Dietary Indole-3-Carbinol Alleviated Spleen Enlargement, Enhanced IgG Response in C3H/HeN Mice Infected with Citrobacter rodentium
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Cr Infection
2.3. Sample Collection
2.4. Histological Analysis
2.5. Gene Expression Analysis
2.6. Serum Cytokines Analysis
2.7. Immunoglobulin Analysis
2.8. Statistcal Analysis
3. Results
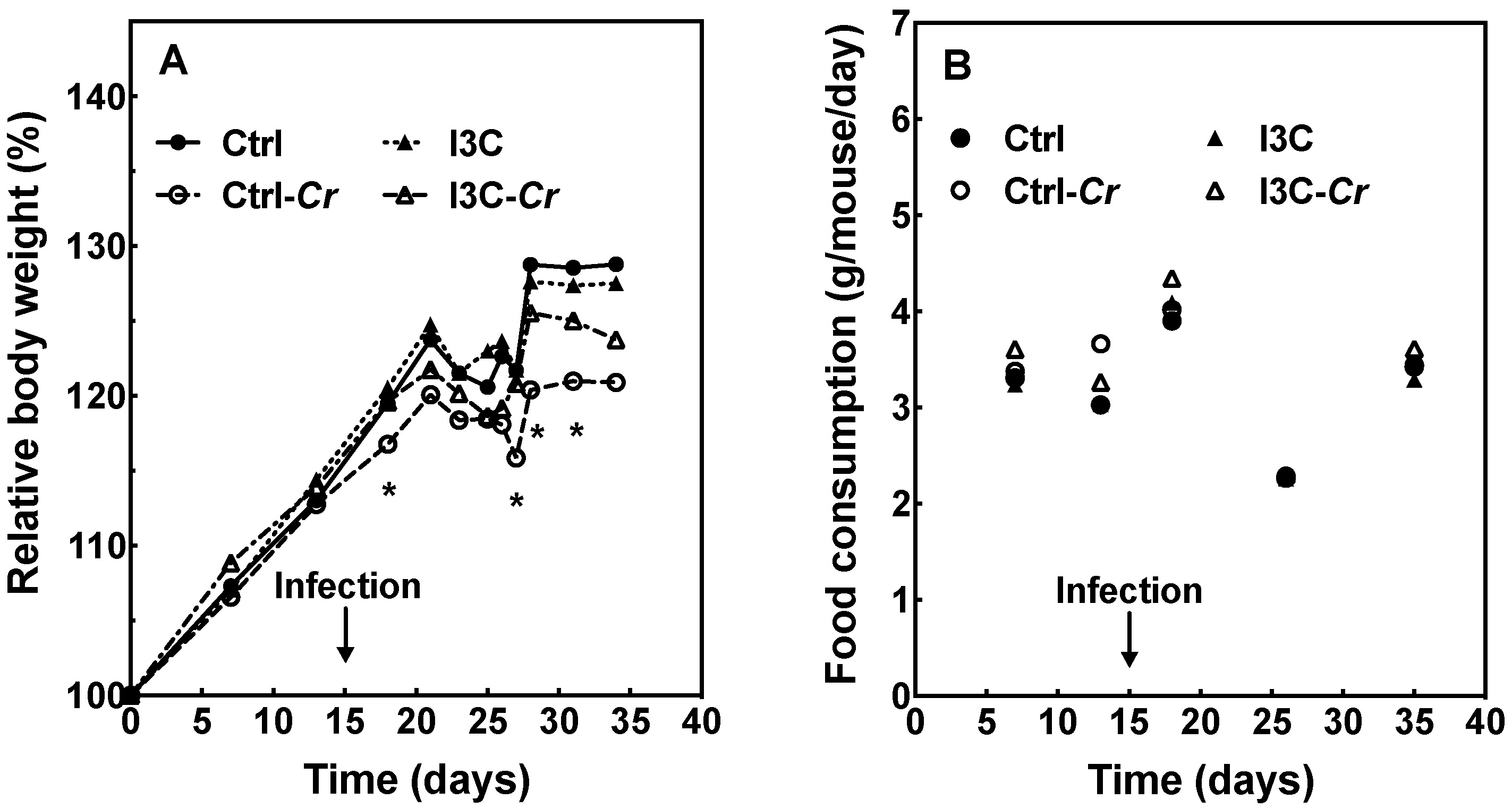
3.1. Effect of Cr Infection and I3C Supplementation on Food Intake and Body Weight
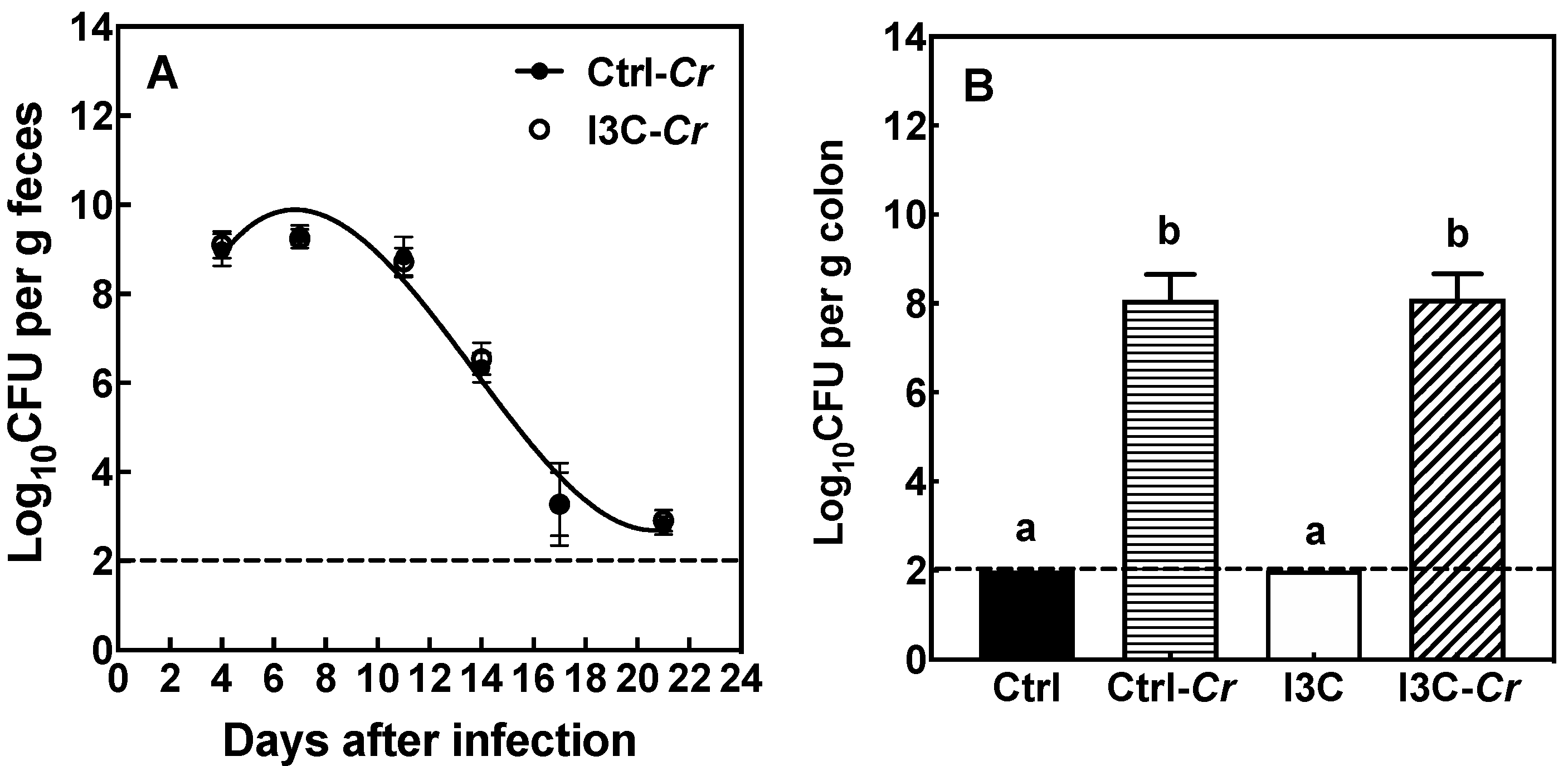
3.2. Effects of Dietary I3C on Cr Colonization in Feces and Colon Tissue
3.3. Effects of Dietary I3C on Colon and Cecum Weight in Mice
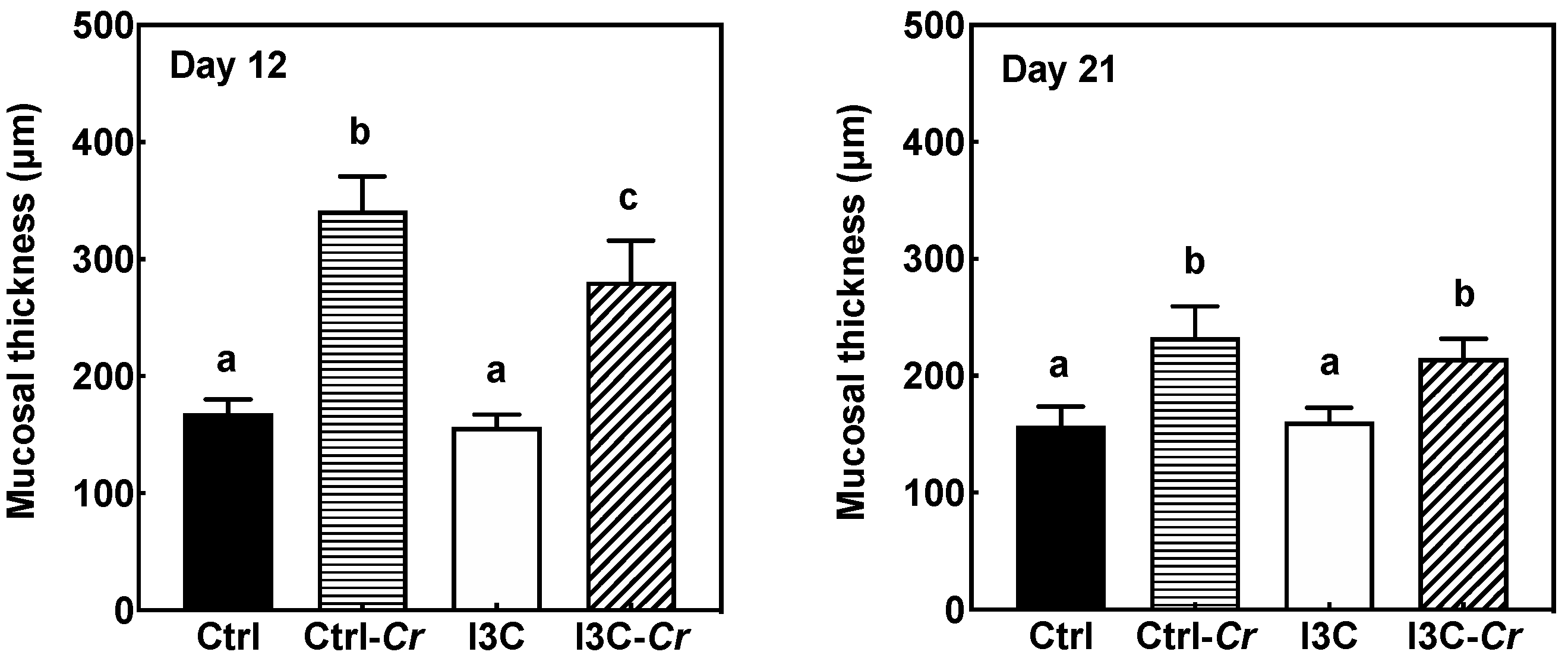
3.4. Effects of Dietary I3C on Histologic Changes in Colon of Mice
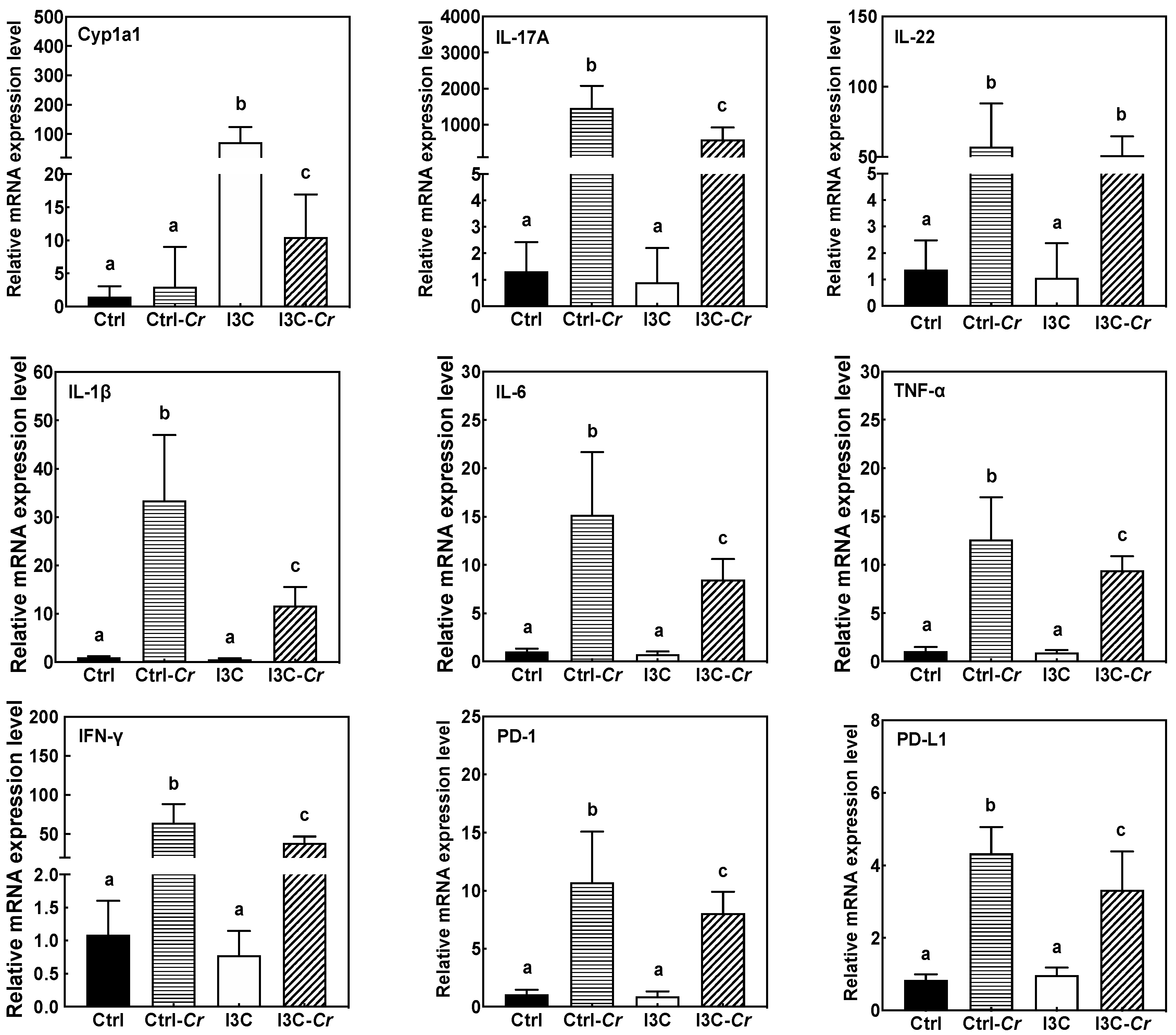
3.5. Effects of Dietary I3C on AhR and Immune Markers in Colonic Tissues
