Effect of High-Oleic Peanut Intake on Aging and Its Hippocampal Markers in Senescence-Accelerated Mice (SAMP8)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Animals and Diet
2.3. Amino Acid, TBARS, and Proteome Analyses
2.4. Statistics
3. Results and Discussion
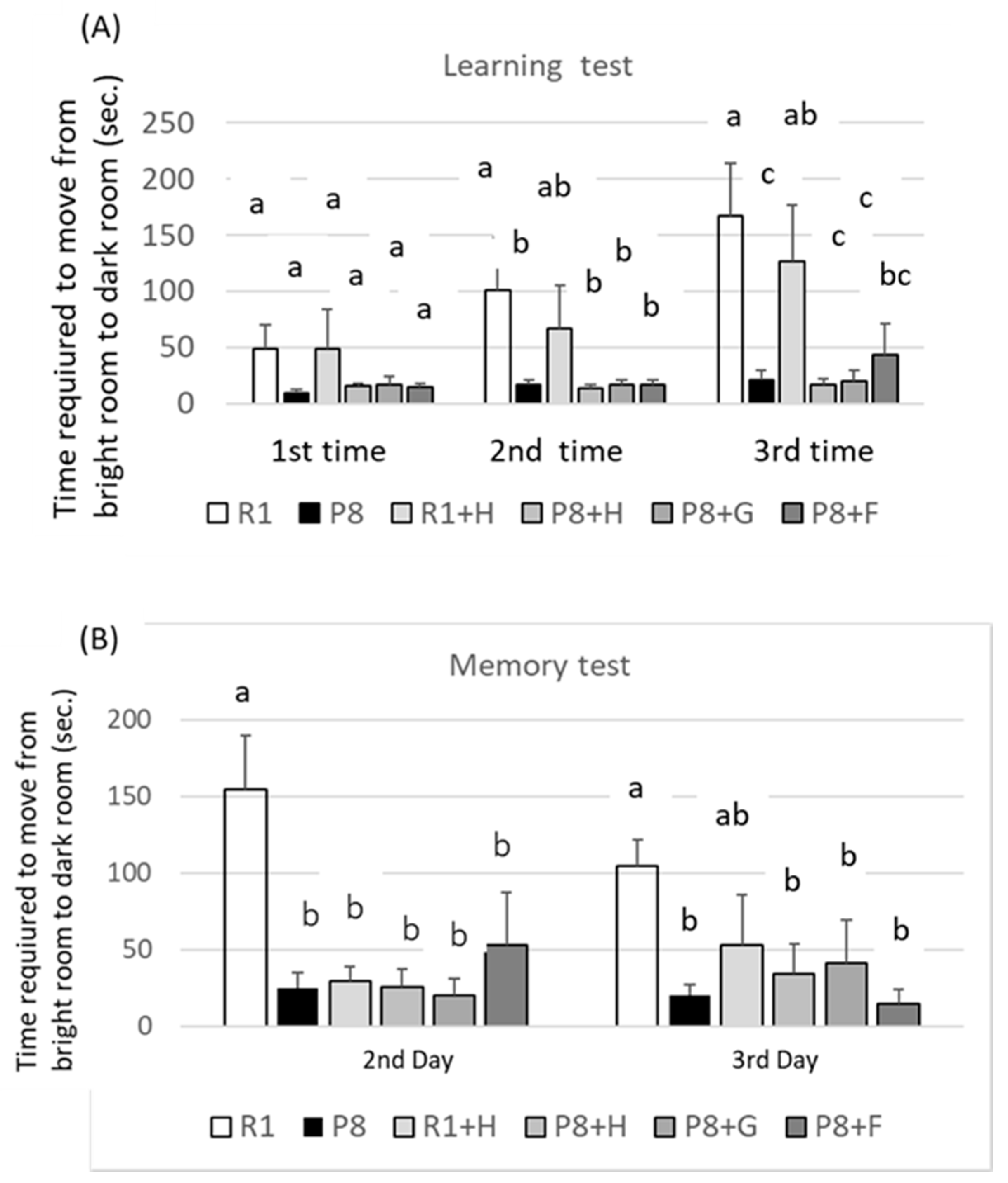
3.1. Suppression of Peanut on Aging of SAMP8 in Appearance (Phenotype) and Learning and Memory Abilities
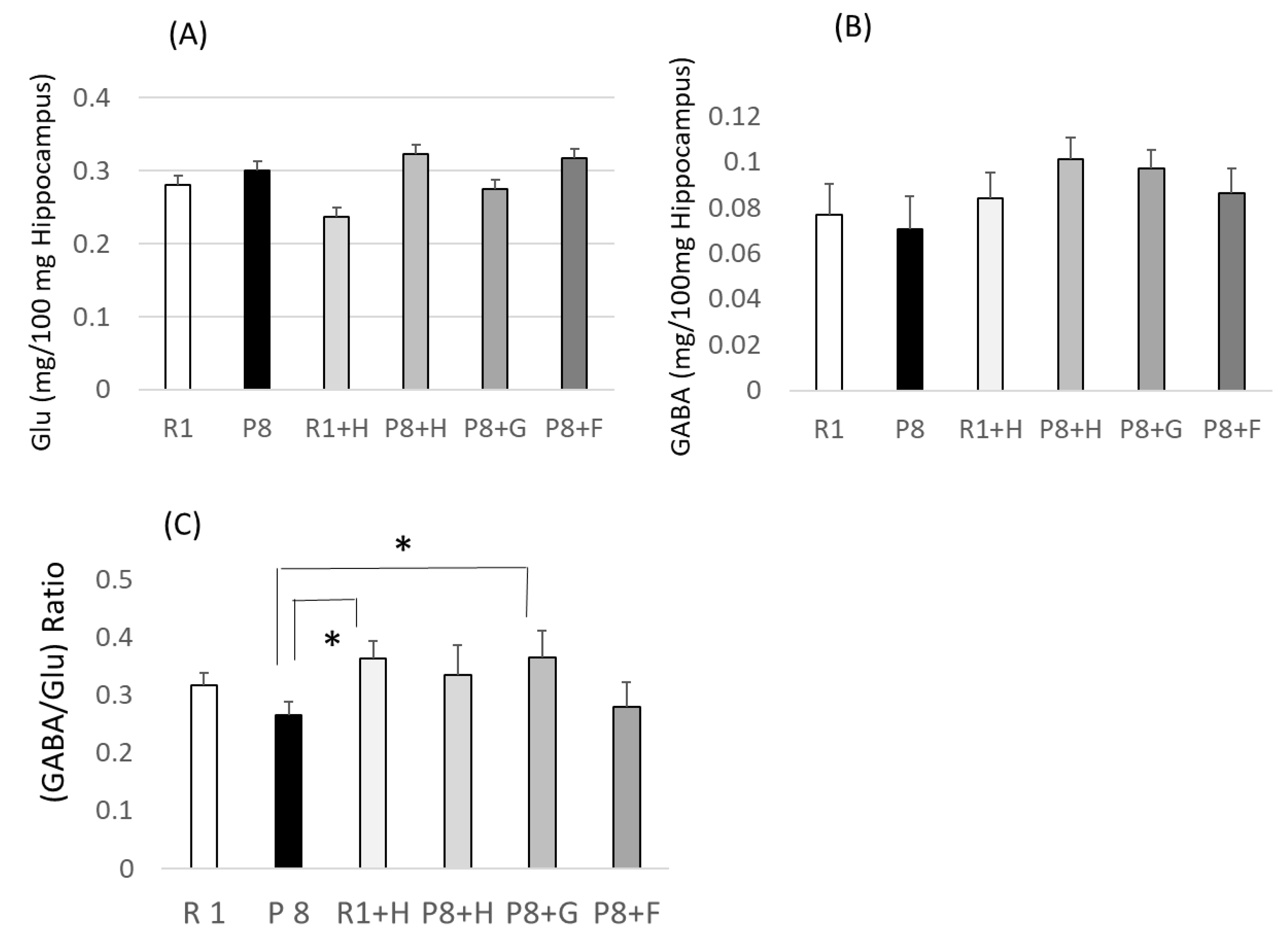
3.2. Relation between (GABA/Glutamic Acid) Ratio as a New Senescense Marker and Peroxidation in the Hippocampus
3.3. Regulation of Glial Protein Degradation and Antioxidant Enzymes by Dietary Peanuts
3.4. Regulation of the Expression of Proteins Related to Glutamic Acid Transport and Metabolism by Dietary Peanut
3.5. Regulation of the Expression of Protein Related to Formation of Neuromodulator by Dietary Peanut
3.6. Tublin and Hemoglobin Subunit Levels in Hippocampus of Mice Given Peanut
3.7. Hemoglobin Subunit Alpha and Beta Levels in Hippocampus of Mice Given Peanuts
3.8. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barbour, J.A.; Howe, P.R.C.; Buckley, J.D.; Bryan, J.; Coates, A.M. Cerebrovascular and cognitive benefits of high-oleic peanut consumption in healthy overweight middle-aged adults. Nutr. Neurosci. 2017, 20, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Parks, C.H.; Noh, J.S.; Roh, S.S. Role of oligomeric procyanidins derived from an extract of persimmon fruits in the oxidative stress-related aging process. Molecules 2014, 19, 6707–6726. [Google Scholar] [CrossRef] [PubMed]
- Muthaiyah, B.; Essa, M.M.; Lee, M.; Chauhan, V.; Kaur, K.; Chauhan, A. Dietary supplementation of walnuts improves memory deficits and learning skills in transgenic mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 42, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Okereke, O.; Devore, E.; Rosner, B.; Breteler, M.; Grodstein, F. Long-term intake of nuts in relation to cognitive function in older women. J. Nutr. Health Aging 2014, 18, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Sowell, R.A.; Owen, J.B.; Butterfield, D.A. Proteomics in animal models of Alzheimer’s and Parkinson’s diseases. Ageing Res. Rev. 2009, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tarbit, I.; Perry, E.K.; Perry, R.H.; Blessed, G.; Tomlinson, B.E. Hippocampal free amino acids in Alzheimer’s disease. J. Neurochem. 