The Effect of Soluble Fiber Dextrin on Subjective and Physiological Markers of Appetite: A Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
2.3. Test Foods and Beverages
2.4. Subjective Appetite and Food Intake
2.5. Hormones and Breath Hydrogen
2.6. Statistical Analysis
3. Results
3.1. Participant Demographics
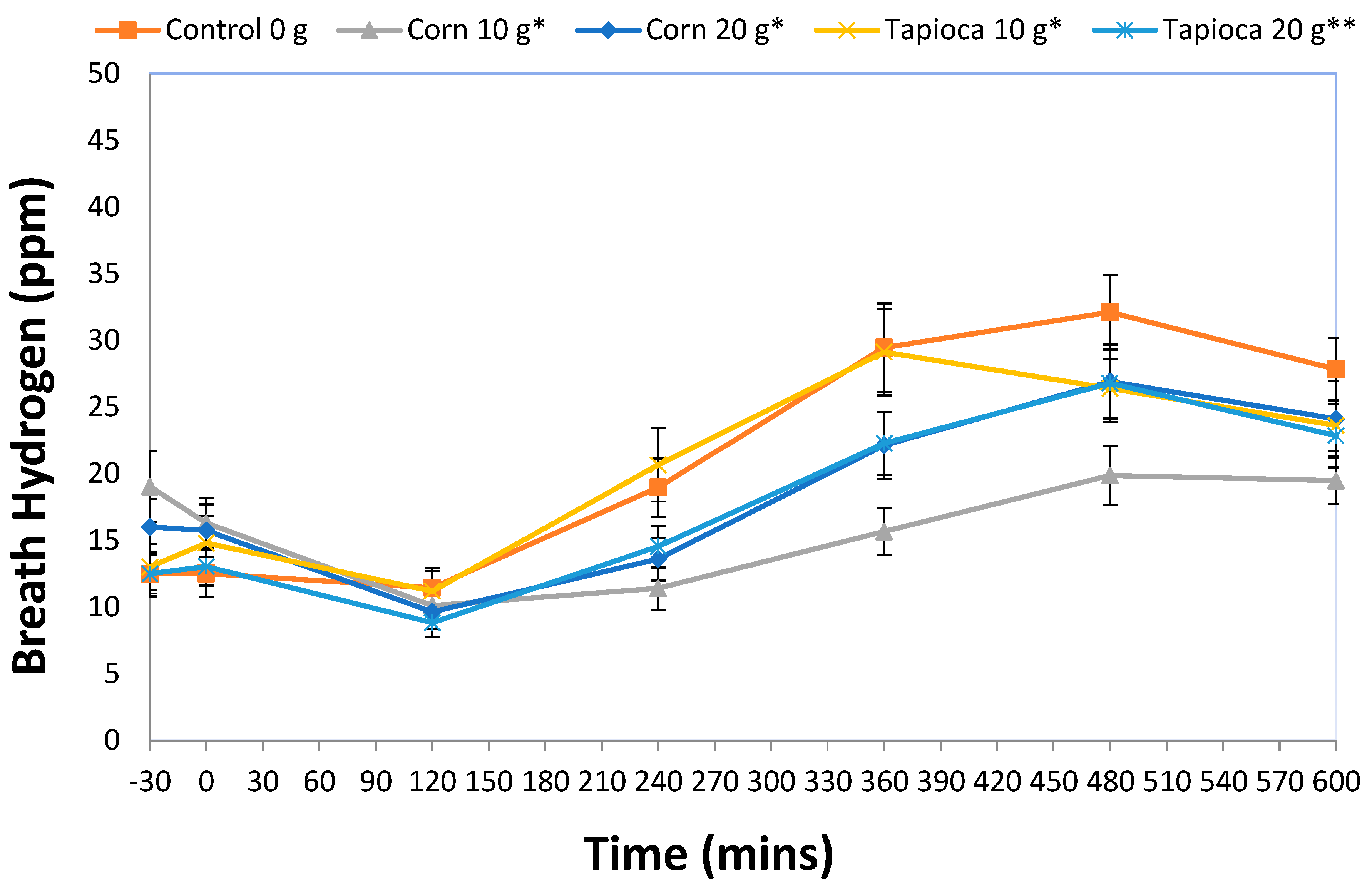
3.2. Breath Hydrogen
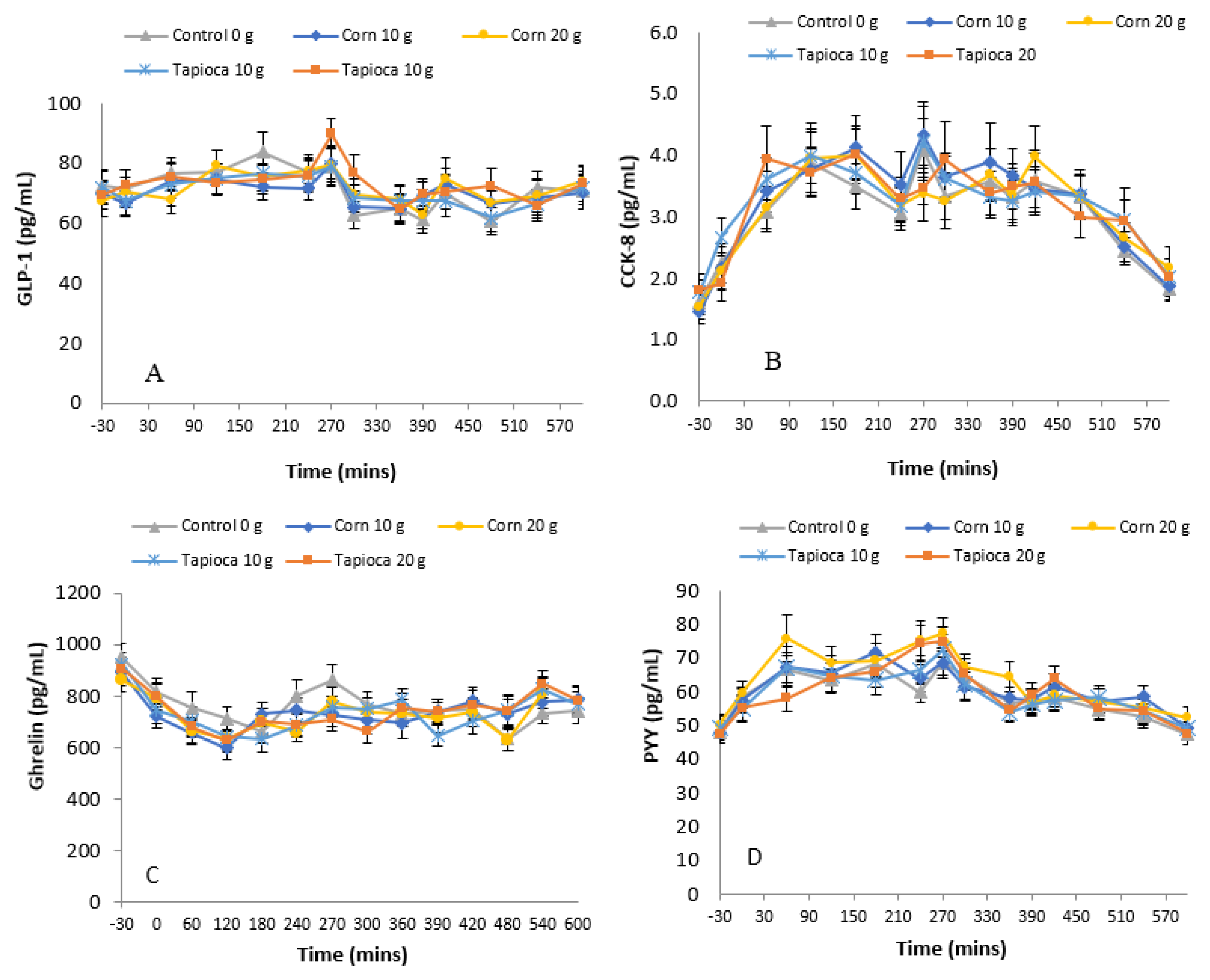
3.3. Hormones
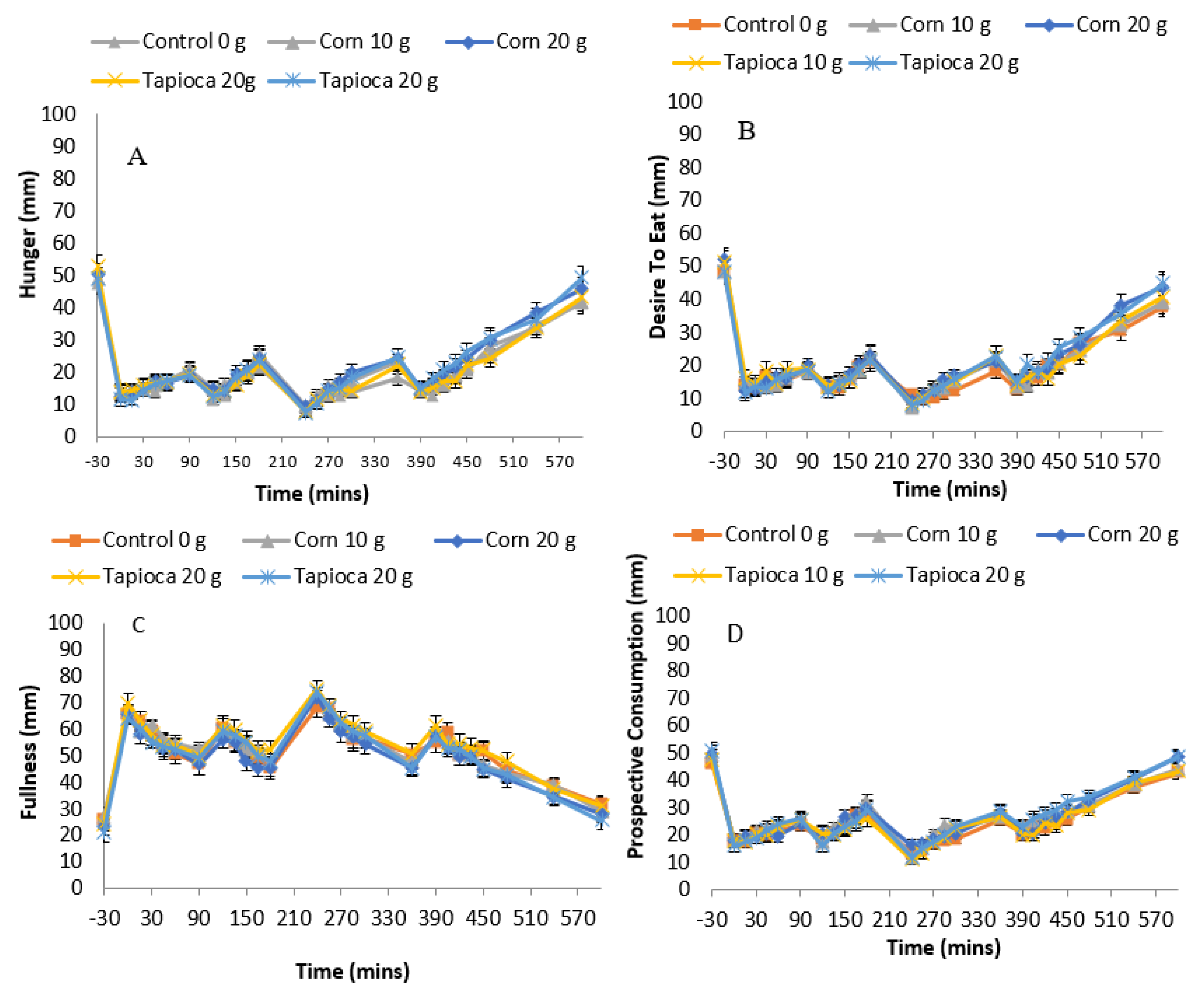
3.4. Subjective Appetite
3.5. Food Intake
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gallagher, E.J.; LeRoith, D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol. Rev. 2015, 95, 727–748. [Google Scholar] [CrossRef]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.-P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Overweight, obesity and risk of liver cancer: A meta-analysis of cohort studies. Br. J. Cancer 2007, 97, 1005–1008. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, Y.; Chen, B.; Wan, H.-W.; Jia, G.-Q.; Bai, H.L.; Wu, X.-T. Overweight, obesity and gastric cancer risk: Results from a meta-analysis of cohort studies. Eur. J. Cancer 2009, 45, 2867–2873. [Google Scholar] [CrossRef]
- Clark, M.J.; Slavin, J.L. The effect of fiber on satiety and food intake: A systematic review. J. Am. Coll. Nutr. 2013, 32, 200–211. [Google Scholar] [CrossRef]
- Wanders, A.J.; Feskens, E.J.M.; Jonathan, M.C.; Schols, H.A.; de Graaf, C.; Mars, M. Pectin is not pectin: A randomized trial on the effect of different physicochemical properties of dietary fiber on appetite and energy intake. Physiol. Behav. 2014, 128, 212–219. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef]
- Aziz, A.A.; Kenney, L.S.; Goulet, B.; Abdel-Aal, E.-S. Dietary starch type affects body weight and glycemic control in freely fed but not energy-restricted obese rats. J. Nutr. 2009, 139, 1881–1889. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; King, R.A.; Christophersen, C.T.; Bird, A.R. Dietary resistant starch dose-dependently reduces adiposity in obesity-prone and obesity-resistant male rats. Nutr. Metab. 2012, 9. [Google Scholar] [CrossRef]
- Keenan, M.J.; Zhou, J.; McCutcheon, K.L.; Raggio, A.M.; Bateman, H.G.; Todd, E.; Jones, C.K.; Tulley, R.T.; Melton, S.; Martin, R.J.; et al. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity 2006, 14, 1523–1534. [Google Scholar] [CrossRef]
- Willis, H.J.; Eldridge, A.L.; Beiseigel, J.; Thomas, W.; Slavin, J.L. Greater satiety response with resistant starch and corn bran in human subjects. Nutr. Res. 2009, 29, 100–105. [Google Scholar] [CrossRef]
- Monsivais, P.; Carter, B.E.; Christiansen, M.; Perrigue, M.M.; Drewnowski, A. Soluble fiber dextrin enhances the satiating power of beverages. Appetite 2011, 56, 9–14. [Google Scholar] [CrossRef]
- Van den Heuvel, E.G.H.M.; Wils, D.; Pasman, W.J.; Bakker, M.; Saniez, M.-H.; Kardinaal, A.F.M. Short-term digestive tolerance of different doses of NUTRIOSE® FB, a food dextrin, in adult men. Eur. J. Clin. Nutr. 2004, 58, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Emilien, C.H.; Zhu, Y.; Hsu, W.H.; Williamson, P.; Hollis, J.H. The effect of soluble fiber dextrin on postprandial appetite and subsequent food intake in healthy adults. Nutrition 2018, 47, 6–12. [Google Scholar] [CrossRef]
- Stewart, M.L.; Savarino, V.; Slavin, J.L. Assessment of dietary fiber fermentation: Effect of Lactobacillus reuteri and reproducibility of short-chain fatty acid concentrations. Mol. Nutr. Food Res. 2009, 53, S114–S120. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1160–E1166. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.; Salem, V.; Long, C.J.; Makwana, A.; Newbould, R.D.; Rabiner, E.A.; Ghatei, M.A.; Bloom, S.R.; Matthews, P.M.; Beaver, J.D.; et al. The gut hormones PYY3-36 and GLP-1(7-36) amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011, 14, 700–706. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Messick, S. The 3-fator eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Imamura, M.; Yamauchi, H.; Nakajima, Y.; Shin, S. Improved radioimmunoassay of cholecystokinin (CCK) using OAL-656 by ethanol extraction of human plasma. Tohoku J. Exp. Med. 1993, 170, 53–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, Y.; Hsu, W.H.; Hollis, J.H. Increasing the number of masticatory cycles is associated with reduced appetite and altered postprandial plasma concentrations of gut hormones, insulin and glucose. Br. J. Nutr. 2013, 110, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hsu, W.H.; Hollis, J.H. Increased number of chews during a fixed-amount meal suppresses postprandial appetite and modulates glycemic response in older males. Physiol. Behav. 2014, 133, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Flood-Obbagy, J.E.; Rolls, B.J. The effect of fruit in different forms on energy intake and satiety at a meal. Appetite 2009, 52, 416–422. [Google Scholar] [CrossRef]
- DiMeglio, D.P.; Mattes, R.D. Liquid versus solid carbohydrate: Effects on food intake and body weight. Int. J. Obes. 2000, 24, 794–800. [Google Scholar] [CrossRef]
- Mattes, R.D.; Campbell, W.W. Effects of food form and timing of ingestion on appetite and energy intake in lean young adults and in young adults with obesity. J. Am. Diet. Assoc. 2009, 109, 430–437. [Google Scholar] [CrossRef]
- Mourao, D.M.; Bressan, J.; Campbell, W.W.; Mattes, R.D. Effects of food form on appetite and energy intake in lean and obese young adults. Int. J. Obes. 2007, 31, 1688–1695. [Google Scholar] [CrossRef]
- Van’t Riet, J.; Sijtsema, S.J.; Dagevos, H.; De Bruijn, G.-J. The importance of habits in eating behaviour. An overview and recommendations for future research. Appetite 2011, 57, 585–596. [Google Scholar] [CrossRef]
- Blundell, J.E.; Stubbs, R.J.; Hughes, D.A.; Whybrow, S.; King, N.A. Cross talk between physical activity and appetite control: Does physical activity stimulate appetite? Proc. Nutr. Soc. 2003, 62, 651–661. [Google Scholar] [CrossRef]
- Wanders, A.J.; Mars, M.; Borgonjen-van den Berg, K.J.; de Graaf, C.; Feskens, E.J.M. Satiety and energy intake after single and repeated exposure to gel-forming dietary fiber: Post-ingestive effects. Int. J. Obes. 2014, 38, 794–800. [Google Scholar] [CrossRef]
- Oku, T.; Nakamura, S. Comparison of digestibility and breath hydrogen gas excretion of fructo-oligosaccharide, galactosyl-sucrose, and isomalto-oligosaccharide in healthy human subjects. Eur. J. Clin. Nutr. 2003, 57, 1150–1156. [Google Scholar] [CrossRef][Green Version]
- Cani, P.D.; Lecourt, E.; Dewulf, E.M.; Sohet, F.M.; Pachikian, B.D.; Naslain, D.; De Backer, F.; Neyrinck, A.M.; Delzenne, N.M. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am. J. Clin. Nutr. 2009, 90, 1236–1243. [Google Scholar] [CrossRef]
- Klosterbuer, A.S.; Thomas, W.; Slavin, J.L. Resistant starch and pullulan reduce postprandial glucose, insulin, and GLP-1, but have no effect on satiety in healthy humans. J. Agric. Food. Chem. 2012, 60, 11928–11934. [Google Scholar] [CrossRef]
- Habib, A.M.; Richards, P.; Rogers, G.J.; Reimann, F.; Gribble, F.M. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia 2013, 56, 1413–1416. [Google Scholar] [CrossRef]
- Cherbut, C.; Ferrier, L.; Rozé, C.; Anini, Y.; Blottière, H.; Lecannu, G.; Galmiche, J.-P. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 1998, 275, G1415–G1422. [Google Scholar] [CrossRef]
- Wallach, J.D.; Sullivan, P.G.; Trepanowski, J.F.; Sainani, K.L.; Steyerberg, E.W.; Ioannidis, J.P.A. Evaluation of evidence of statistical support and corroboration of subgroup claims in randomized clinical trials. JAMA Intern. Med. 2017, 177, 554–560. [Google Scholar] [CrossRef]
- Monrroy, H.; Borghi, G.; Pribic, T.; Galan, C.; Nieto, A.; Amigo, N.; Accarino, A.; Correig, X.; Azpiroz, F. Biological response to meal ingestion: Gender differences. Nutrients 2019, 11, 702. [Google Scholar] [CrossRef]
- Dye, L.; Blundell, J.E. Menstrual cycle and appetite control: Implications for weight regulation. Hum. Reprod. 1997, 12, 1142–1151. [Google Scholar] [CrossRef]
- Drewnowski, A. Obesity and the food environment: Dietary energy density and diet costs. Am. J. Prev. Med. 2004, 27, 154–162. [Google Scholar] [CrossRef]
- Blundell, J.; De Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; Van Der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef]
- Emilien, C.; Hollis, J.H. A brief review of salient factors influencing adult eating behaviour. Nutr. Res. Rev. 2017, 30, 233–246. [Google Scholar] [CrossRef]
| Treatment | Lunch | Afternoon Snack | Dinner | Food Logs | Total (Including Breakfast and Morning Snack) |
|---|---|---|---|---|---|
| Control | 441 ± 33 | 263 ± 18 | 499 ± 38 | 290 ± 44 | 2342 ± 90 |
| Corn10 | 452 ± 33 | 282 ± 20 | 534 ± 38 | 358 ± 60 | 2489 ± 94 |
| Corn20 | 457 ± 27 | 268 ± 19 | 511 ± 36 | 337 ± 60 | 2440 ± 85 |
| Tapioca10 | 444 ± 31 | 264 ± 18 | 481 ± 29 | 360 ± 51 | 2412 ± 84 |
| Tapioca20 | 422 ± 30 | 239 ± 18 | 502 ± 36 | 276 ± 48 | 2316 ± 95 |
| F(4,39) | 0.808 | 1.583 | 0.798 | 0.741 | 2.040 |
| Main Effect p-Value | 0.528 | 0.198 | 0.534 | 0.570 | 0.108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emilien, C.H.; Hsu, W.H.; Hollis, J.H. The Effect of Soluble Fiber Dextrin on Subjective and Physiological Markers of Appetite: A Randomized Trial. Nutrients 2020, 12, 3341. https://doi.org/10.3390/nu12113341
Emilien CH, Hsu WH, Hollis JH. The Effect of Soluble Fiber Dextrin on Subjective and Physiological Markers of Appetite: A Randomized Trial. Nutrients. 2020; 12(11):3341. https://doi.org/10.3390/nu12113341
Chicago/Turabian StyleEmilien, Christine H., Walter H. Hsu, and James H. Hollis. 2020. "The Effect of Soluble Fiber Dextrin on Subjective and Physiological Markers of Appetite: A Randomized Trial" Nutrients 12, no. 11: 3341. https://doi.org/10.3390/nu12113341
APA StyleEmilien, C. H., Hsu, W. H., & Hollis, J. H. (2020). The Effect of Soluble Fiber Dextrin on Subjective and Physiological Markers of Appetite: A Randomized Trial. Nutrients, 12(11), 3341. https://doi.org/10.3390/nu12113341