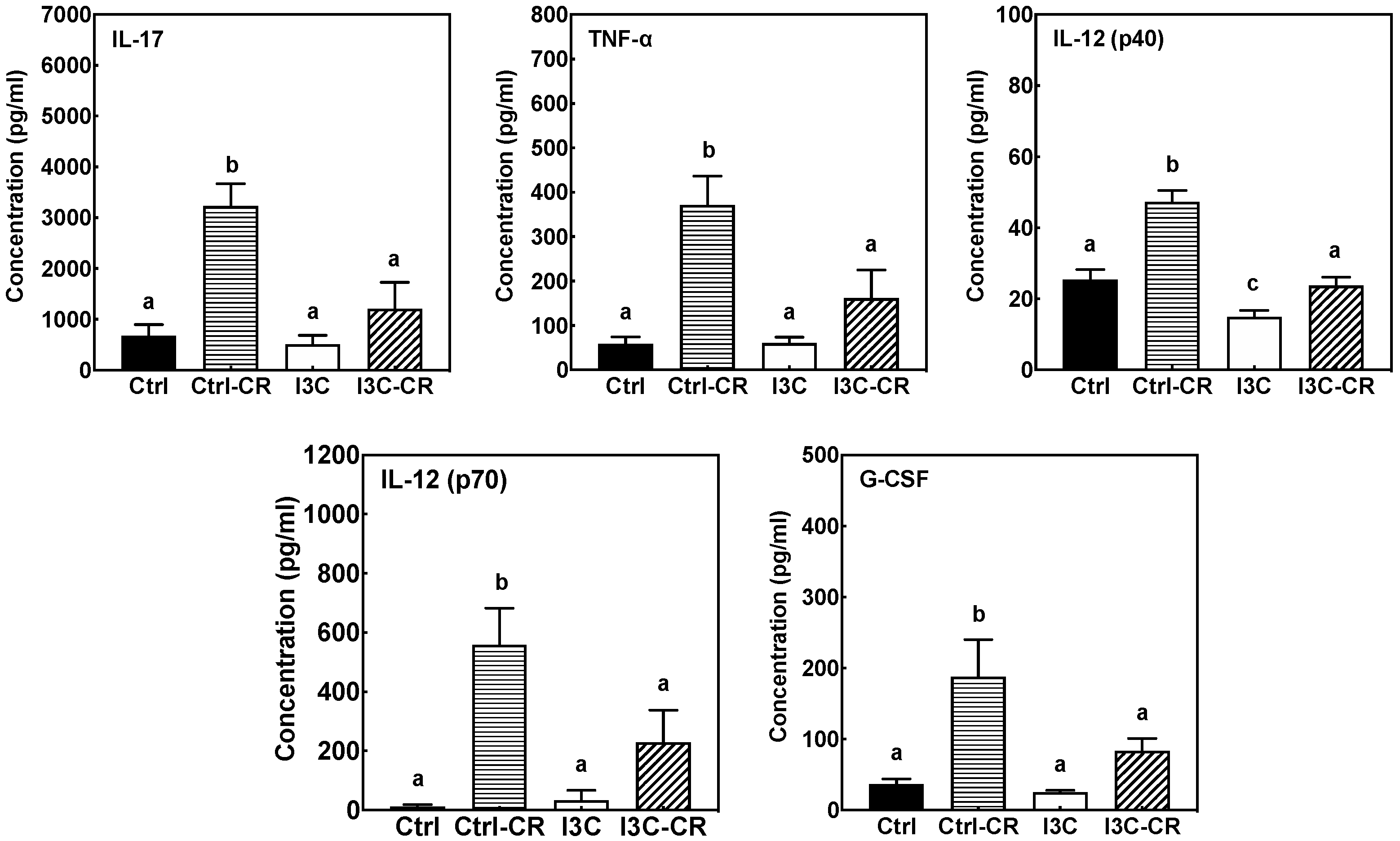
3.6. Effects of Dietary I3C on Cytokine Levels in Serum
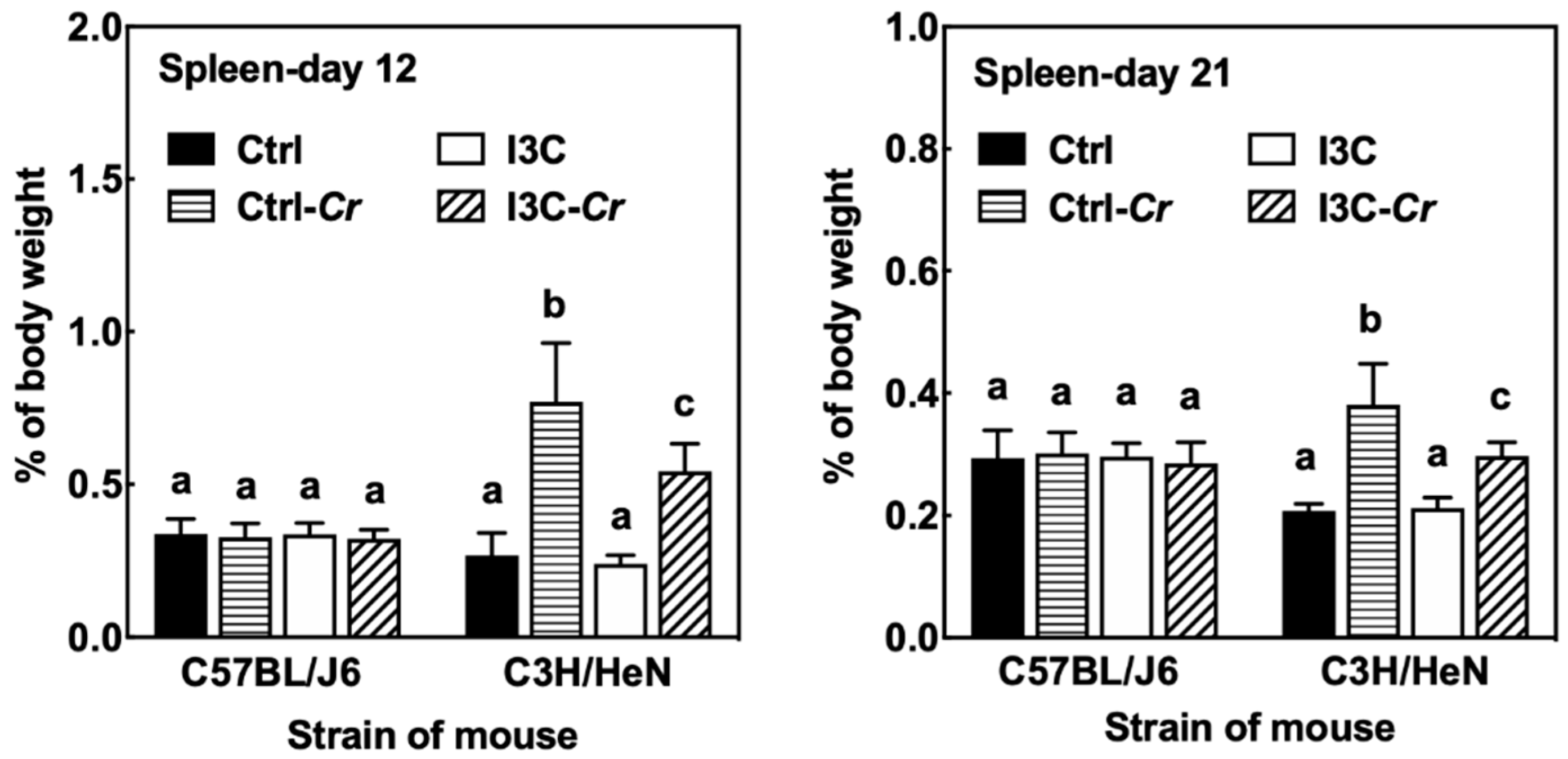
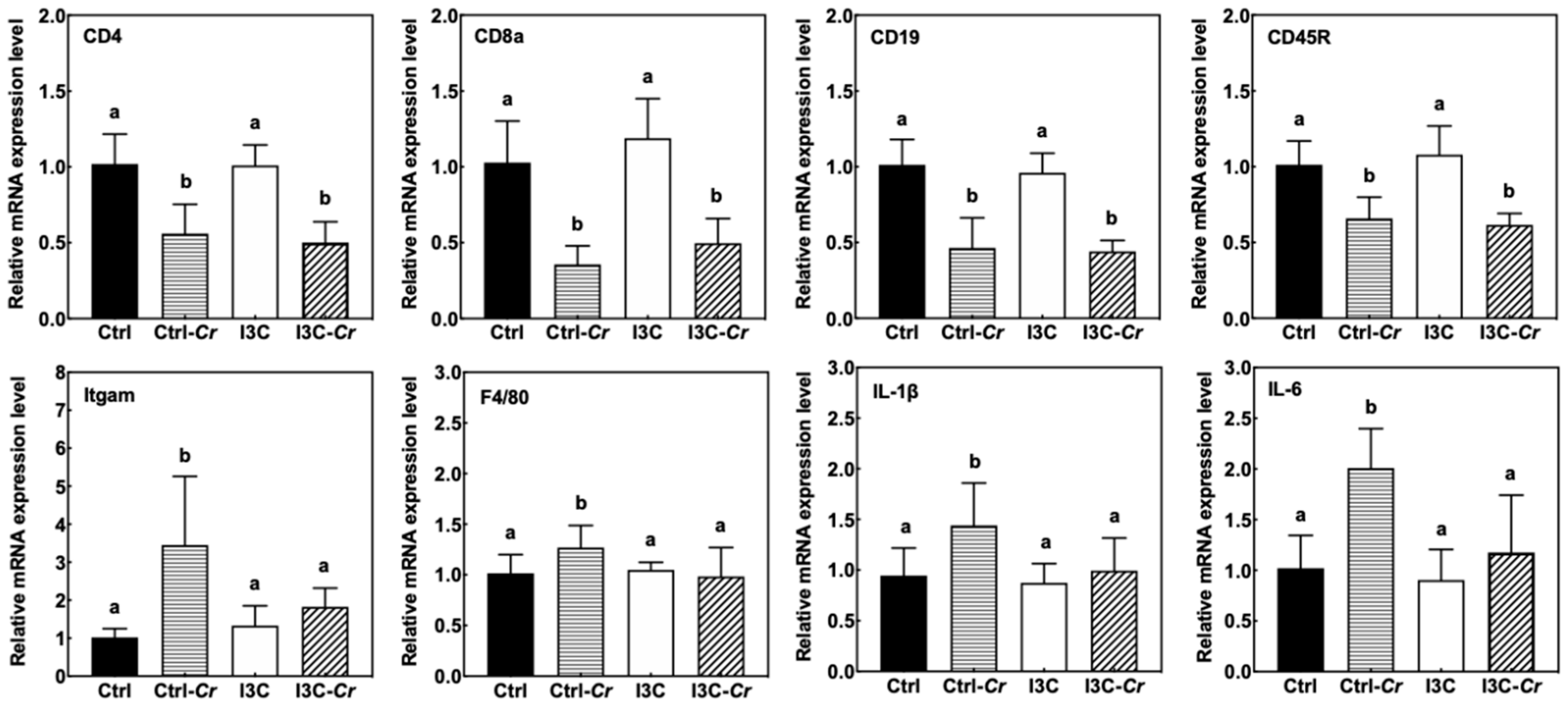
3.7. Effects of Dietary I3C on Spleen Size and Immune Cell Molecular Markers
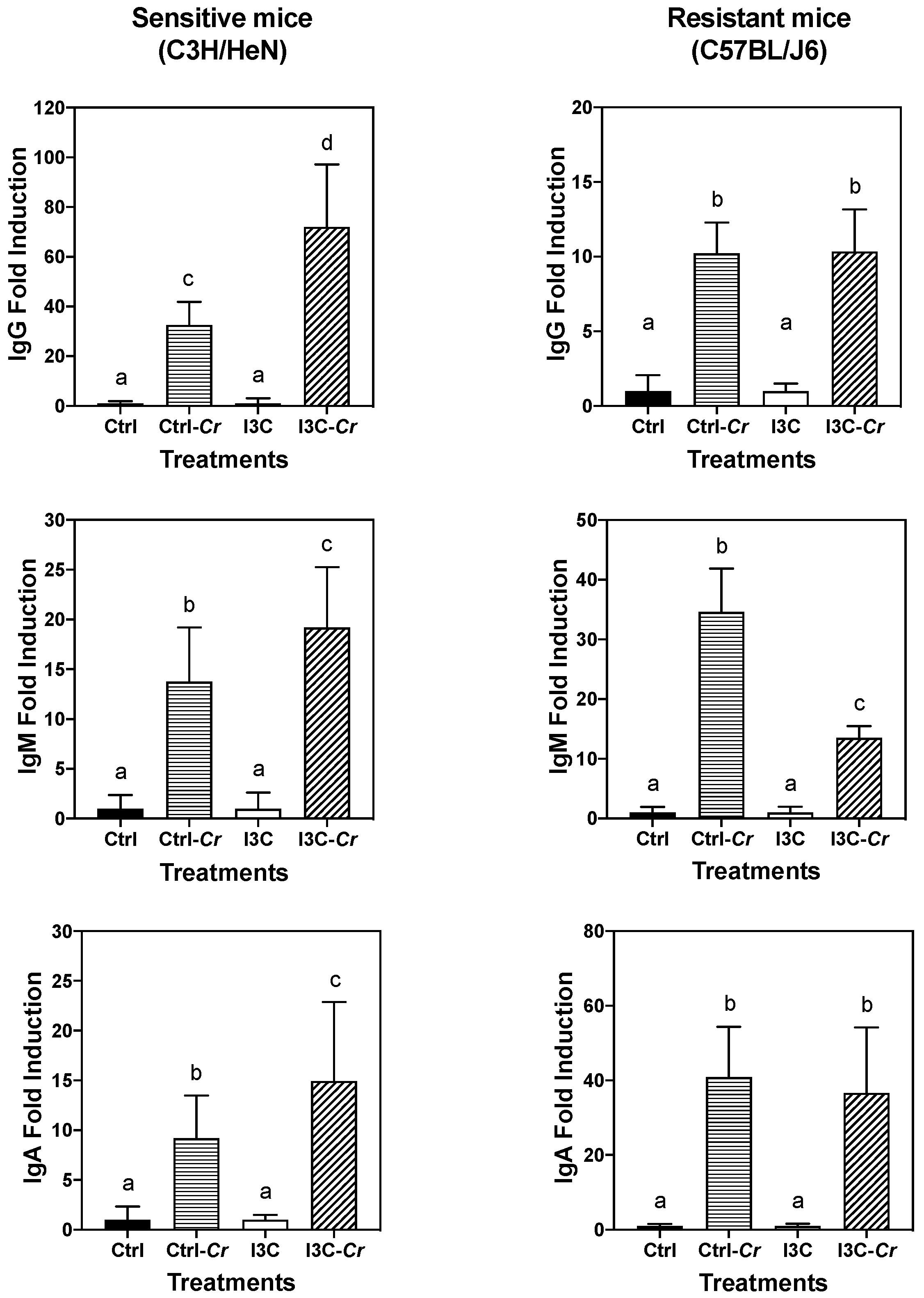
3.8. Effects of Dietary I3C on Cr-Specific Antibody Levels in Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petty, N.K.; Bulgin, R.; Crepin, V.F.; Cerdeño-Tárraga, A.M.; Schroeder, G.N.; Quail, M.A.; Lennard, N.; Corton, C.; Barron, A.; Clark, L. The Citrobacter rodentium genome sequence reveals convergent evolution with human pathogenic Escherichia coli. J. Bacteriol. 2010, 192, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Hartland, E.L.; Leong, J. Enteropathogenic and enterohemorrhagic E. coli: Ecology, pathogenesis, and evolution. Front. Cell. Infect. Microbiol. 2013, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.D.; Frankel, G. Enteropathogenic Escherichia coli: Unravelling pathogenesis. FEMS Microbiol. Rev. 2005, 29, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.A.; Tauxe, R.V.; Hosek, G.W.; Wells, J.G.; Stoesz, P.A.; McFadden, H.W., Jr.; Smith, P.W.; Wright, G.F.; Blake, P.A. Escherichia coli O157: H7 diarrhea in a nursing home: Clinical, epidemiological, and pathological findings. J. Infect. Dis. 1986, 154, 631–638. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.A.R.; Gould, I.M.; Curnow, J. Epidemiology of infection due to Escherichia coli O157: A 3-year prospective study. Epidemiol. Infect. 1996, 116, 279–284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petty, N.K.; Zakour, N.L.B.; Stanton-Cook, M.; Skippington, E.; Totsika, M.; Forde, B.M.; Phan, M.-D.; Moriel, D.G.; Peters, K.M.; Davies, M. Global dissemination of a multidrug resistant Escherichia coli clone. Proc. Natl. Acad. Sci. USA 2014, 111, 5694–5699. [Google Scholar] [CrossRef]
- Chandrakesan, P.; Roy, B.; Jakkula, L.; Ahmed, I.; Ramamoorthy, P.; Tawfik, O.; Papineni, R.; Houchen, C.; Anant, S.; Umar, S. Utility of a bacterial infection model to study epithelial–mesenchymal transition, mesenchymal–epithelial transition or tumorigenesis. Oncogene 2014, 33, 2639–2654. [Google Scholar] [CrossRef]
- Higgins, L.M.; Frankel, G.; Douce, G.; Dougan, G.; MacDonald, T.T. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect. Immun. 1999, 67, 3031–3039. [Google Scholar] [CrossRef]
- Mundy, R.; MacDonald, T.T.; Dougan, G.; Frankel, G.; Wiles, S. Citrobacter rodentium of mice and man. Cell. Microbiol. 2005, 7, 1697–1706. [Google Scholar] [CrossRef]
- Papapietro, O.; Teatero, S.; Thanabalasuriar, A.; Yuki, K.E.; Diez, E.; Zhu, L.; Kang, E.; Dhillon, S.; Muise, A.M.; Durocher, Y. R-spondin 2 signalling mediates susceptibility to fatal infectious diarrhoea. Nat. Commun. 2013, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Borenshtein, D.; McBee, M.E.; Schauer, D.B. Utility of the Citrobacter rodentium infection model in laboratory mice. Curr. Opin. Gastroenterol. 2008, 24, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Wiles, S.; Clare, S.; Harker, J.; Huett, A.; Young, D.; Dougan, G.; Frankel, G. Organ specificity, colonization and clearance dynamics in vivo following oral challenges with the murine pathogen Citrobacter rodentium. Cell. Microbiol. 2004, 6, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of Glucosinolates in Vegetable Crops of Brassica o leracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef]
- Padilla, G.; Cartea, M.E.; Velasco, P.; de Haro, A.; Ordás, A. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry 2007, 68, 536–545. [Google Scholar] [CrossRef]
- Keck, A.-S.; Finley, J.W. Cruciferous vegetables: Cancer protective mechanisms of glucosinolate hydrolysis products and selenium. Integr. Cancer Ther. 2004, 3, 5–12. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Ichikawa, H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 2005, 4, 1201–1215. [Google Scholar] [CrossRef]
- Kim, Y.S.; Milner, J.A. Targets for indole-3-carbinol in cancer prevention. J. Nutr. Biochem. 2005, 16, 65–73. [Google Scholar] [CrossRef]
- Vallance, B.A.; Deng, W.; Knodler, L.A.; Finlay, B.B. Mice lacking T and B lymphocytes develop transient colitis and crypt hyperplasia yet suffer impaired bacterial clearance during Citrobacter rodentium infection. Infect. Immun. 2002, 70, 2070–2081. [Google Scholar] [CrossRef]
- Hall, L.J.; Murphy, C.T.; Hurley, G.; Quinlan, A.; Shanahan, F.; Nally, K.; Melgar, S. Natural killer cells protect against mucosal and systemic infection with the enteric pathogen Citrobacter rodentium. Infect. Immun. 2013, 81, 460–469. [Google Scholar] [CrossRef]