1980, 35, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yin, X.; Edden, R.A.E.; Evans, A.C.; Xu, J.; Gao, G.; Li, H.; Li, M.; Zhao, B.; Wang, J.; et al. Altered hippocampal GABA and glutamate levels and uncoupling from functional connectivity in multiple sclerosis. Hippocampus 2018, 28, 813–823. [Google Scholar] [CrossRef]
- Asamoto, H.; Minamisawa, H.; Imai, K. Proteomic analysis of aged rat hippocampus by HPLC with fluorescence detection using a fluorogenic derivatization reagent. Bunseki Kagaku 2012, 61, 547–553. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Poon, H.F.; Castegna, A.; Farr, S.A.; Thongboonkerd, V.; Lynn, B.C.; Banks, W.A.; Morley, J.E.; Klein, J.B.; Butterfield, D.A. Quantitative proteomics analysis of specific protein expression and oxidative modification in aged senescence-accelerated-prone 8 mice. Neuroscience 2004, 126, 915–926. [Google Scholar] [CrossRef]
- Shi, C.; Xiao, S.; Liu, J.; Guo, K.; Wu, F.; Yew, D.T.; Xu, J. Ginkgo biloba extract EGb761 protects against aging-associated mitochondrial dysfunction in platelets and hippocampi of SAMP8 mice. Platelets 2010, 21, 379. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Zhang, Z.; Pei, X.; Wang, J.; Li, Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009, 256, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Poon, H.F. The senescence-accelerated prone mouse (SAMP8): A model of age-related cognitive decline with relevance to alternations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp. Gerontol. 2005, 40, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Neuner, S.M.; Wilmott, L.A.; Hoffmannb, B.A.; Mozhuic, K.; Kaczorowski, C.C. Hippocampal proteomics defines pathways associated with memory decline and resilience in normal aging and Alzheimer’s disease mouse models. Behav. Brain Res. 2017, 322, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Sakura, M.; Chiba, Y. The Grading Score System: A Method For Evaluating The Degree Of Senescence In SAM Strains Of Mice. In The Senescence-Accelerated Mouse (SAM)-Achievement and Future Directions; Takeda, T., Akiguchi, I., Higuchi, K., Hosokawa, M., Hosokawa, T., Nomura, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 561–567. [Google Scholar]
