Anti-Obesity Effect of an Ethanol Extract of Cheongchunchal In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
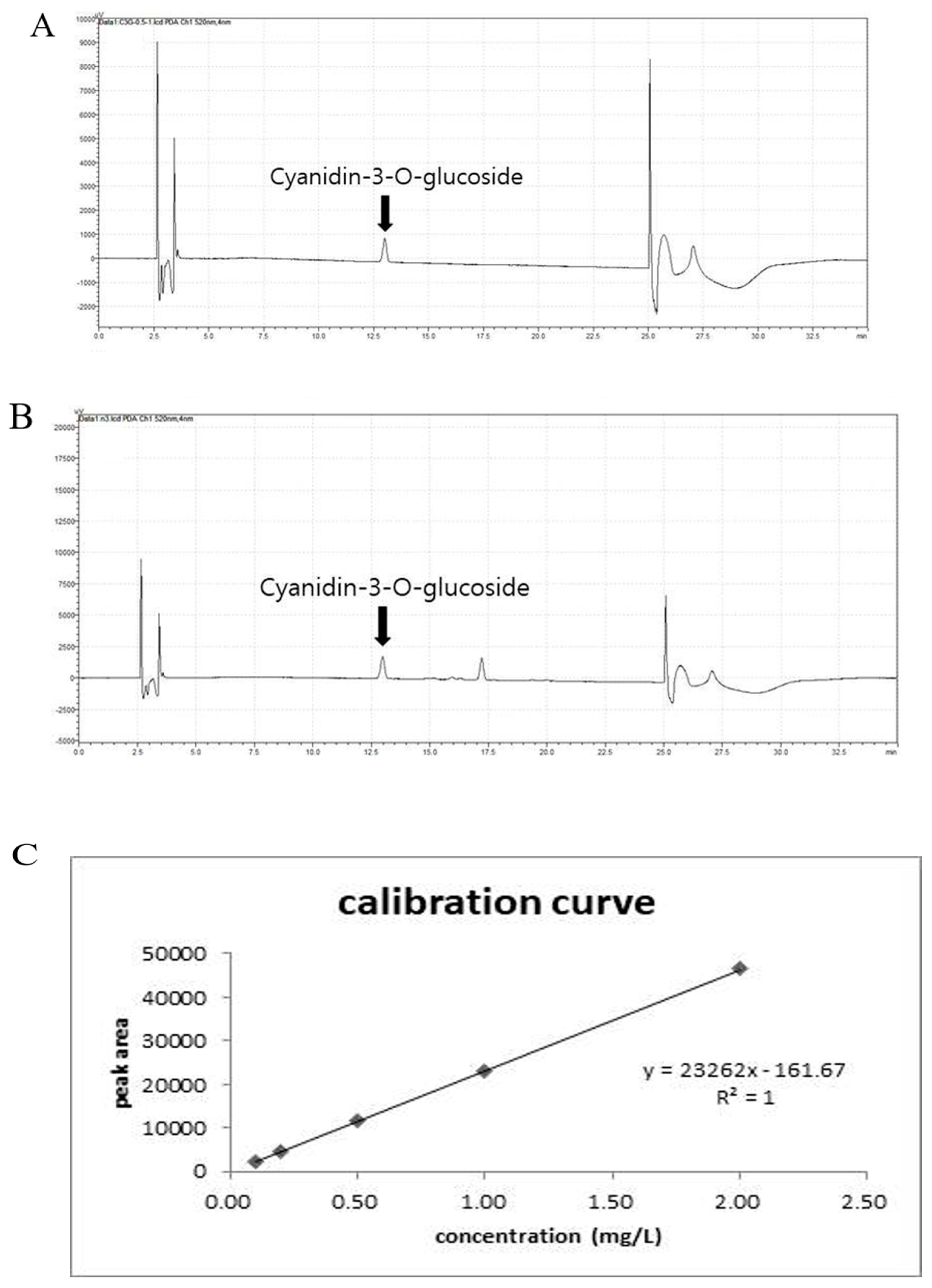
2.2. Quantitative Analysis of the Cyanidin-3-O-Glucoside Chloride (Anthocyanin) Content in the CE Using High-Performance Liquid Chromatography (HPLC)
2.3. 3T3-L1 Preadipocyte Cell Culture
2.4. WST-1 Assay
2.5. Differentiation Induction
2.6. Oil Red O Staining
2.7. Triglyceride Assay
2.8. Western Blotting
2.9. Experimental Animal Breeding and Diet
2.10. Body Weight and Feed Efficiency Measurements
2.11. Blood and Tissue Collection and Analysis
2.12. Statistical Analyses
3. Results
3.1. Qualitative Analysis of Anthocyanin in CE by HPLC
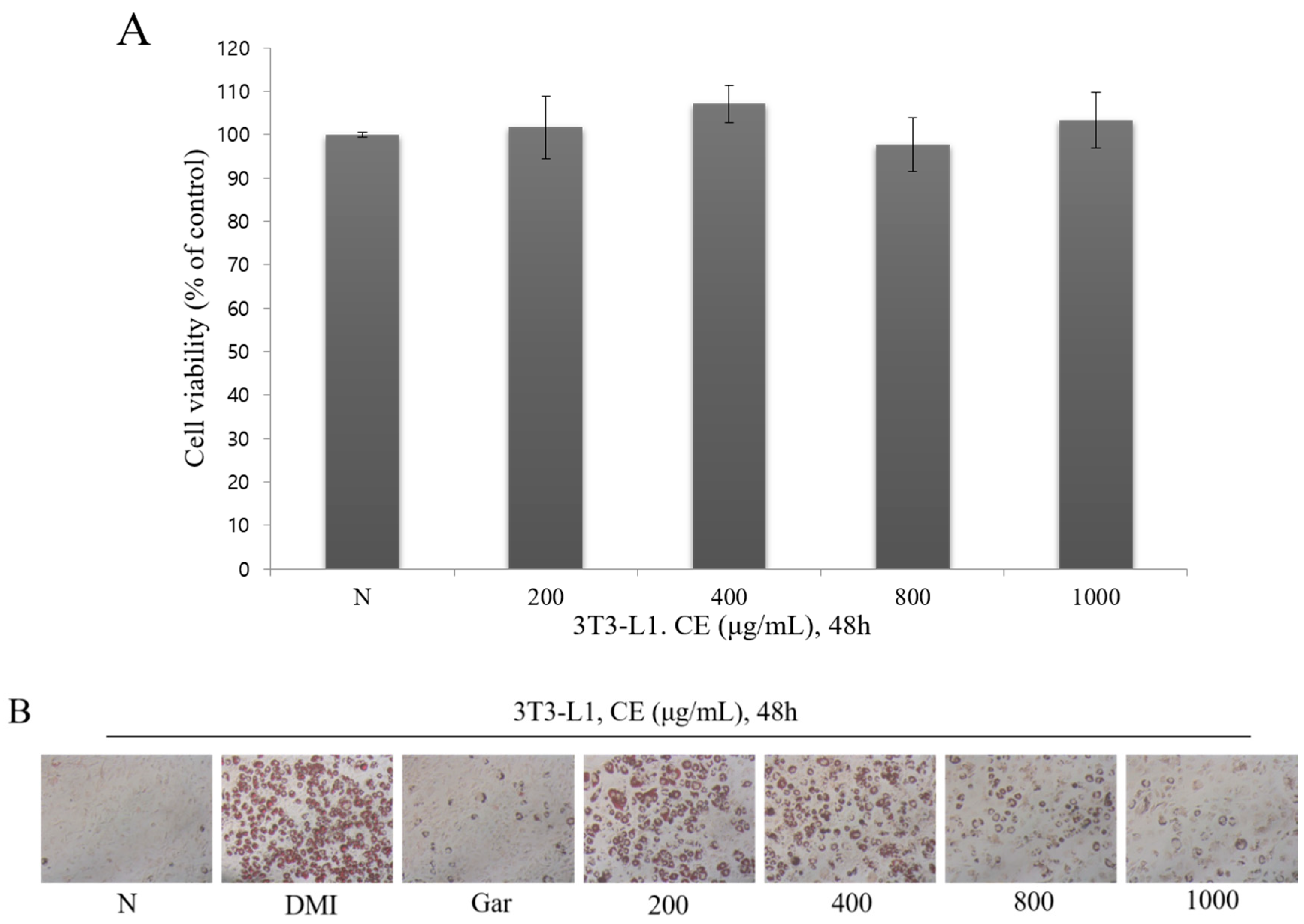
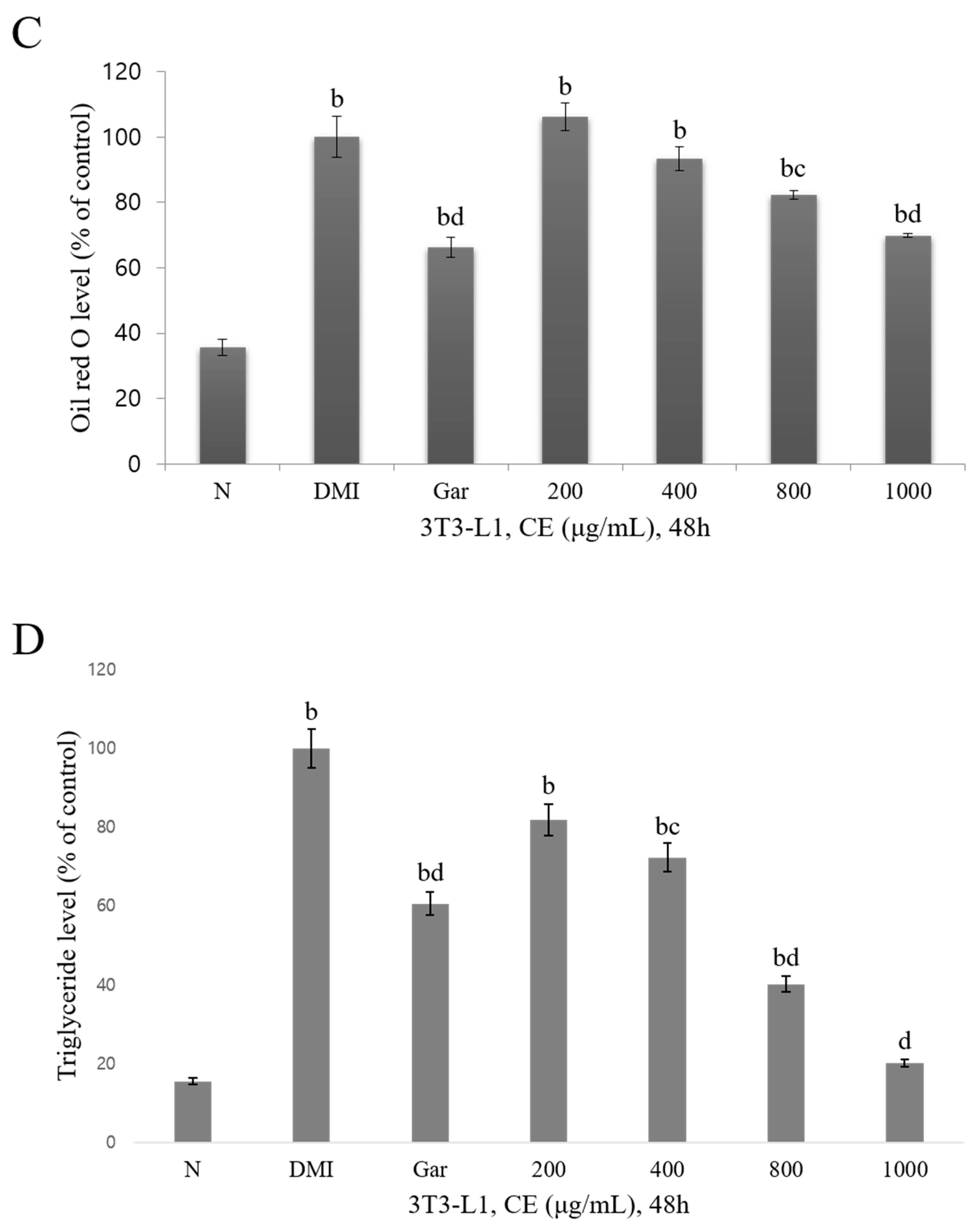
3.2. Confirmation of the Cytotoxic and Inhibitory Effects on Adipocyte Differentiation
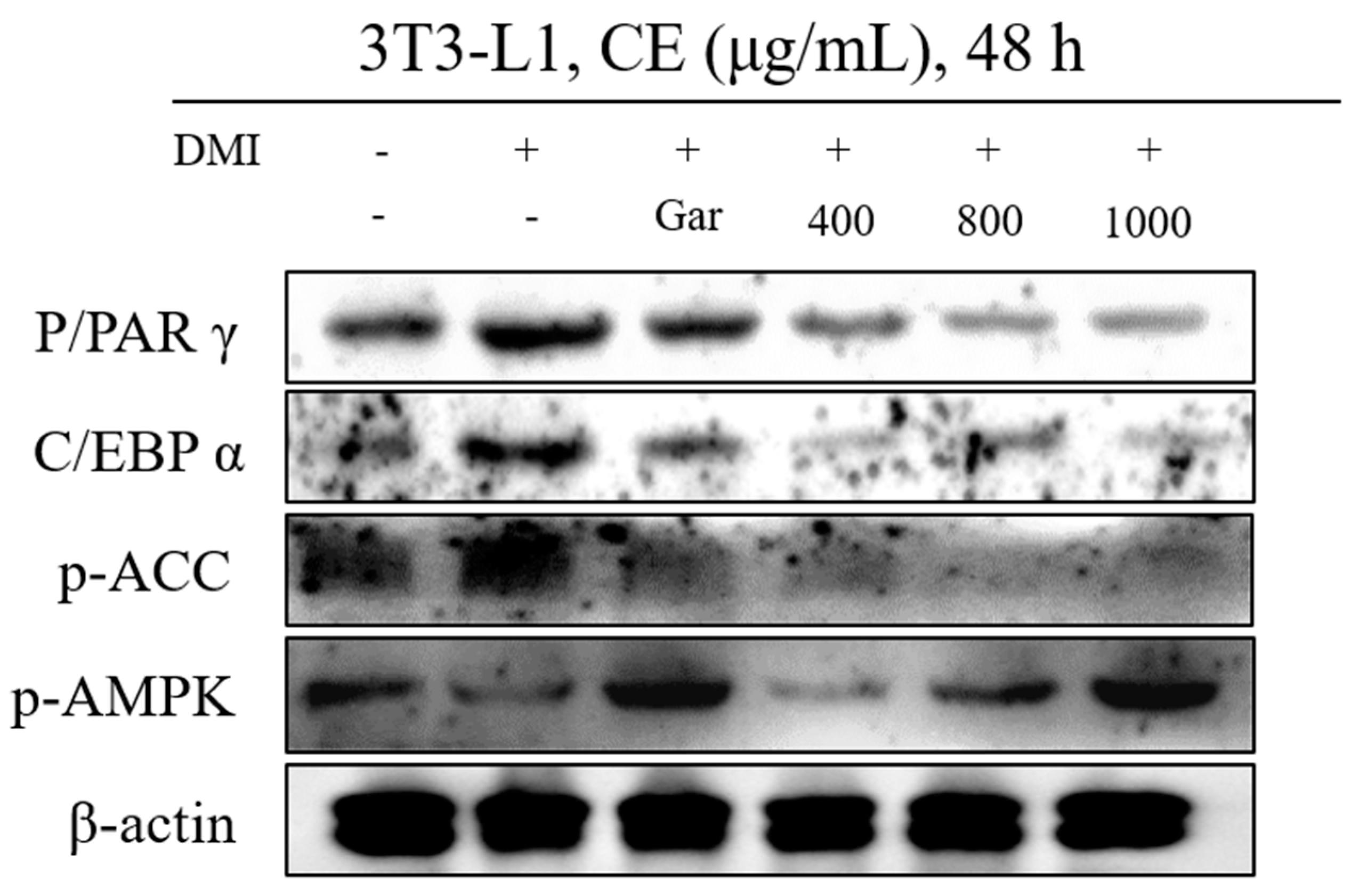
3.3. Confirmation of CE-Induced Changes in the Expression of Proteins Related to Adipocyte Differentiation
3.4. Confirmation of CE-Induced Changes in the Experimental Animals’ Body and Tissue Weights, Food Intake, and FER
3.5. Confirmation of Blood Biochemical Changes Induced by CE
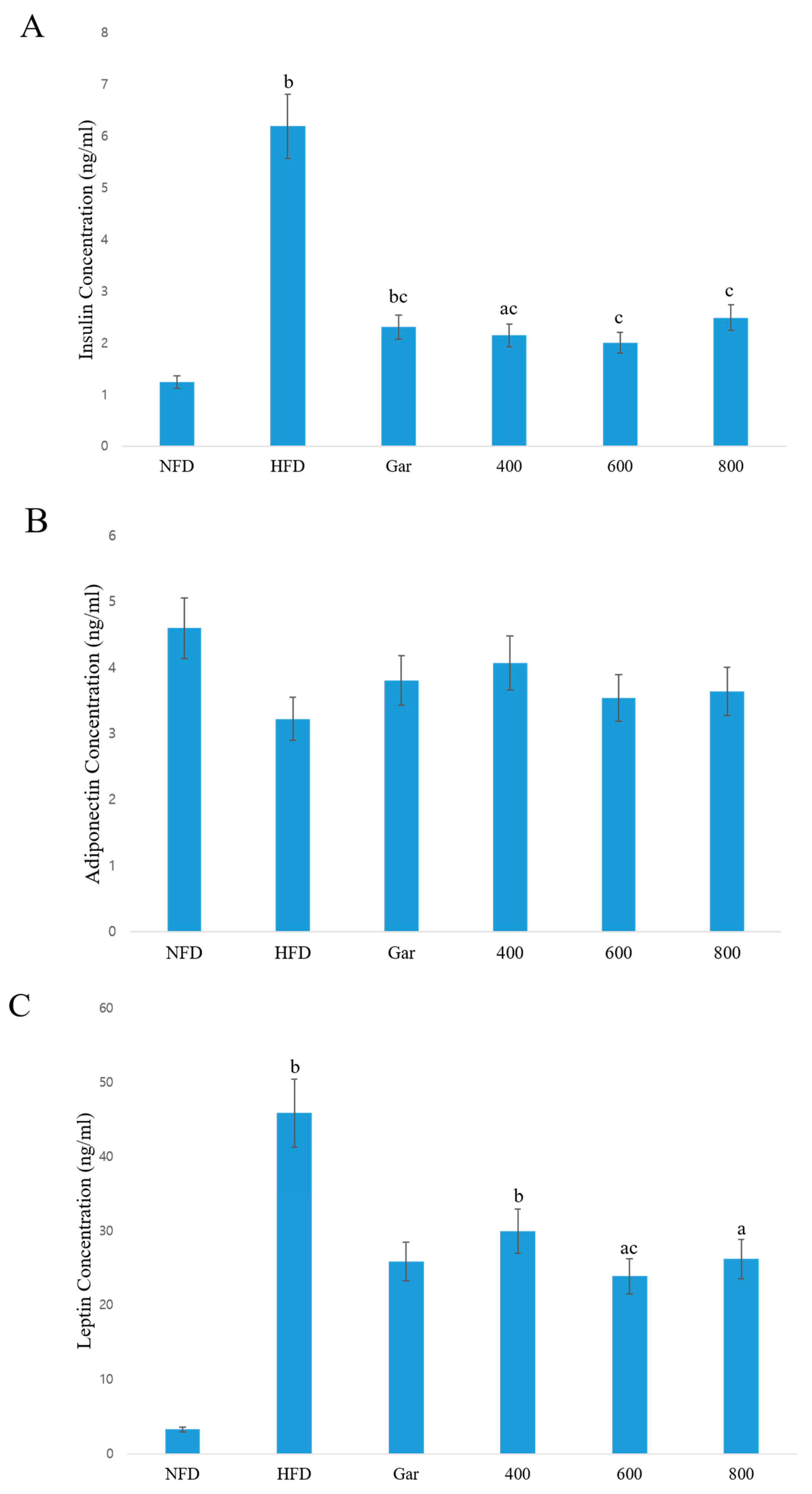
3.6. Confirmation of Changes in the Serum Concentrations of Insulin, Adiponectin, and Leptin
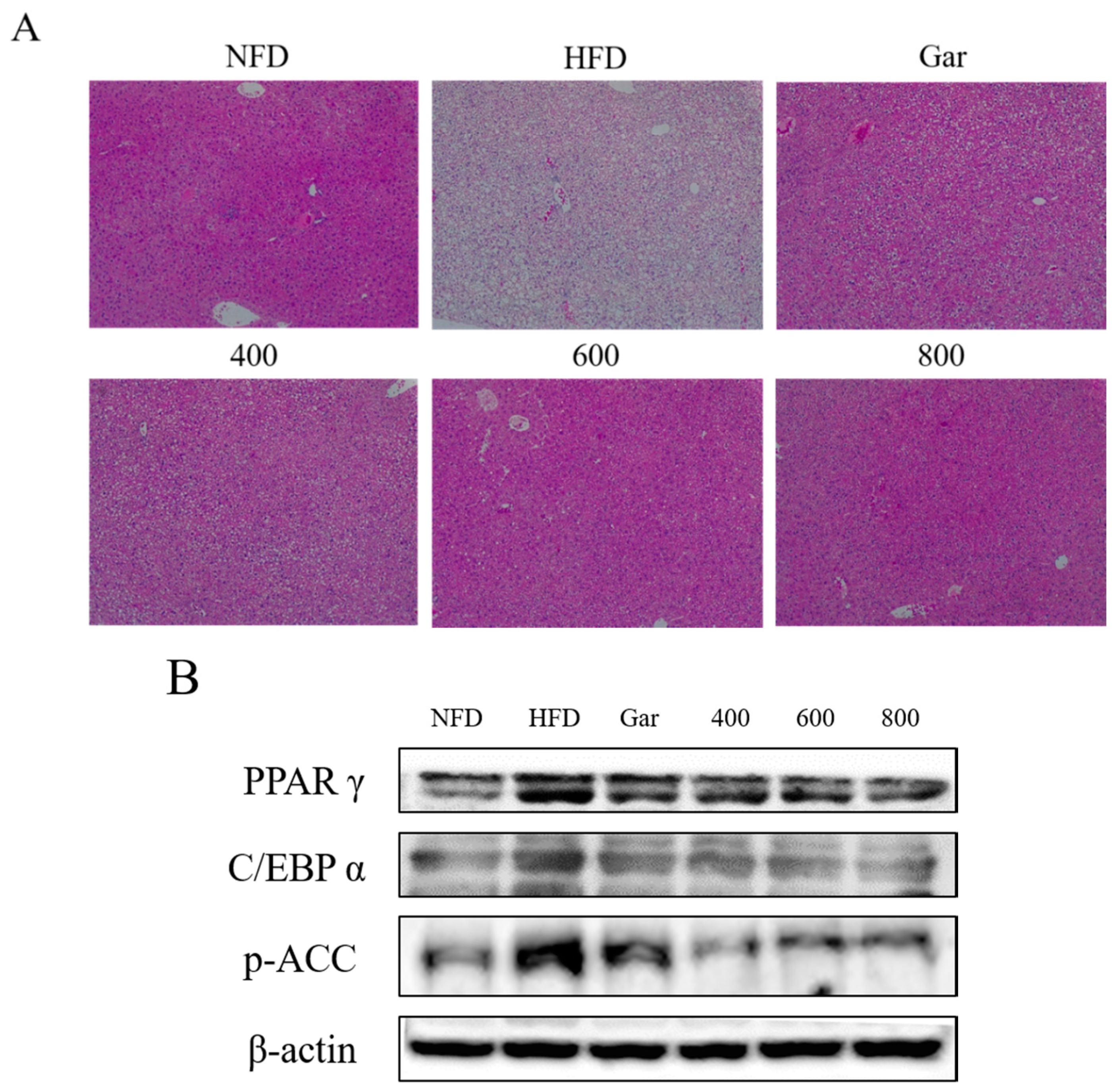