- Schreiber, H.A.; Loschko, J.; Karssemeijer, R.A.; Escolano, A.; Meredith, M.M.; Mucida, D.; Guermonprez, P.; Nussenzweig, M.C. Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium. J. Exp. Med. 2013, 210, 2025–2039. [Google Scholar] [CrossRef]
- Simmons, C.P.; Clare, S.; Ghaem-Maghami, M.; Uren, T.K.; Rankin, J.; Huett, A.; Goldin, R.; Lewis, D.J.; MacDonald, T.T.; Strugnell, R.A. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 2003, 71, 5077–5086. [Google Scholar] [CrossRef]
- Ota, N.; Wong, K.; Valdez, P.A.; Zheng, Y.; Crellin, N.K.; Diehl, L.; Ouyang, W. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat. Immunol. 2011, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Geddes, K.; Rubino, S.J.; Magalhaes, J.G.; Streutker, C.; Le Bourhis, L.; Cho, J.H.; Robertson, S.J.; Kim, C.J.; Kaul, R.; Philpott, D.J. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat. Med. 2011, 17, 837–844. [Google Scholar] [CrossRef]
- Kiss, E.A.; Vonarbourg, C.; Kopfmann, S.; Hobeika, E.; Finke, D.; Esser, C.; Diefenbach, A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011, 334, 1561–1565. [Google Scholar] [CrossRef]
- Wu, Y.; He, Q.; Yu, L.; Pham, Q.; Cheung, L.; Kim, Y.S.; Wang, T.T.Y.; Smith, A.D. Indole-3-Carbinol Inhibits Citrobacter rodentium Infection through Multiple Pathways Including Reduction of Bacterial Adhesion and Enhancement of Cytotoxic T Cell Activity. Nutrients 2020, 12, 917. [Google Scholar] [CrossRef]
- Kassie, F.; Matise, I.; Negia, M.; Upadhyaya, P.; Hecht, S.S. Dose-dependent inhibition of tobacco smoke carcinogen–induced lung tumorigenesis in A/J mice by indole-3-carbinol. Cancer Prev. Res. 2008, 1, 568–576. [Google Scholar] [CrossRef]
- Fletcher, A.; Huang, H.; Yu, L.; Pham, