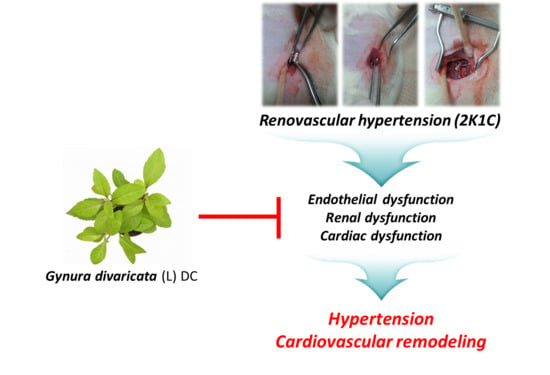
Antihypertensive Effects of Gynura divaricata (L.) DC in Rats with Renovascular Hypertension
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Gynura divaricata Extract Preparation
2.2. Major Metabolite Isolation and Structural Analysis
2.3. 2K1C Hypertensive Rat Model
2.4. Aortic Tissue Immunohistochemistry
2.5. Aldosterone and Ang II Radioimmunoassay
2.6. Blood Analysis
2.7. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (Q-PCR) Analysis
2.8. Statistical Analysis
3. Results
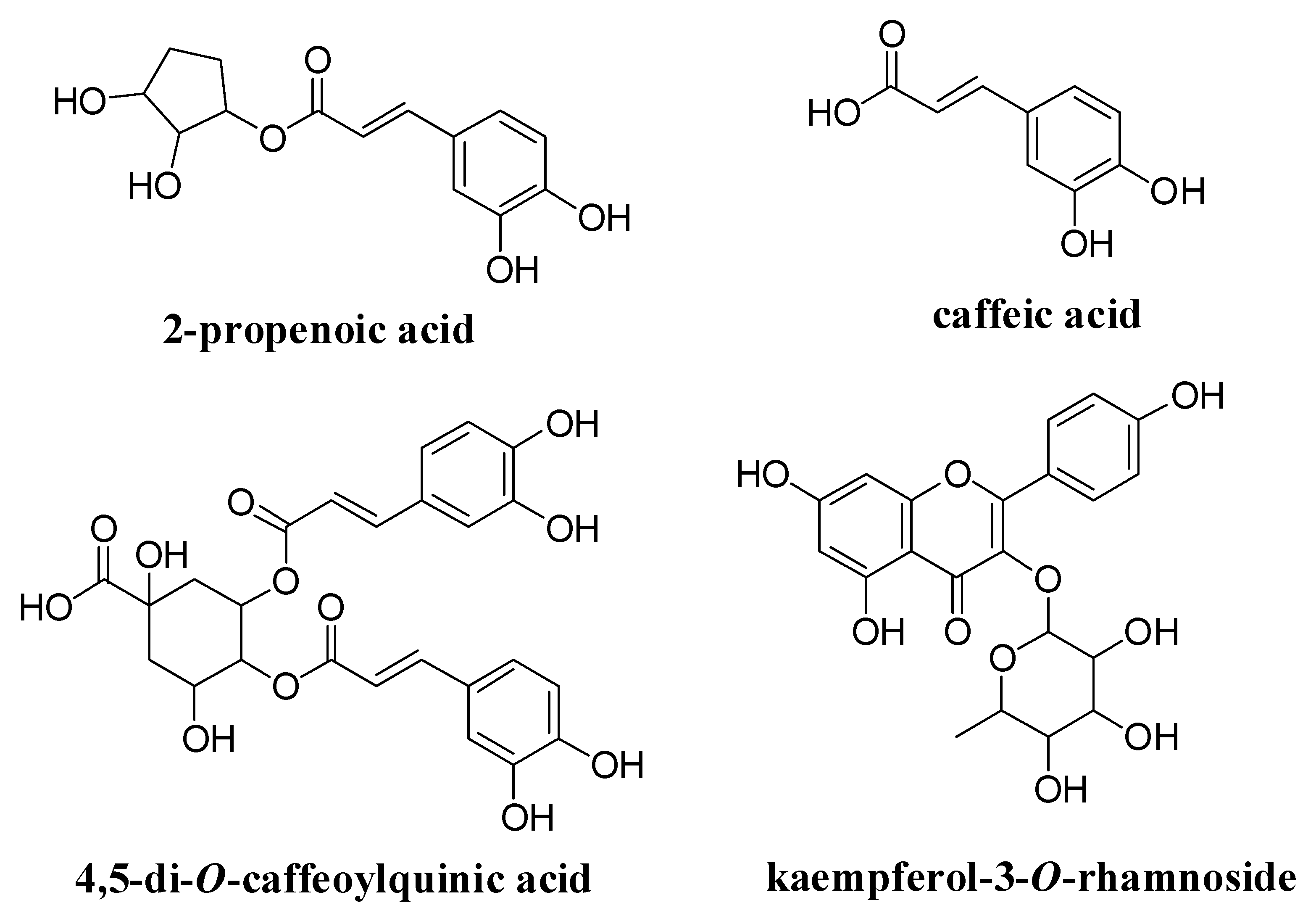
3.1. High-Performance Liquid Chromatography Analysis of GD
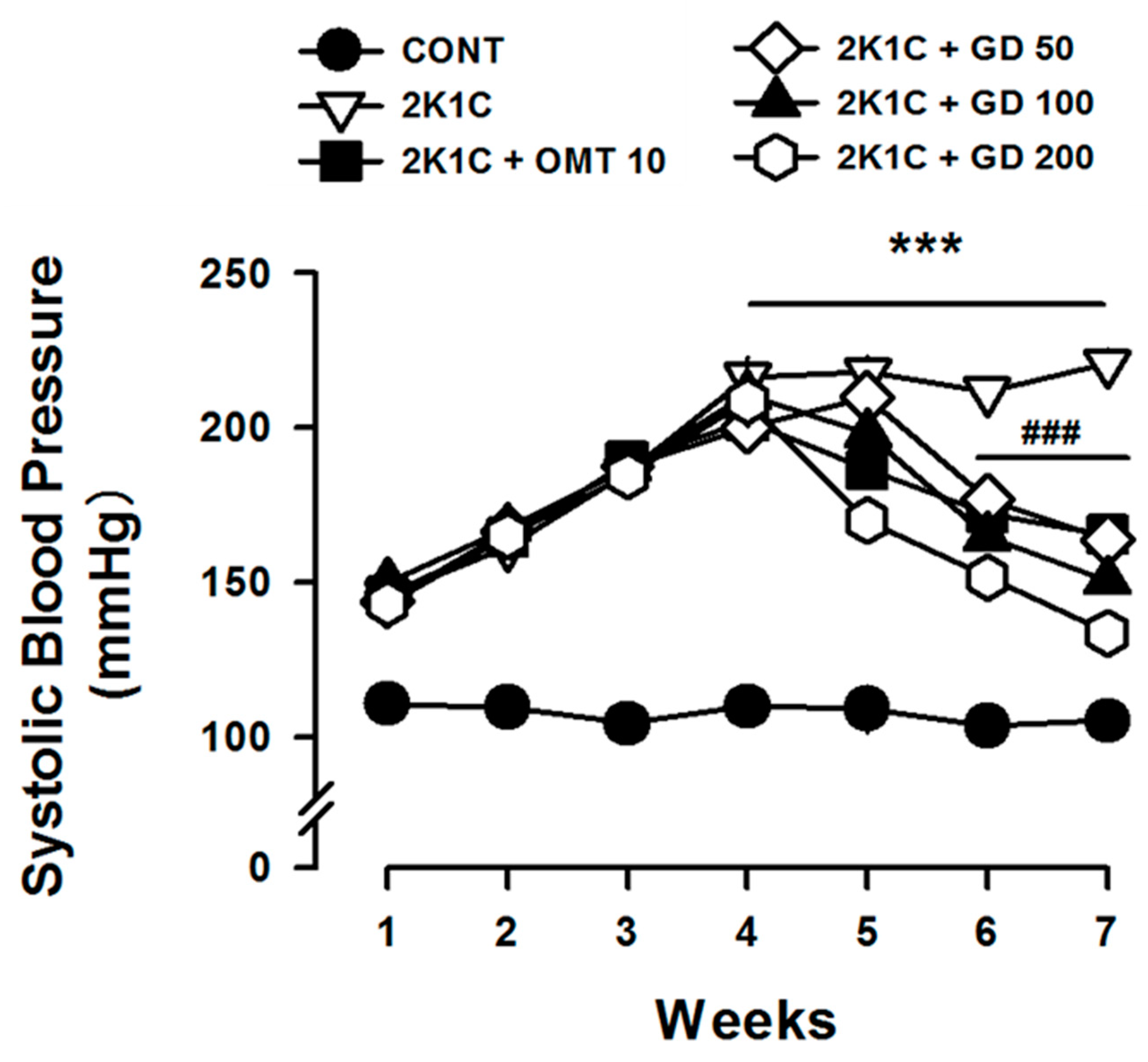
3.2. GD Effect on Systolic Blood Pressure and Vascular Tension
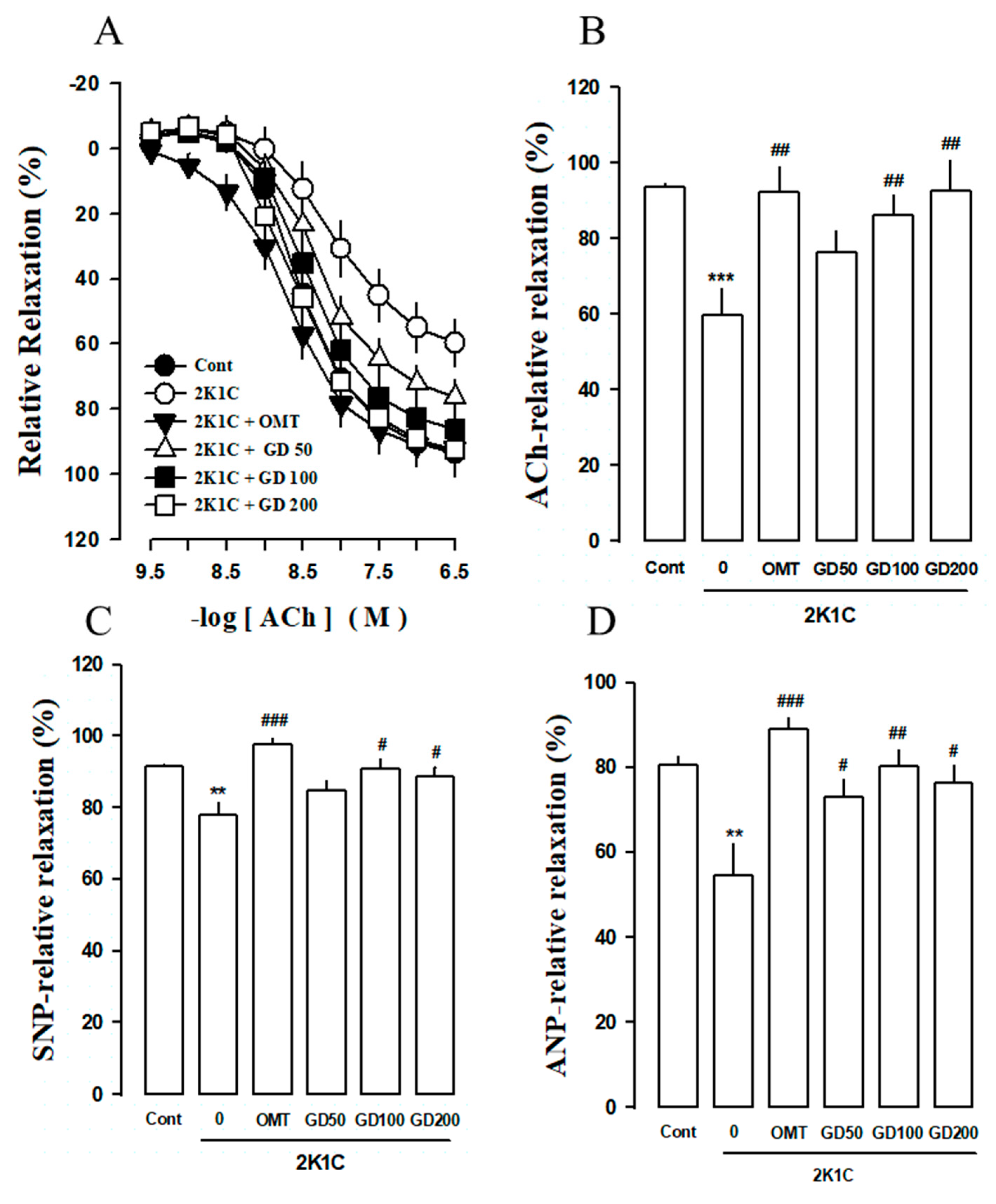
3.3. Effects of GD on Vascular Responses
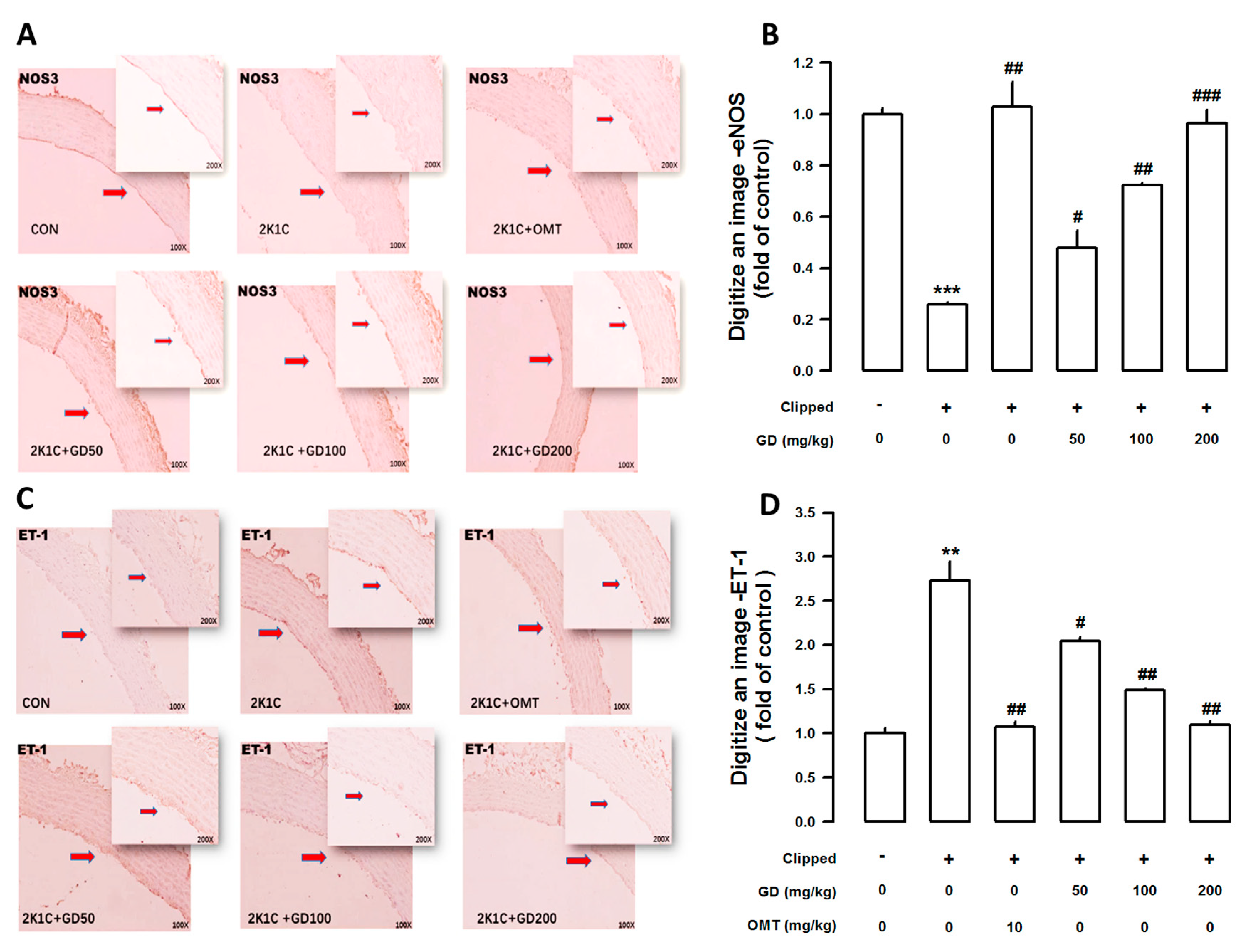
3.4. Effects of GD on Endothelial Nitric Oxide Synthase (eNOS) and Endothelin-1 (ET-1) Immunoreactivity in the Thoracic Aorta
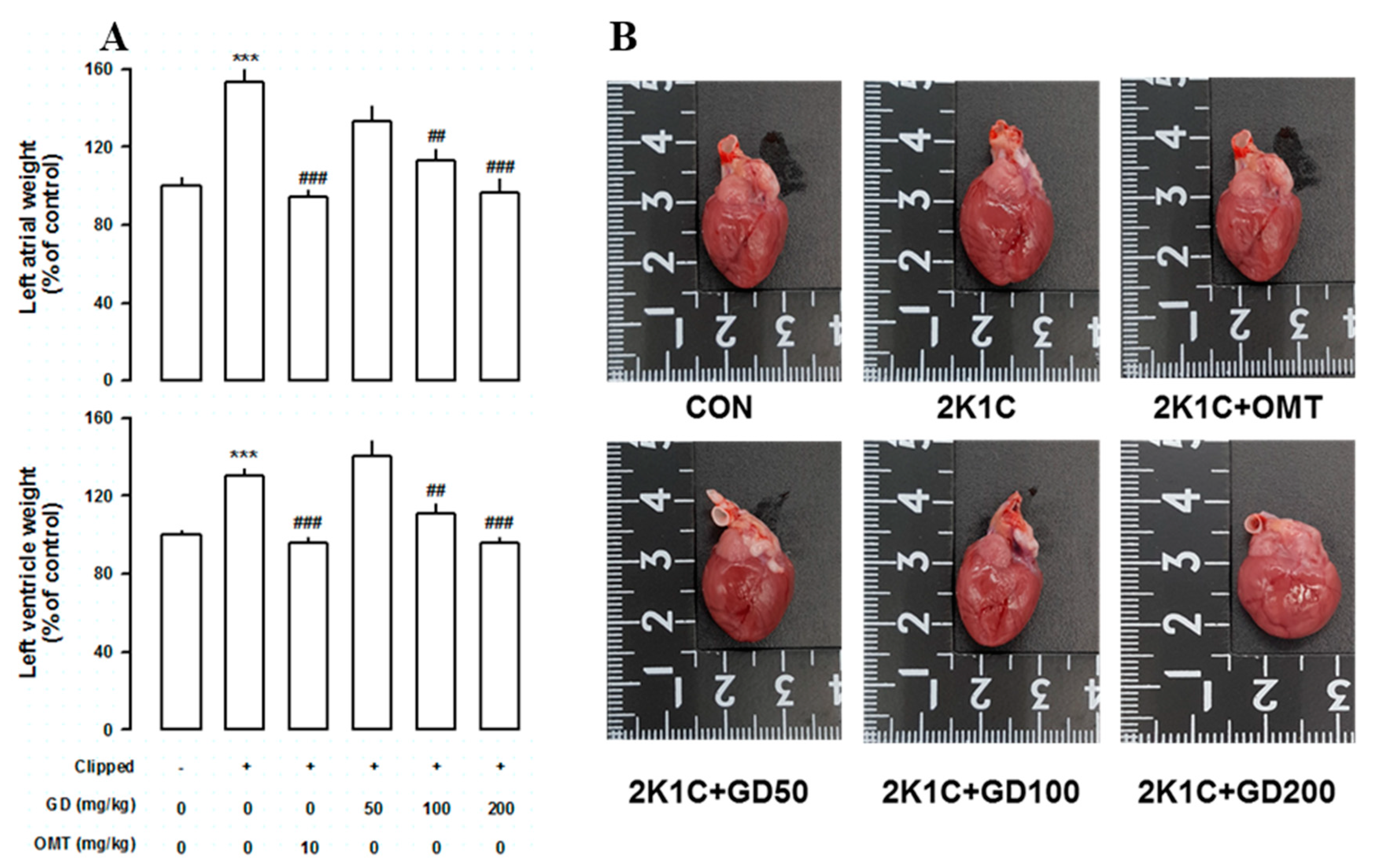
3.5. Effects of GD on the Cardiac Function
3.6. Effects of GD on Renal Function
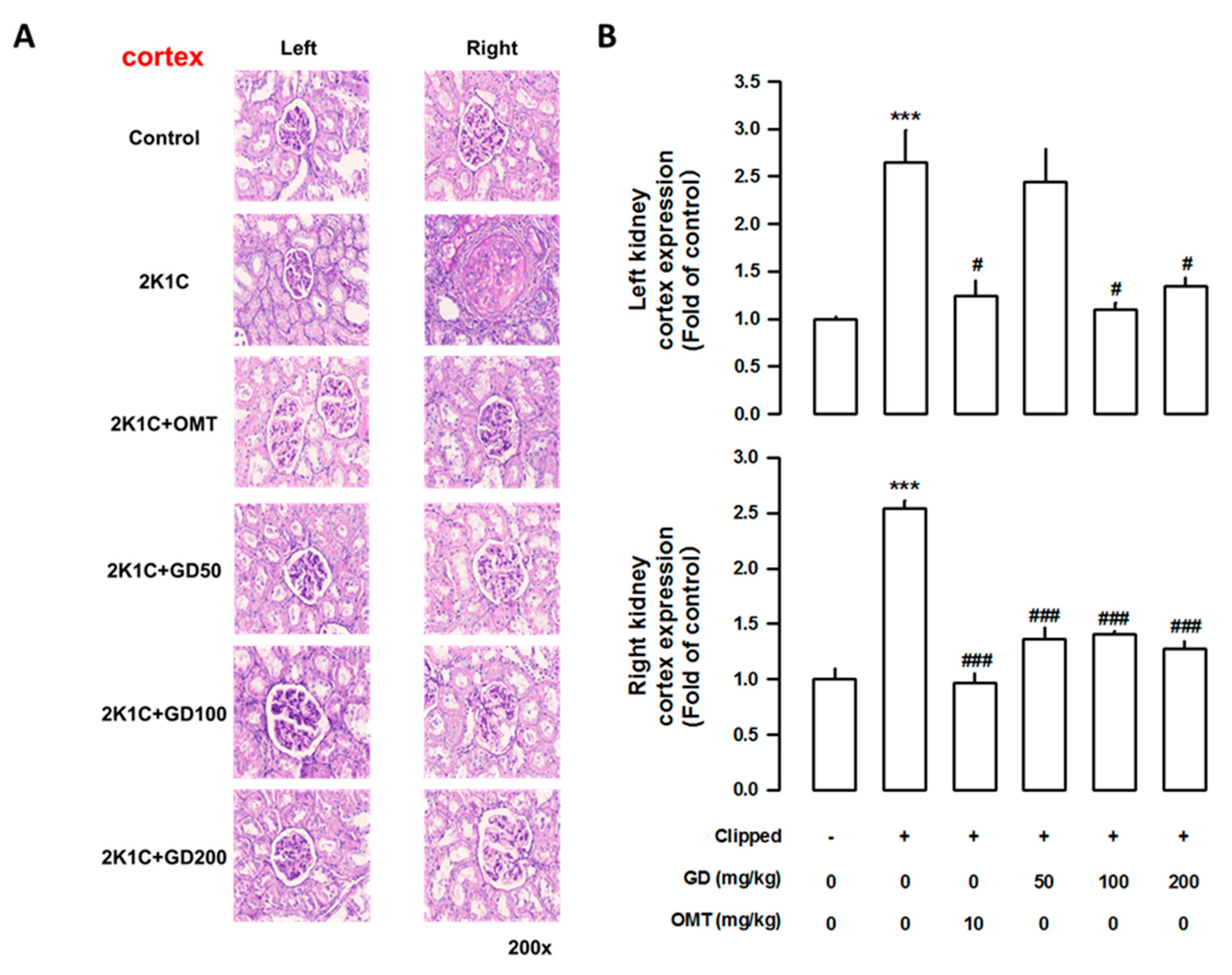
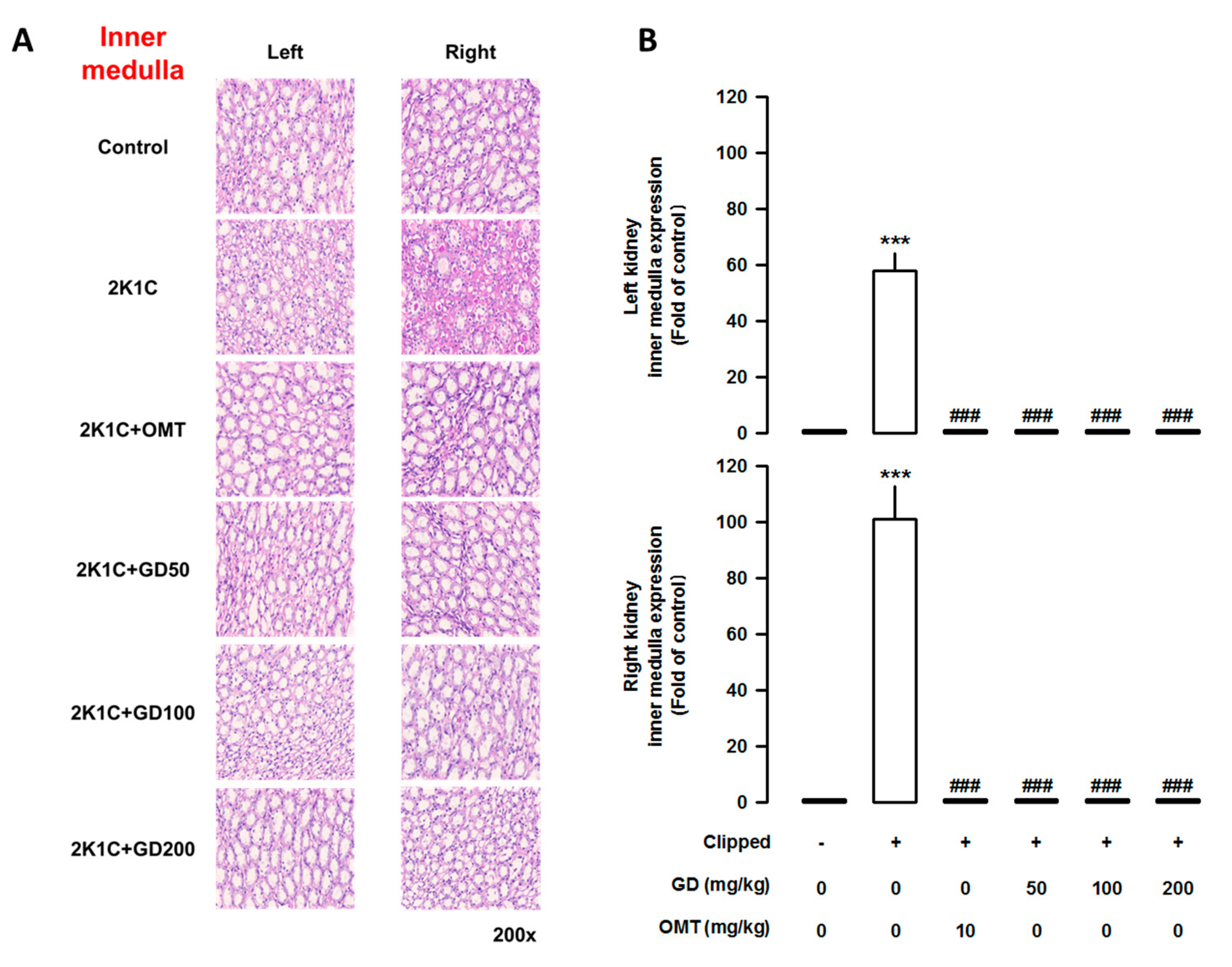
3.7. Effects of GD on Kidney Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lacruz, M.E.; Kluttig, A.; Hartwig, S.; Loer, M.; Tiller, D.; Greiser, K.H.; Werdan, K.; Haerting, J. Prevalence and incidence of hypertension in the general adult population. Medicine 2015, 94, e952. [Google Scholar] [CrossRef]
- Bloch, M.J. Worldwide prevalence of hypertension exceeds 1.3 billion. J. Am. Soc. Hypertens. 2016, 10, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Bolad, I.; Delafontaine, P. Endothelial dysfunction: Its role in hypertensive coronary disease. Curr. Opin. Cardiol. 2005, 20, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.T.; Babcock, M.C.; Watso, J.C.; Brian, M.S.; Migdal, K.U.; Wenner, M.M.; Farquhar, W.B. Relation between resting sympathetic outflow and vasoconstrictor responses to sympathetic nerve bursts: Sex differences in healthy young adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, 463–471. [Google Scholar] [CrossRef]
- Peramaiyan, R.; Thamaraiselvan, R.; Jayakumar, T.; Yutaka, N.; Dhanapal, S.; Gautam, S.; Ikuo, N. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar]
- Tousoulis, D.; Kampoli, A.M.; Tentolouris, N.P.C.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- King, A.L.; Polhemus, D.J.; Bhushan, D.; Otsuka, H.; Kazuhisa, K.; Nicholson, C.K.; Bradley, J.M.; Islam, K.N.; Calvert, J.W.; Tao, Y.X. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. USA 2014, 111, 3182–3187. [Google Scholar] [CrossRef]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 222–231. [Google Scholar] [CrossRef]
- Natarajan, M.; Habib, S.; Reddick, R.J.; Delma, C.R.; Manickam, K.; Prihoda, T.J.; Wemer, S.L.; Mohan, S. Endothelial cell-specific overexpression of endothelial nitric oxide synthase in Ins2Akita mice exacerbates diabetic nephropathy. J. Diabetes Complicat. 2018, 33, 23–32. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Cao, X.X.; Wen, H.X.; Zang, H.Y. Correlation analysis of levels of inflammatory cytokines and nitric oxide in peripheral blood with urine proteins and renal function in patients with gestational hypertension. Exp. Ther. Med. 2019, 13, 657–662. [Google Scholar] [CrossRef]
- Schulz, E.; Gori, T.; Münzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar]
- Hilde, J.M.; Skjørten, I.; Grøtta, O.J.; Hansteen, V.; Melsom, M.N.; Hisdal, J.; Humerfelt, S.; Steine, K. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, 1103–1111. [Google Scholar]