- Ivanisevic, J.; Stauch, K.L.; Petrascheck, M.; Benton, H.P.; Epstein, A.A.; Fang, S.; Gorantla, M.; Tran, M.; Hoang, L.; Kurczy, M.E.; et al. Metabolic drift in the aging brain. Aging 2016, 8, 1–21. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissue by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Zhao, G.; Gao, H.; Qiu, J.; Lu, W.; Wei, X. Molecular mechanism of Pprotective effects of grape seed proanthocyanidin extract on reperfusion arrhythmias in rats in vivo. Biol. Pharm. Bull. 2010, 33, 759–767. [Google Scholar] [CrossRef]
- Chan, Y.C.; Hsu, C.C.; Wang, M.F.; Liao, J.W.; Su, T.Y. Beneficial effect of yam on the amyloid-beta protein, monoamine oxidase B and cognitive deficit in mice with accelerated senescence. J. Sci. Food Agric. 2006, 86, 1517–1525. [Google Scholar] [CrossRef]
- McEntee, W.J.; Crook, T.H. Glutamate: Its role in learning, memory, and the aging brain. Psychopharmacology 1993, 111, 391–401. [Google Scholar] [CrossRef]
- Farr, S.A.; Poon, H.F.; Dogrukol-Ak, D.; Drake, J.; Banks, W.A.; Eyerman, E.; Butterfield, D.A.; Morley, J.E. The antioxidant alpha-lipoic acid and N-acetyl cysteine reserve memory impairment and brain oxidative stress in aged SAMP8. J. Neurochem. 2003, 84, 1173–1183. [Google Scholar] [CrossRef]
- Chou, M.-Y.; Chen, Y.J.; Lin, L.H.; Nakao, A.; Lim, L.; Wang, M.F.; Yong, S.M. Protective effects of hydrolyzed chicken extract (Probeptigen®/Cmi-168) on memory retention and brain oxidative stress in senescence-accelerated mice. Nutrients 2019, 11, 1870. [Google Scholar] [CrossRef]
- Schubert, K.O.; Focking, M.; Wynne, K.; Cotter, D. Proteome and pathway of chronic haloperidol treatment in mouse hippocampus. Proteomics 2016, 16, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Lee, S.E.; Boo, J.H.; Kim, M.; Yoon, Y.D.; Kim, S.; Inhee, M.J. Profiling proteins related to amyloid deposited brain of Tg2576 mice. Proteomics 2004, 4, 3359–3368. [Google Scholar] [CrossRef] [PubMed]
- Espey, M.G.; Kustova, Y.; Sei, Y.; Basile, A.S. Extracellular glutamate levels are chronically elevated in the brains of LP-BM5-infected mice: A mechanism of retrovirus-induced encephalopathy. J. Neurochem. 1998, 71, 2079–2087. [Google Scholar] [CrossRef]
- Takagi, M.; Ishigaki, Y.; Uno, K.; Sawada, S.; Imai, J.; Kaneko, K.; Hasegawa, Y.; Yamada, T.; Tokita, A.; Iseki, K.; et al. Cognitive dysfunction associated with anti-glutamic acid decarboxylase autoimmunity: A case-control study. BMC Neurol. 2013, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, A.-Q.; Yew, D.T. Age related changes of various markers of astrocytes in senescence-accelerated mice hippocampus. Neurochem. Int. 2005, 46, 565–574. [Google Scholar] [CrossRef]
- Kurokawa, T.; Asada, S.; Nishitani, S.; Hazeki, O. Age-related changes in manganese superoxide dismutase activity in the cerebral cortex of senescence-accelerated prone and resistant mouse. Neurosci. Lett. 2001, 298, 135–138. [Google Scholar] [CrossRef]
- Yamauchi, T. Neuronal Ca2+/calmodulin-dependent protein kinase II—Discovery, progress in a quarter of a century, and perspective: Implication for learning and memory. Biol. Pharm. Bull. 2005, 28, 1342–1354. [Google Scholar] [CrossRef]
- Ferreira, A.; Han, H.Q.; Greengard, P.; Kosik, K.S. Suppression of synapsin II inhibits the formation and maintenance of synapses in hippocampal culture. Proc. Natl. Acad. Sci. USA 1995, 92, 9225–9229. [Google Scholar] [CrossRef]
- Corradi, A.; Zanardi, A.; Giacomini, C.; Onofri, F.; Valtorta, F.; Zoliand, M.; Benfenati, F. Synapsin-I- and synapsin-II-null mice display an increased age-dependent cognitive impairment. J. Cell. Sci. 2008, 121, 3042–3051. [Google Scholar] [CrossRef][Green Version]
- Rusu, M.; Mocan, A.; Ferreira, I.C.F.R.; Popa, D.S. Health benefits of nut consumption in middle-aged and elderly population. Antioxidants 2019, 8, 302. [Google Scholar] [CrossRef]



| Expreimental Groups | R1 | P8 | R1 + H | P8 + H | P8 + G | P8 + F |
|---|---|---|---|---|---|---|
| Mouse strains | SAMR1 | SAMP8 | SAMR1 | SAMP8 | SAMP8 | SAMP8 |
| Casein | 20 | 20 | 20 | 20 | 20 | 20 |
| α-Cornstarch | 55.95 | 55.95 | 52.47 | 52.47 | 52.03 | 55.75 |
| Sucrose | 7 | 7 | 7 | 7 | 7 | 7 |
| Cellulose powder | 5 | 5 | 5 | 5 | 5 | 5 |
| Corn oil | 7 | 7 | 3.48 | 3.48 | 3.92 | 7 |
| Mineral mixture a | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin mixture b | 1 | 1 | 1 | 1 | 1 | 1 |
| L-Cystine | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Peanuts wholegrains | 7 | 7 | ||||
| Germ-rich fraction | 7 | |||||
| Ferulic acid | 0.2 | |||||
| Total (%) | 100 | 100 | 100 | 100 | 100 | 100 |
| Accession | Description | (R1 + H)/R1 | P8/R1 | (P8 + H)/P8 | (P8 + G)/P8 | (P8 + F)/P8 | |
|---|---|---|---|---|---|---|---|
| Glial fibrillary acidic protein | |||||||
| GFAP_MOUSE | Glial fibrillary acidic protein OS = Mus musculus GN = Gfap PE = 1 SV = 4 | ||||||
| Fold change (ratio) | 1.2 | 1.52 | 1.15 | 1.16 | 0.93 | ||
| p Value | 0.94 | 1 | 0.98 | 0.99 | 0.16 | ||
| A0MTM0_MOUSE | Glial fibrillary acidic protein (Fragment) OS = Mus musculus GN = Gfap PE = 2 SV = 1 | ||||||
| Fold change (ratio) | 1.28 | 1.09 | 1.07 | ||||
| p Value | 0.98 | 0.72 | 0.69 | ||||
| SOD | |||||||
| Q3U8W4_MOUSE | Superoxide dismutase OS=Mus musculus GN = Sod2 PE = 2 SV = 1 | ||||||
| Fold change (ratio) | NU | NU | 1.16 | 1.12 | 1.04 | ||
| p Value | 0.65 | 0.65 | 0.56 | ||||
| Q4FJX9_MOUSE | Superoxide dismutase OS = Mus musculus GN = Sod2 PE = 2 SV = 1 | ||||||
| Fold change (ratio) | NU | NU | 1.25 | 1.12 | 1.12 | ||
| p Value | 0.74 | 0.62 | 0.64 | ||||
| SODM_MOUSE | Superoxide dismutase [Mn], mitochondrial OS = Mus musculus GN = Sod2 PE = 1 SV = 3 | ||||||
| Fold change (ratio) | NU | NU | 1.23 | 1 | 1.16 | ||
| p Value | 0.73 | 0.5 | 0.62 | ||||
| Glutathione S-transferase | |||||||
| D3YVP6_MOUSE | Glutathione S-transferase Mu 7 OS = Mus musculus GN = Gstm7 PE = 4 SV = 2 | ||||||
| Fold change (ratio) | 1.02 | 1.04 | 0.94 | 0.83 | 0.89 | ||
| p Value | 0.59 | 0.67 | 0.33 | 0.12 | 0.2 | ||
| D3YX76_MOUSE | Glutathione S-transferase Mu 2 OS = Mus musculus GN = Gstm2 PE = 3 SV = 1 | ||||||
| Fold change (ratio) | 1.03 | 1.02 | 1.04 | 0.83 | |||
| p Value | 0.57 | 0.63 | 0.6 | 0.08 | |||
| A2AE91_MOUSE | Glutathione S-transferase, mu 4 OS = Mus musculus GN = Gstm4 PE = 3 SV = 1 | ||||||
| Fold change (ratio) | 1.01 | 1.05 | 0.93 | 0.83 | 0.85 | ||
| p Value | 0.52 | 0.69 | 0.3 | 0.11 | 0.1 | ||
| Q8R5I6_MOUSE | Glutathione S-transferase mu 4 OS = Mus musculus GN = Gstm4 PE = 2 SV = 1 | ||||||
| Fold change (ratio) | 1.02 | 1.09 | 0.92 | 0.81 | 0.91 | ||
| p Value | 0.55 | 0.83 | 0.3 | 0.08 | 0.25 | ||
| Glial high affinity glutamate transporter | |||||||
| A2APL7_MOUSE | Solute carrier Family 1 (Glial high affinity glutamate transporter), member 2 OS = Mus musculus GN = Slc1a2 PE = 2 SV = 1 | ||||||
| Fold change (ratio) | 0.72 | 0.91 | 1.04 | 0.86 | 0.78 | ||
| p Value | 0.02 | 0.22 | 0.61 | 0.14 | 0.16 | ||
| A2APL8_MOUSE | Solute carrier family 1 (Glial high affinity glutamate transporter), member 2 OS = Mus musculus GN = Slc1a2 PE = 4 SV = 1 | ||||||
| Fold change (ratio) | 0.73 | 1.05 | 0.93 | 0.73 | 1.25 | ||
| p Value | 0.01 | 0.61 | 0.29 | 0.02 | 0.92 | ||
| Q543U3_MOUSE | Solute carrier family 1 (Glial high affinity glutamate transporter), member 3 OS = Mus musculus GN = Slc1a3 PE = 2 SV = 1 | ||||||
| Fold change (ratio) | 0.93 | 1.19 | 0.82 | 0.84 | 1.07 | ||
| p Value | 0.38 | 0.87 | 0.13 | 0.17 | 0.63 | ||
| A2APM7_MOUSE | Solute carrier family 1 (Glial high affinity glutamate transporter), member 2 OS = Mus musculus GN = Slc1a2 PE = 4 SV = 1 | ||||||
| Fold change (ratio) | 0.79 | 0.94 | 0.63 | 0.41 | 1.45 | ||
| p Value | 0.11 | 0.31 | 0.15 | 0 | 0.96 | ||
| Q3UYK6_MOUSE | Solute carrier family 1 (Glial high affinity glutamate transporter), member 2 OS = Mus musculus GN = Slc1a2 PE = 2 SV = 1 | ||||||
| Fold change (ratio) | 0.79 | 0.81 | 0.94 | 0.76 | 1.25 | ||
| p Value | 0.08 | 0.19 | 0.35 | 0.06 | 0.94 | ||
| Calcium/calmodulin-dependent protein kinase | |||||||
| KCC2B_MOUSE | Calcium/calmodulin-dependent protein kinase type II sub-unit beta OS = Mus musculus GN = Camk2b PE = 1 SV = 2 | ||||||
| Fold change (ratio) | 0.99 | 1.3 | 0.64 | 0.94 | 1.17 | ||
| p Value | 0.39 | 0.99 | 0 | 0.27 | 0.99 | ||
| KCC2A_MOUSE | Calcium/calmodulin-dependent protein kinase type II sub-unit alpha OS = Mus musculus GN = Camk2a PE = 1 SV = 2 | ||||||
| Fold change (ratio) | 0.97 | 1.01 | 0.85 | 0.89 | 1.11 | ||
| p Value | 0.3 | 0.5 | 0.03 | 0.06 | 0.98 | ||
| E9Q1V9_MOUSE | Calcium/calmodulin-dependent protein kinase type II sub-unit delta OS = Mus musculus GN = Camk2d PE = 4 SV = 1 | ||||||
| Fold change (ratio) | 0.93 | 1.07 | 0.7 | 0.91 | 1.2 | ||
| p Value | 0.21 | 0.86 | 0 | 0.22 | 0.99 | ||
| Sodium- and chloride-dependent GABA transporter | |||||||
| S6A11_MOUSE | Sodium- and chloride-dependent GABA transporter 3 OS = Mus musculus GN = Slc6a11 PE = 1 SV = 2 | ||||||
| Fold change (ratio) | 1.14 | 0.92 | 1.13 | 1.31 | 1.67 | ||
| p Value | 0.78 | 0.29 | 0.86 | 0.9 | 0.99 | ||
| Glutamine synthase | |||||||
| GLNA_MOUSE | Glutamine synthetase OS = Mus musculus GN = Glul PE = 1 SV = 6 | ||||||
| Fold change (ratio) | 0.9 | 1 | 1.01 | 0.91 | 0.83 | ||
| p Value | 0.23 | 0.51 | 0.56 | 0.27 | 0.06 | ||
| Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein | |||||||
| A2A5N2_MOUSE | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, | ||||||
| beta OS = Mus musculus GN = Ywhab PE = 2 SV = 1 | |||||||
| Fold change (ratio) | 0.95 | 1.03 | 0.82 | 0.81 | 0.88 | ||
| p Value | 0.24 | 0.75 | 0 | 0.01 | 0.05 | ||
| Q5SS40_MOUSE | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, | ||||||
| epsilon polypeptide OS = Mus musculus GN = Ywhae PE = 2 SV = 1 | |||||||
| Fold change (ratio) | 1.01 | 1.04 | 0.9 | 0.9 | 0.89 | ||
| p Value | 0.53 | 0.83 | 0.04 | 0.03 | 0.01 | ||
| Dihydropteridine reductase | |||||||
| D3YWR7_MOUSE | Dihydropteridine reductase OS = Mus musculus GN = Qdpr PE = 4 SV = 1 | ||||||
| Fold change (ratio) | NU | NU | NU | 1.28 | NU | ||
| p Value | 0.79 | ||||||
| DHPR_MOUSE | Dihydropteridine reductase OS = Mus musculus GN = Qdpr PE = 1 SV = 2 | ||||||
| Fold change (ratio) | NU | NU | NU | 1.55 | NU | ||
| p Value | 0.88 | ||||||
| Tubulin alpha | |||||||
| TBA1A_MOUSE | Tubulin alpha-1A chain OS = Mus musculus GN = Tuba1a PE = 1 SV = 1 | ||||||
| Fold change (ratio) | 0.99 | 1.08 | 0.96 | 0.97 | 0.96 | ||
| p Value | 0.19 | 1 | 0 | 0.04 | 0.03 | ||
| TBA1C_MOUSE | Tubulin alpha-1C chain OS = Mus musculus GN = Tuba1c PE = 1 SV = 1 | ||||||
| Fold change (ratio) | 0.99 | 1.07 | 0.95 | 0.97 | 0.97 | ||
| p Value | 0.39 | 1 | 0.01 | 0.02 | 0.05 | ||
| O89052_MOUSE | Alpha-tubulin (Fragment) OS = Mus musculus GN = Tuba1b PE = 2 SV = 1 | ||||||
| Fold change (ratio) | 1 | 1.16 | 0.9 | 0.97 | 1.02 | ||
| p Value | 0.46 | 1 | 0 | 0.12 | 0.77 | ||
| Synapsin-2 | |||||||
| SYN2_MOUSE | Synapsin-2 OS = Mus musculus GN = Syn2 PE = 1 SV = 2 | ||||||
| Fold change (ratio) | 0.84 | 0.97 | 0.77 | 0.79 | 1.01 | ||
| p Value | 0.01 | 0.31 | 0 | 0 | 0.59 | ||
| Hemoglobin subunit alpha | |||||||
| HBA_MOUSE | Hemoglobin subunit alpha OS = Mus musculus GN = Hba PE = 1 SV = 2 | ||||||
| Fold change (ratio) | 0.97 | 0.8 | 1.2 | 1.19 | 1.06 | ||
| p Value | 0.31 | 0 | 1 | 1 | 0.88 | ||
| Sodium- and chloride-dependent GABA transporter | |||||||
| S6A11_MOUSE | Sodium- and chloride-dependent GABA transporter 3 OS = Mus musculus GN = Slc6a11 PE = 1 SV = 2 | ||||||
| Fold change (ratio) | 1.14 | 0.92 | 1.13 | 1.31 | 1.67 | ||
| p Value | 0.78 | 0.29 | 0.86 | 0.9 | 0.99 | ||
| Hemoglobin subunit beta | |||||||
| HBB1_MOUSE | Hemoglobin subunit beta-1 OS = Mus musculus GN = Hbb-b1 PE = 1 SV = 2 | ||||||
| Fold change (ratio) | 0.94 | 0.79 | 1.14 | 1.17 | 0.99 | ||
| p Value | 0.06 | 0 | 1 | 1 | 0.37 | ||
| E9Q223_MOUSE | Hemoglobin subunit beta-1 (Fragment) OS = Mus musculus GN = Hbb-b1 PE = 3 SV = 1 | ||||||
| Fold change (ratio) | 0.9 | 0.79 | 1.12 | 1.11 | 0.92 | ||
| p Value | 0.01 | 0 | 0.97 | 0.97 | 0.1 | ||
| HBB2_MOUSE | Hemoglobin subunit beta-2 OS = Mus musculus GN = Hbb-b2 PE = 1 SV = 2 | ||||||
| Fold change (ratio) | 0.98 | 0.84 | 1.12 | 1.16 | 0.99 | ||
| p Value | 0.32 | 0 | 0.98 | 1 | 0.44 | ||
| Amino Acid Ratio and Proteins Could Become Markers Used for Evaluation of Aging Status and Cognitive Impairment | Expression | |
|---|---|---|
| Expression in SAMP8, Compared to SAMR1 | Expression in SAMP8 Given High Oleic Peanut, Compared to SAMP8 | |
| Hippocampus (GABA/glutamic acid) ratio | ↓ | ↑ |
| Glial fibrillary acidic protein | ↑ | ↓ |
| SOD | → | ↑ |
| Glutathione S-trnaserase | → | ↓ |
| Solute carrier family 1 (Glial high affinity glutamate transporter), member 2 | → | ↓ |
| Calcium/calmodulin-dependent protein kinase type II sub-unit beta | ↑ | ↓ |
| Calcium/calmodulin-dependent protein kinase type II sub-unit alpha | → | ↓ |
| Sodium- and chloride-dependent GABA transporter | ↓ | ↑ |
| Tyrosine 3-monooxygenase/tryptpphan 5-monooxygenase activation protein, beta | ↑→ | ↓ |
| Dihydropteridine reductase | ↓→ | ↑ |
| Tubulin alpha | ↑ | ↓ |
| Synapsin-2 | → | ↓ |
| Hemoglobin subunit alpha | ↓ | ↑ |
| Hemoglobin subunit beta | ↓ | ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igarashi, K.; Kurata, D. Effect of High-Oleic Peanut Intake on Aging and Its Hippocampal Markers in Senescence-Accelerated Mice (SAMP8). Nutrients 2020, 12, 3461. https://doi.org/10.3390/nu12113461
Igarashi K, Kurata D. Effect of High-Oleic Peanut Intake on Aging and Its Hippocampal Markers in Senescence-Accelerated Mice (SAMP8). Nutrients. 2020; 12(11):3461. https://doi.org/10.3390/nu12113461
Chicago/Turabian StyleIgarashi, Kiharu, and Daisuke Kurata. 2020. "Effect of High-Oleic Peanut Intake on Aging and Its Hippocampal Markers in Senescence-Accelerated Mice (SAMP8)" Nutrients 12, no. 11: 3461. https://doi.org/10.3390/nu12113461
APA StyleIgarashi, K., & Kurata, D. (2020). Effect of High-Oleic Peanut Intake on Aging and Its Hippocampal Markers in Senescence-Accelerated Mice (SAMP8). Nutrients, 12(11), 3461. https://doi.org/10.3390/nu12113461





