarkers Measured by Liquid Chromatography Tandem Mass Spectrometry to Assess Polyphenol Intake and Their Association with Overweight and Obesity in Free-Living Healthy Subjects. Oxidative Med. Cell. Longev. 2019, 2019, 4809836. [Google Scholar] [CrossRef] [Green Version]
- Appeldoorn, M.M.; Vincken, J.-P.; Aura, A.-M.; Hollman, P.C.H.; Gruppen, H. Procyanidin Dimers Are Metabolized by Human Microbiota with 2-(3,4-Dihydroxyphenyl)acetic Acid and 5-(3,4-Dihydroxyphenyl)-γ-valerolactone as the Major Metabolites. J. Agric. Food Chem. 2009, 57, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut BacteriumAkkermansia muciniphilaand Attenuate High-Fat Diet–Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masumoto, S.; Terao, A.; Yamamoto, Y.; Mukai, T.; Miura, T.; Shoji, T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci. Rep. 2016, 6, 31208. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef] [Green Version]
- Jantscher-Krenn, E.; Bode, L. Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Minerva Pediatr. 2012, 64, 83–99. [Google Scholar]
- Good, M.; Sodhi, C.P.; Yamaguchi, Y.; Jia, H.; Lu, P.; Fulton, W.B.; Martin, L.Y.; Prindle, T.; Nino, D.F.; Zhou, Q.; et al. The human milk oligosaccharide 2′-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br. J. Nutr. 2016, 116, 1175–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sillner, N.; Walker, A.; Lucio, M.; Maier, T.V.; Bazanella, M.; Rychlik, M.; Haller, D.; Schmitt-Kopplin, P. Longitudinal profiles of dietary and microbial metabolites in formula- and breastfed infants. J. bioRxiv 2020. [Google Scholar] [CrossRef]
- Elsen, L.W.J.; Garssen, J.; Burcelin, R.; Verhasselt, V. Shaping the Gut Microbiota by Breastfeeding: The Gateway to Allergy Prevention? Front. Pediatr. 2019, 7. [Google Scholar] [CrossRef]

| HM | MF | SF | p-Value | |
|---|---|---|---|---|
| n | 93 | 80 | 76 | |
| Child Sex, N (%) | 0.271 1 | |||
| Female | 43 (46.2%) | 29 (36.2%) | 27 (35.5%) | |
| Male | 50 (53.8%) | 51 (63.8%) | 49 (64.5%) | |
| Child Race, N (%) | 0.004 1 | |||
| Caucasian | 88 (94.6%) | 71 (88.8%) | 59 (77.6%) | |
| Non-Caucasian | 5 (5.4%) | 9 (11.2%) | 17 (22.4%) | |
| Gestational Age, weeks (SD) | 39.519 (1.082) | 39.112 (0.886) | 39.053 (0.949) | 0.003 2 |
| Birth Weight, kg (SD) | 3.572 (0.333) | 3.512 (0.396) | 3.454 (0.404) | 0.132 2 |
| Birth Length, cm (SD) | 51.647 (2.130) | 51.498 (2.285) | 51.201 (2.236) | 0.423 2 |
| Weight at 3 month, kg (SD) | 6.223 (0.655) | 6.189 (0.674) | 6.092 (0.529) | 0.385 2 |
| Length at 3 month, cm (SD) | 60.368 (1.820) | 60.330 (2.271) | 59.810 (1.576) | 0.136 2 |
| Weight-for-Length Z-score | 0.282 (0.964) | 0.287 (0.960) | 0.333 (0.849) | 0.934 2 |
| Sugar Metabolites | HM 1 | SEM 2 | MF 1 | SEM 2 | SF 1 | SEM 2 | FDR 3 |
|---|---|---|---|---|---|---|---|
| lactulose | 336,279.97 a | 14,022.23 | 247,323.82 b | 12,749.61 | 14,196.64 c | 4591.57 | <0.01 |
| maltose | 34,973.18 a | 2167.14 | 16,006.10 b | 1520.60 | 4519.71 c | 397.87 | <0.01 |
| leucrose | 3676.20 a | 166.62 | 1060.06 b | 75.08 | 1837.64 c | 122.26 | <0.01 |
| raffinose | 1084.35 a | 122.07 | 238.66 b | 9.1 | 732.36 c | 296.15 | <0.01 |
| fucose | 139,782.64 a | 8791.63 | 53,538.91 b | 2002.00 | 64,898.99 b | 3622.54 | <0.01 |
| ribose | 12,310.79 a | 390.34 | 9439.59 b | 348.86 | 8822.64 b | 328.75 | <0.01 |
| arabinose | 11,618.04 a | 436.44 | 8996.94 b | 368.72 | 8884.23 b | 363.02 | <0.01 |
| 1,5-anhydroglucitol | 54,718.26 a | 2247.00 | 17,163.66 b | 812.97 | 40,641.95 c | 2275.23 | <0.01 |
| xylose | 184,198.91 a | 6264.48 | 137,284.11 b | 4142.07 | 142,267.39 b | 6501.21 | <0.01 |
| isoribose | 3285.43 a | 190.48 | 2637.76 b | 144.15 | 2955.26 a,b | 167.3 | 0.05 |
| isomaltose | 5398.49 a,c | 898.31 | 2925.34 b | 112.23 | 4661.21 c | 643.68 | <0.01 |
| sucrose | 2337.10 a,c | 144.04 | 1872.82 b | 186.53 | 4055.60 c | 748.1 | 0.01 |
| mannose | 496,603.14 a,b | 25,463.34 | 439,742.43 b | 22,004.12 | 68,955.42 c | 13,014.98 | <0.01 |
| glucose | 93,916.11 a,b | 4419.51 | 81,781.49 b | 3994.20 | 24,763.18 c | 1457.20 | <0.01 |
| threose | 15,276.43 a,b | 1450.50 | 18,832.83 b | 2002.76 | 9808.84 c | 1108.25 | <0.01 |
| erythrose | 12,508.35 a | 1231.90 | 16,378.82 b | 1559.38 | 8712.12 c | 1031.38 | <0.01 |
| lactitol | 6657.86 a | 365.48 | 3782.18 b | 233.71 | 567.13 c | 55.33 | <0.01 |
| hexitol | 63,063.27 a | 3831.99 | 12,559.48 b | 710.18 | 7928.03 c | 468.42 | <0.01 |
| myo-inositol | 174,330.32 a | 14,214.23 | 89,158.83 b | 7248.63 | 104,181.71 b | 11,103.32 | <0.01 |
| glycerol | 41,361.03 a | 2669.30 | 33,635.40 b | 3302.20 | 33,937.36 b | 3093.07 | <0.01 |
| ribitol | 40,869.38 a,c | 1346.74 | 30,120.87 b | 1216.22 | 36,017.68 c | 1417.21 | <0.01 |
| lyxitol | 43,275.89 a,c | 2702.30 | 34,099.54 b | 2624.39 | 43,992.82 c | 3680.79 | <0.01 |
| galactinol | 2041.95 a | 84.28 | 1104.02 b | 29.26 | 759.29 c | 36.61 | <0.01 |
| mannitol | 28,703.55 a,c | 9513.62 | 14,531.88 b | 642.03 | 20,289.30 c | 2674.27 | <0.01 |
| tartaric acid | 945.04 a,c | 221.94 | 1305.12 b | 168.3 | 1172.62 c | 467.75 | <0.01 |
| Amino Acids | HM 1 | SEM 2 | MF 1 | SEM 2 | SF 1 | SEM 2 | FDR 3 |
|---|---|---|---|---|---|---|---|
| histidine | 83,412.36 a | 6632.23 | 48,203.59 b | 5667.53 | 122,964.25 c | 11,274.22 | <0.01 |
| glycine | 393,582.61 a,b | 31,185.59 | 355,168.56 b | 27,719.71 | 535,661.74 c | 36,454.11 | <0.01 |
| tryptophan | 42,979.28 a,b | 2287.27 | 37,026.27 b | 1964.72 | 53,510.87 c | 2575.93 | <0.01 |
| cystine | 7536.74 a,b | 531.85 | 8513.79 b | 1625.75 | 10,904.34 c | 677.24 | <0.01 |
| asparagine | 6862.53 a,b | 230.49 | 7391.12 b | 231.12 | 8542.12 c | 351.29 | <0.01 |
| alanine | 286,085.48 a | 12,290.38 | 195,293.56 b | 9948.89 | 235,474.88 b | 13,378.32 | <0.01 |
| serine | 25,789.93 a | 2944.29 | 11,442.01 b | 1855.34 | 14,446.06 b | 1539.00 | <0.01 |
| glutamate | 2073.78 a | 310.36 | 1410.73 b | 191.34 | 1166.16 b | 124.82 | <0.01 |
| proline | 15,639.28 a | 2133.72 | 9850.33 b | 1843.49 | 7586.87 b | 613.61 | 0.01 |
| aminomalonate | 10,504.22 a | 674.29 | 8019.96 b | 562.93 | 8139.34 b | 541.41 | 0.01 |
| Fatty Acids | HM 1 | SEM 2 | MF 1 | SEM 2 | SF 1 | SEM 2 | FDR 3 |
|---|---|---|---|---|---|---|---|
| myristic acid | 2223.06 a | 282.29 | 1522.09 b | 60.91 | 1510.69 b | 76.7 | <0.01 |
| arachidic acid | 4545.14 a | 398.59 | 3582.22 b | 121.74 | 3443.39 b | 125.25 | <0.01 |
| lactic acid | 9645.72 a | 784.78 | 6973.41 b | 320.83 | 7781.34 b | 823.55 | 0.01 |
| capric acid | 1554.56 a | 288.65 | 1113.27 a,b | 52.17 | 1014.99 b | 55.32 | <0.01 |
| palmitic acid | 40,491.55 a | 4516.01 | 31,238.59 a,b | 1112.55 | 29,289.79 b | 1124.40 | <0.01 |
| DCAs | HM 1 | SEM 2 | MF 1 | SEM 2 | SF 1 | SEM 2 | FDR 3 |
|---|---|---|---|---|---|---|---|
| methylmalonic acid | 71,256.81 a | 5079.69 | 42,923.66 b | 3670.23 | 42,424.23 b | 2381.65 | <0.01 |
| oxalic acid | 78,654.24 a | 11,197.09 | 76,873.18 b | 9254.18 | 73,285.75 a,b | 10,122.92 | 0.05 |
| succinic acid | 53,680.60 a,b | 4071.73 | 49,757.76 b | 2897.10 | 40,729.92 c | 2349.76 | 0.01 |
| Polyphenol Derivatives | HM 1 | SEM 2 | MF 1 | SEM 2 | SF 1 | SEM 2 | FDR3 |
|---|---|---|---|---|---|---|---|
| 3,4-dihydroxyphenylacetic acid | 2878.32 a,b | 112.05 | 3248.77 b | 171.18 | 4437.78c | 181.23 | <0.01 |
| 3-hydroxyphenylacetic acid | 778.17 a | 46.6 | 1192.32 b | 160.31 | 1470.35 c | 45.52 | <0.01 |
| 4-hydroxyhippuric acid | 11,301.01 a,b | 1303.86 | 11,903.33 b | 1454.27 | 14,253.94 c | 839.55 | <0.01 |
| 4-hydroxyphenylacetic acid | 24,389.05 a | 2827.89 | 33,122.91 b | 2848.73 | 54,340.90 c | 5328.47 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, F.; Mercer, K.E.; Lin, H.; Sims, C.R.; Pack, L.M.; Goode, G.; Badger, T.; Andres, A.; Yeruva, L. Early Infant Formula Feeding Impacts Urinary Metabolite Profile at 3 Months of Age. Nutrients 2020, 12, 3552. https://doi.org/10.3390/nu12113552
Rosa F, Mercer KE, Lin H, Sims CR, Pack LM, Goode G, Badger T, Andres A, Yeruva L. Early Infant Formula Feeding Impacts Urinary Metabolite Profile at 3 Months of Age. Nutrients. 2020; 12(11):3552. https://doi.org/10.3390/nu12113552
Chicago/Turabian StyleRosa, Fernanda, Kelly E. Mercer, Haixia Lin, Clark R. Sims, Lindsay M. Pack, Grace Goode, Thomas Badger, Aline Andres, and Laxmi Yeruva. 2020. "Early Infant Formula Feeding Impacts Urinary Metabolite Profile at 3 Months of Age" Nutrients 12, no. 11: 3552. https://doi.org/10.3390/nu12113552
APA StyleRosa, F., Mercer, K. E., Lin, H., Sims, C. R., Pack, L. M., Goode, G., Badger, T., Andres, A., & Yeruva, L. (2020). Early Infant Formula Feeding Impacts Urinary Metabolite Profile at 3 Months of Age. Nutrients, 12(11), 3552. https://doi.org/10.3390/nu12113552