- Vermeulen Windsant, I.C.; Wit, N.C.J.; Sertorio, J.T.C.; Bijnen, A.A.; Ganushchak, Y.M.; Heijmans, J.H.; Tanus-Santos, J.E.; Jacobs, M.J.; Maessen, J.G.; Buurman, W.A. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front. Physiol. 2014, 5, 340. [Google Scholar] [CrossRef] [PubMed]
- Pijacka, W.; McBryde, F.D.; Marvar, P.J.; Lincevicius, G.S.; Abdala, A.P.L.; Woodward, L.; Li, D.; Paterson, D.J.; Paton, J.F.R. Carotid sinus denervation ameliorates renovascular hypertension in adult Wistar rats. J. Physiol. 2016, 594, 6255–6266. [Google Scholar] [CrossRef] [PubMed]
- Natalin, H.M.; Garcia, A.F.E.; Ramalho, L.N.Z.; Restini, C.B.A. Resveratrol improves vasoprotective effects of captopril on aortic remodeling and fibrosis triggered by renovascular hypertension. Cardiovasc. Pathol. 2016, 25, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhou, Z.; Zhang, Q.; Cai, W.; Zhou, Y.; Sun, H.; Qiu, L. Vaccarin administration ameliorates hypertension and cardiovascular remodeling in renovascular hypertensive rats. J. Cell. Biochem. 2018, 119, 926–937. [Google Scholar] [CrossRef]
- Guimarães, D.D.; Cruz, J.C.; Carvalho-Galvão, A.; Zhuge, Z.; Marques, S.M.; Naves, L.M.; Persson, A.E.G.; Weitzberg, E.; Lundberg, J.O.; Balarini, C.M. Dietary nitrate reduces blood pressure in rats with angiotensin II–induced hypertension via mechanisms that involve reduction of sympathetic hyperactivity. Hypertension 2019, 73, 839–848. [Google Scholar] [CrossRef]
- Yu, T.T.; Guo, K.; Chen, H.C.; Lan, C.Z.; Wang, J.; Huang, L.L.; Wang, X.H. Effects of traditional Chinese medicine Xin-Ji-Er-Kang formula on 2K1C hypertensive rats: Role of oxidative stress and endothelial dysfunction. BMC Complement. Altern. Med. 2013, 13, 173. [Google Scholar] [CrossRef]
- Alam, M.A.; Chowdhury, M.R.H.; Jain, P.; Sagor, M.A.T.; Reza, H.M. DPP-4 inhibitor sitagliptin prevents inflammation and oxidative stress of heart and kidney in two kidney and one clip (2K1C) rats. Diabetol. Metab. Syndr. 2015, 7, 107. [Google Scholar] [CrossRef]
- Campagnaro, B.P.; Gava, A.L.; Meyrelles, S.S.; Vasquez, E.C. Cardiac-autonomic imbalance and baroreflex dysfunction in the renovascular angiotensin-dependent hypertensive mouse. Int. J. Hypertens. 2012, 968123. [Google Scholar] [CrossRef]
- Li, J.; Feng, J.; Wei, H.; Liu, Q.; Yang, T.; Hou, S.; Zhao, Y.; Zhang, B.; Yang, C. The aqueous extract of Gynura divaricata (L.) DC improves glucose and lipid metabolism and ameliorates type 2 diabetes mellitus. Evid. Based Complement. Alternat. Med. 2018, 2018, 8686297. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhou, X.; Deng, Y.; Jing, Q.; Li, M.; Yuan, L. In vitro studies of Gynura divaricata (L.) DC extracts as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. J. Ethnopharmacol. 2011, 136, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.Q.; Zhang, U.Q. Bioactive components of Gynura divaricate and its potential use in health, food and medicine: A mini-review. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 113–127. [Google Scholar] [PubMed]
- Jamjang, C.; Kijpakorn, S.; Angkanaporn, K. Effect of dietary inclusion of Gynura divaricate (L.) on growth performance, hematology, and carcass fat deposition in broilers. J. Poult. Sci. 2020, 57, 114–123. [Google Scholar]
- Hoe, S.Z.; Kamaruddin, M.H.; Lam, S.K. Inhibition of angiotensin-converting enzyme activity by partially purified fraction of Gynura procumbens in spontaneously hypertensive rats. Med. Princ. Pract. 2007, 16, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M. International Society of Hypertension Global Hypertension Practice Guidelines. J. Hypertens. 2020, 75, 1334–1357. [Google Scholar]
- Kitt, J.; Fox, R.; Tucker, K.L.; McManus, R.J. New approaches in hypertension management: A review of current and developing technologies and their potential impact on hypertension care. Curr. Hypertens. Rep. 2019, 21, 44. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High blood pressure and cardiovascular disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Bak, M.; Thomsen, K.; Christiansen, T.; Flyvbjerg, A. Renal enlargement precedes renal hyperfiltration in early experimental diabetes in rats. J. Am. Soc. Nephrol. 2000, 11, 1287–1292. [Google Scholar]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S. The assessment of endothelial function-from research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef]
- Nagasu, H.; Satoh, M.; Kidokoro, K.; Nishi, Y.; Channon, K.M.; Sasaki, T.; Kashihara, N. Endothelial dysfunction promotes the transition from compensatory renal hypertrophy to kidney injury after unilateral nephrectomy in mice. Am. J. Physiol. Renal Physiol. 2012, 302, 1402–1408. [Google Scholar] [CrossRef]
- Zuccolo, E.; Lim, D.; Kheder, D.A.; Perna, A.; Catarsi, P.; Botta, L.; Rosti, V.; Riboni, L.; Sancini, G.; Tanzi, F. Acetylcholine induces intracellular Ca2+ oscillations and nitric oxide release in mouse brain endothelial cells. Cell Calcium 2017, 66, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Martínez, P.; Muñoz, M.; Benedito, S.; García-Sacristán, A.; Hernández, M.; Prieto, D. Endothelin-1 contributes to endothelial dysfunction and enhanced vasoconstriction through augmented superoxide production in penile arteries from insulin-resistant obese rats: Role of ETA and ETB receptors. Br. J. Pharmacol. 2014, 171, 5682–5695. [Google Scholar] [CrossRef]
- Adiarto, S.; Heiden, S.; Vignon-Zellweger, N.; Nakayama, K.; Yagi, K.; Yanagisawa, M.; Emoto, N. ET-1 from endothelial cells is required for complete angiotensin II-induced cardiac fibrosis and hypertrophy. Life Sci. 2012, 91, 651–657. [Google Scholar] [CrossRef]
- Te Riet, L.; Van Esch, J.H.; Roks, A.J.; Van den Meiracker, A.H.; Danser, A.H. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Pan, C.; Yu, T.T.; Guo, K.; Wang, X.H.; Zhang, J.Y.; Wang, H.Z.; Gao, S. Beneficial therapeutic effect of Chinese herbal Xinji’Erkang formula on hypertension-induced renal injury in the 2-kidney-1-clip hypertensive rats. Arf. J. Tradit. Complement. Altern. Med. 2014, 11, 16–27. [Google Scholar] [CrossRef]
- Ronco, C.; Di Lullo, L. Cardiorenal syndrome. Heart Fail. Clin. 2014, 10, 251–280. [Google Scholar] [CrossRef] [PubMed]
- House, A.A. Cardio-renal syndrome type 4: Epidemiology, pathophysiology and treatment. Semin. Nephrol. 2012, 32, 40–48. [Google Scholar] [CrossRef]
- Guo, K.; Lan, C.Z.; Yu, T.T.; Huang, L.L.; Wang, X.H.; Pan, C.; Gao, S. Effects of Xin-Ji-Er-Kang formula on 2K1C-induced hypertension and cardiovascular remodeling in rats. J. Ethnopharmacol. 2014, 155, 1227–1235. [Google Scholar] [CrossRef]
- Marra, A.M.; Bossone, E.; Salzano, A.; D’Assante, R.; Monaco, F.; Ferrara, F.; Arcopinto, M.; Vriz, O.; Suzuki, T.; Cittadini, A. Biomarkers in pulmonary hypertension. Heart Fail. Clin. 2018, 14, 393–402. [Google Scholar] [CrossRef]
- Yamashita, T.; Seino, Y.; Ogawa, A.; Ogata, K.I.; Fukushima, M.; Tanaka, K.; Mizuno, K. N-terminal pro-BNP is a novel biomarker for integrated cardio-renal burden and early risk stratification in patients admitted for cardiac emergency. J. Cardiol. 2010, 55, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, Y.; Sun, W.; De Lemos, J.A.; Taffet, G.E.; Virani, S.S.; Ndumele, C.E.; Mosley, T.H.; Hoogeveen, R.C.; Coresh, J.; Wright, J.D. High sensitivity troponin T and cardiovascular events in systolic blood pressure categories: Atherosclerosis risk in communities study. Hypertension 2015, 65, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.; Gopal, D.; Kipper, B.A.; Landa, A.D.L.P.; Aramin, H.; Lee, E.; Shah, S.; Maisel, A.S. Cardiorenal biomarkers in acute heart failure. J. Geriatr. Cardiol. 2012, 9, 292–304. [Google Scholar] [PubMed]
- Cruz, D.N.; Fard, A.; Clementi, A.; Ronco, C.; Maisel, A. Role of biomarkers in the diagnosis and management of cardio-renal syndromes. Semin. Nephrol. 2011, 32, 79–92. [Google Scholar] [CrossRef]

| Gene | Primer Nucleotide Sequence |
|---|---|
| TNT | Forward: 5′-CGT GGC TTG GGT TTG GTG T-3′ Reverse: 5′-AGA CTG GAG TGA AGA AGA GGA GGA C-3′ |
| BNP | Forward: 5′-TGA TTC CTG CTC CTG CTT TTC-3′ Reverse: 5′-GTG GAT TGT TCT GGA GAC TG-3′ |
| GAPDH | Forward: 5′-CAG TGC CAG CCT CGT CTC-3′ Reverse: 5′-AGG GGC CAT CCA CAG TCT-3′ |
| CONT | 2K1C | OMT 10 (mg/kg/day) | GD 50 (mg/kg/day) | GD 100 (mg/kg/day) | GD 200 (mg/kg/day) | |
|---|---|---|---|---|---|---|
| Aldosterone (pg/mL) | 248.3 ± 54.5 | 1693 ± 496.6 *** | 253.1 ± 46.3 ### | 212.6 ± 69.5 ### | 108.4 ± 13.6 ### | 188 ± 20 ### |
| Angiotensin (pg/mL) | 532.1 ± 36.8 | 1699.8 ± 144 *** | 1168.6 ± 88.1 # | 1250.6 ± 119.5 # | 910 ± 109 ## | 623 ± 112.6 ### |
| Albumin (g/dL) | 3.1 ± 0.03 | 2.5 ± 0.06 *** | 3.3 ± 0.05 ### | 3.1 ± 0.05 ### | 3.2 ± 0.06 ### | 3.1 ± 0.12 ## |
| Bun (mg/dL) | 15.7 ± 0.5 | 38.6 ± 7.9 ** | 16.5 ± 0.7 ## | 18.8 ± 2.2 # | 18.2 ± 1.6 # | 18.2 ± 1.2 ## |
| Ccr (mL/min) | 527.9 ± 143.2 | 35.2 ± 27.4 * | 466.7 ± 100.1 ## | 426.9 ± 63.7 # | 399.8 ± 100.6 ## | 487.9 ± 92.2 ## |
| CONT | 2K1C | OMT 10 (mg/kg/day) | GD 50 (mg/kg/day) | GD 100 (mg/kg/day) | GD 200 (mg/kg/day) | |
|---|---|---|---|---|---|---|
| Body weight (g) | 458.6 ± 3.4 | 336.2 ± 18.1 *** | 415 ± 7.3 ## | 418.7 ± 6.3 ## | 414.3 ± 3 ## | 423.1 ± 7.8 ## |
| Water intake (m/day) | 29.5 ± 1.8 | 61.5 ± 6.3 *** | 38.5 ± 2.3 ## | 38.8 ± 2.2 ## | 29.5 ± 1.6 ### | 30 ± 2.7 ## |
| Urine volume (g/23 h) | 11.3 ± 0.8 | 47.3 ± 5.9 *** | 19.8 ± 2.6 ### | 17 ± 1.8 ### | 15.5 ± 2 ### | 15.6 ± 1.4 ### |
| Urine Cl− (mmol/L) | 274.5 ± 22.7 | 96 ± 6.1 *** | 208.2 ± 36.5 # | 222 ± 15.4 ### | 198.7 ± 14.4 ### | 273 ± 31.1 ### |
| Urine Na+ (mmol/L) | 165.7 ± 19.2 | 46.5 ± 6.1 *** | 124.8 ± 19.3 ## | 133.8 ± 11.9 ### | 108 ± 10.6 ## | 120.6 ± 23 ## |
| Urine K+ (mmol/L) | 241.3 ± 18 | 78.3 ± 7 *** | 182.5 ± 32.3 ## | 208.9 ± 13.4 ### | 186.7 ± 14.4 ### | 201 ± 24.7 ### |
| Osmolality (mosm/kg) | 1786 ± 111.1 | 570.8 ± 40.3 *** | 1560.3 ± 241.4 ### | 1540.2 ± 1.7.6 ### | 1609 ± 151.3 ### | 1597.8 ± 139.9 ### |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.H.; Jin, X.J.; Yoon, J.J.; Lee, Y.J.; Oh, H.C.; Lee, H.S.; Kim, H.Y.; Kang, D.G. Antihypertensive Effects of Gynura divaricata (L.) DC in Rats with Renovascular Hypertension. Nutrients 2020, 12, 3321. https://doi.org/10.3390/nu12113321
Hong MH, Jin XJ, Yoon JJ, Lee YJ, Oh HC, Lee HS, Kim HY, Kang DG. Antihypertensive Effects of Gynura divaricata (L.) DC in Rats with Renovascular Hypertension. Nutrients. 2020; 12(11):3321. https://doi.org/10.3390/nu12113321
Chicago/Turabian StyleHong, Mi Hyeon, Xian Jun Jin, Jung Joo Yoon, Yun Jung Lee, Hyun Cheol Oh, Ho Sub Lee, Hye Yoom Kim, and Dae Gill Kang. 2020. "Antihypertensive Effects of Gynura divaricata (L.) DC in Rats with Renovascular Hypertension" Nutrients 12, no. 11: 3321. https://doi.org/10.3390/nu12113321
APA StyleHong, M. H., Jin, X. J., Yoon, J. J., Lee, Y. J., Oh, H. C., Lee, H. S., Kim, H. Y., & Kang, D. G. (2020). Antihypertensive Effects of Gynura divaricata (L.) DC in Rats with Renovascular Hypertension. Nutrients, 12(11), 3321. https://doi.org/10.3390/nu12113321