3.7. Confirmation of Changes in Liver Morphological- and Adipocyte Differentiation-Related Factors
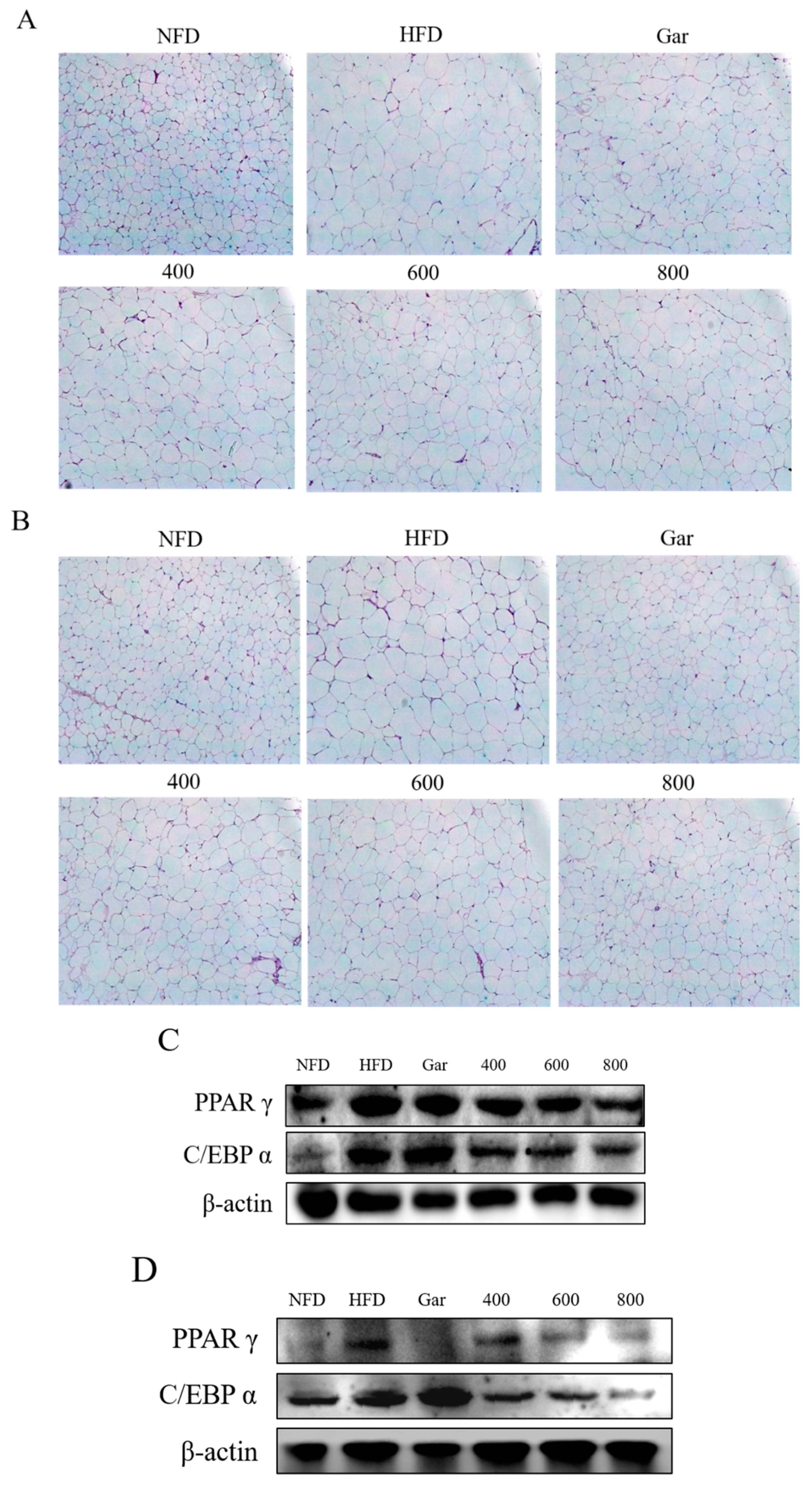
3.8. Confirmation of Changes in the Morphological- and Adipocyte Differentiation-Related Factors of Abdominal and Epididymal Fat
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arner, P.; Spalding, K.L. Fat cell turnover in humans. Biochem. Biophys. Res. Commun. 2010, 396, 101–104. [Google Scholar] [CrossRef]
- Larsson, B.; Björntorp, P.; Tibblin, G. The health consequences of moderate obesity. Int. J. Obes. 1981, 5, 97–116. [Google Scholar]
- Krauss, R.M. Triglycerides and atherogenic lipoproteins: Rationale for lipid management. Am. J. Med. 1998, 105, 58S–62S. [Google Scholar] [CrossRef]
- Golan, M.; Fainaru, M.; Weizman, A. Role of behaviour modification in the treatment of childhood obesity with the parents as the exclusive agents of change. Int. J. Obes. 1998, 22, 1217–1224. [Google Scholar] [CrossRef]
- Seo, M.H.; Lee, W.Y.; Kim, S.S.; Kang, J.H.; Kang, J.H.; Kim, K.K.; Kim, B.Y.; Kim, Y.H.; Kim, W.J.; Kim, E.M.; et al. 2018 Korean Society for the Study of Obesity guideline for the management of obesity in Korea. J. Obes. Metab. Syndr. 2019, 28, 40. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.G.; Apovian, C.M.; Aronne, L.J. New Drug Targets for the Treatment of Obesity. Clin. Pharmacol. Ther. 2011, 90, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Padwal, R.S.; Majumdar, S.R. Drug treatments for obesity: Orlistat, sibutramine, and rimonabant. Lancet 2007, 369, 71–77. [Google Scholar] [CrossRef]
- De Simone, G.; D’Addeo, G. Sibutramine: Balancing weight loss benefit and possible cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 337–341. [Google Scholar] [CrossRef]
- Mayer, M.A.; Höcht, C.; Puyo, A.; Taira, A.C. Recent Advances in Obesity Pharmacotherapy. Curr. Clin. Pharmacol. 2009, 4, 53–61. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, C.-Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef]
- Costa, A.G.V.; Garcia-Diaz, D.F.; Jimenez, P.; Silva, P.I. Bioactive compounds and health benefits of exotic tropical red–black berries. J. Funct. Foods 2013, 5, 539–549. [Google Scholar] [CrossRef]
- Jou, P.-C.; Ho, B.-Y.; Hsu, Y.-W.; Pan, T.-M. The Effect ofMonascusSecondary Polyketide Metabolites, Monascin and Ankaflavin, on Adipogenesis and Lipolysis Activity in 3T3-L1. J. Agric. Food Chem. 2010, 58, 12703–12709. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Heber, D. Herbal preparations for obesity: Are they useful? Prim. Care Clin. Off. Pract. 2003, 30, 441–463. [Google Scholar] [CrossRef]
- Park, H.J.; Cho, J.-Y.; Kim, M.K.; Koh, P.-O.; Cho, K.-W.; Kim, C.H.; Lee, K.-S.; Chung, B.Y.; Kim, G.-S. Anti-obesity effect of Schisandra chinensis in 3T3-L1 cells and high fat diet-induced obese rats. Food Chem. 2012, 134, 227–234. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Antioxidant Capacity and Polyphenols Components of Teas: Implications for Altering In Vivo Antioxidant Status. Exp. Biol. Med. 1999, 220, 255–261. [Google Scholar] [CrossRef]
- Klösgen, R.B.; Gierl, A.; Schwarz-Sommer, Z.; Saedler, H. Molecular analysis of the waxy locus of Zea mays. Mol. Genet. Genom. 1986, 203, 237–244. [Google Scholar] [CrossRef]
- Harakotr, B.; Suriharn, B.; Tangwongchai, R.; Scott, M.P.; Lertrat, K. Anthocyanin, phenolics and antioxidant activity changes in purple waxy corn as affected by traditional cooking. Food Chem. 2014, 164, 510–517. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, J.-B.; Cho, S.M.; Chung, M.N.; Lee, Y.M.; Chu, S.M.; Che, J.H.; Na Kim, S.; Kim, S.Y.; Cho, Y.S.; et al. Anthocyanin changes in the Korean purple-fleshed sweet potato, Shinzami, as affected by steaming and baking. Food Chem. 2012, 130, 966–972. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Hucl, P.; Rabalski, I. Compositional and antioxidant properties of anthocyanin-rich products prepared from purple wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Trigueros, L.; Peña, S.; Ugidos, A.V.; Sayas-Barberá, E.; Pérez-Álvarez, J.A.; Sendra, E. Food Ingredients as Anti-Obesity Agents: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G. The search for compounds that stimulate thermogenesis in obesity management: From pharmaceuticals to functional food ingredients. Obes. Rev. 2011, 12, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-H.; Lee, J.-J.; Son, H.-K.; Kim, B.-H.; Byun, J.; Ha, J.-H. Antiobesity Effects of Extract from Spergularia marina Griseb in Adipocytes and High-Fat Diet-Induced Obese Rats. Nutrients 2020, 12, 336. [Google Scholar] [CrossRef]
- Choi, B.-R.; Kim, H.; Lee, Y.J.; Ku, S.-K. Anti-diabetic Obesity Effects of Wasabia Japonica Matsum Leaf Extract on 45% Kcal High-Fat Diet-Fed Mice. Nutrients 2020, 12, 2837. [Google Scholar] [CrossRef]
- Ko, J.W.; Chung, Y.-S.; Kwak, C.S.; Kwon, Y.-H. Doenjang, A Korean Traditional Fermented Soybean Paste, Ameliorates Neuroinflammation and Neurodegeneration in Mice Fed a High-Fat Diet. Nutrients 2019, 11, 1702. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinonen, M. Antioxidant Activity of Anthocyanins and Their Aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef]
- Miguel, M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Azzini, E.; Giacometti, J.; Russo, G.L. Antiobesity Effects of Anthocyanins in Preclinical and Clinical Studies. Oxidative Med. Cell. Longev. 2017, 2017, 2740364. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Lopez, E.; Castañeda-Ovando, A.; González-Olivares, L.G.; Añorve-Morga, J.; Jaimez-Ordaz, J. Effect of Light on Stability of Anthocyanins in Ethanolic Extracts of Rubus fruticosus. Food Nutr. Sci. 2014, 5, 488–494. [Google Scholar]
| Time (min) | Mobile phase A(%) 1 | Mobile phase B(%) 2 |
|---|---|---|
| 0 | 90 | 10 |
| 20 | 75 | 25 |
| 21 | 0 | 100 |
| 22 | 0 | 100 |
| 23 | 90 | 10 |
| 35 | 90 | 10 |
| Ingredient (g/kg) | Normal-Fat Diet | High-Fat Diet |
|---|---|---|
| Casein | 200 | 265.0 |
| L-cysteine | 3 | 4.0 |
| Corn starch | 150 | - |
| Maltodextrin | - | 160.0 |
| Sucrose | 500 | 90.0 |
| Cellulose | 50 | 65.5 |
| Soybean Oil | 50 | 30.0 |
| Lard | - | 310.0 |
| Mineral mixture | 35 | 48.0 |
| Vitamin mixture | 10 | 21.0 |
| Choline Bitartrate | 2 | 3.0 |
| Energy (kcal/g) | 4 | 5.1 |
| Blue Food Color | - | 0.1 |
| Protein (% kcal) | 20 | 18.3 |
| Carbohydrate (% kcal) | 64 | 21.4 |
| Fat (% kcal) | 16 | 60.3 |
| Measurements | NFD 1 | HFD 2 | Gar 3 | 400 4 | 600 5 | 800 6 |
|---|---|---|---|---|---|---|
| Weight (g) | ||||||
| 1th weeks (start supplement) | 21.00 ± 1.00 | 21.10 ± 0.75 | 21.50 ± 0.87 | 21.48 ± 0.53 | 21.92 ± 0.13 | 21.23 ± 0.78 |
| 9th weeks (end supplement) | 24.63 ± 0.64 | 37.25 ± 2.17 b | 34.05 ± 1.40 b | 33.48 ± 0.45 b,c | 32.65 ± 2.31 b,c | 32.03 ± 1.36 b,c |
| Weight gain (g) | 3.63 ± 0.68 | 16.15 ± 2.44 b | 12.55 ± 1.69 b | 11.98 ± 0.94 b,c | 10.73 ± 2.26 b,c | 10.8 ± 1.47 b,c |
| Intake | ||||||
| Food intake (g/day) | 3.21 ± 0.64 | 2.55 ± 0.43 a | 2.59 ± 0.63 | 2.32 ± 0.45 b | 2.26 ± 0.35 b | 2.27 ± 0.50 b |
| FER7 | 0.018 ± 0.003 | 0.101 ± 0.02 b | 0.077 ± 0.01 b | 0.082 ± 0.01 b | 0.075 ± 0.02 b | 0.075 ± 0.01 b |
| Tissue weight (g) | ||||||
| Liver | 0.93 ± 0.04 | 1.38 ± 0.14 b | 1.27 ± 0.11 b | 1.23 ± 0.05 b | 1.05 ± 0.06 a,d | 1.06 ± 0.08 a,c |
| Kidney | 0.31 ± 0.01 | 0.40 ± 0.01 b | 0.39 ± 0.04 a | 0.37 ± 0.02 b,c | 0.34 ± 0.02 d | 0.35 ± 0.01 b,d |
| Adipose tissue weight (g) | ||||||
| Abdominal fat tissue | 0.385 ± 0.08 | 1.820 ± 0.10 b | 1.283 ± 0.51 a | 1.317 ± 0.08 b,d | 1.211 ± 0.24 b,d | 1.363 ± 0.36 b |
| Epididymal fat tissue | 0.052 ± 0.01 | 0.301 ± 0.04 b | 0.141 ± 0.05 a,d | 0.192 ± 0.01 b,d | 0.159 ± 0.03 b,d | 0.168 ± 0.04 b,d |
| Visceral fat tissue | 0.442 ± 0.08 | 1.357 ± 0.07 b | 1.117 ± 0.42 a | 1.293 ± 0.07 b | 1.121 ± 0.19 b | 1.215 ± 0.27 b |
| Subcutaneous fat tissue | 0.411 ± 0.09 | 2.759 ± 0.12 b | 1.668 ± 0.88 a | 1.860 ± 0.34 b,c | 1.201 ± 0.44 b,d | 1.371 ± 0.51 b,d |
| Measurements | NFD 1 | HFD 2 | Gar 3 | 400 4 | 600 5 | 800 6 |
|---|---|---|---|---|---|---|
| ALT (u/L) | 39.83 ± 2.78 | 110.97 ± 19.21 b | 246.13 ± 94.34 a,d | 60.30 ± 10.09 a,c | 90.27 ± 14.18 a | 69.03 ± 20.34 |
| AST (u/L) | 23.33 ± 0.58 | 116.00 ± 50.48 a | 136.00 ± 22.54 a | 37.67 ± 9.07 c | 76.00 ± 9.64 a | 75.67 ± 17.10 a |
| Glucose (mg/dL) | 220.67 ± 13.05 | 370.67 ± 7.57 b | 242.00 ± 24.25 d | 250.00 ± 33.06 d | 269.00 ± 56.40 c | 290.33 ± 40.80 a,c |
| Triglyceride (mg/dL) | 84.33 ± 0.58 | 193.67 ± 7.23 b | 189.67 ± 4.04 b | 167.33 ± 9.07 b,c | 151.33 ± 11.37 b,d | 169.00 ± 16.52 b |
| Total-cholesterol (mg/dL) | 56.67 ± 4.04 | 116.00 ± 18.03 b | 79.33 ± 5.13 b,c | 71.67 ± 1.53 b,c | 63.00 ± 3.61 d | 74.33 ± 9.71 a,c |
| HDL-cholesterol (mg/dL) | 109.00 ± 2.65 | 61.67 ± 0.58 b | 117.00 ± 3.61 a,d | 115.00 ± 7.55 d | 106.33 ± 5.03 d | 109.33 ± 3.21 d |
| LDL-cholesterol (mg/dL) | 10.33 ± 0.58 | 21.33 ± 1.53 b | 20.00 ± 2.65 b | 16.00 ± 2.00 b,c | 15.00 ± 1.73 a,d | 17.33 ± 0.58 b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawk, H.W.; Nam, G.-H.; Kim, M.J.; Kim, S.-Y.; Kim, G.N.; Kim, Y.-M. Anti-Obesity Effect of an Ethanol Extract of Cheongchunchal In Vitro and In Vivo. Nutrients 2020, 12, 3453. https://doi.org/10.3390/nu12113453
Kawk HW, Nam G-H, Kim MJ, Kim S-Y, Kim GN, Kim Y-M. Anti-Obesity Effect of an Ethanol Extract of Cheongchunchal In Vitro and In Vivo. Nutrients. 2020; 12(11):3453. https://doi.org/10.3390/nu12113453
Chicago/Turabian StyleKawk, Hye Won, Gun-He Nam, Myeong Jin Kim, Sang-Yong Kim, Gi No Kim, and Young-Min Kim. 2020. "Anti-Obesity Effect of an Ethanol Extract of Cheongchunchal In Vitro and In Vivo" Nutrients 12, no. 11: 3453. https://doi.org/10.3390/nu12113453
APA StyleKawk, H. W., Nam, G.-H., Kim, M. J., Kim, S.-Y., Kim, G. N., & Kim, Y.-M. (2020). Anti-Obesity Effect of an Ethanol Extract of Cheongchunchal In Vitro and In Vivo. Nutrients, 12(11), 3453. https://doi.org/10.3390/nu12113453







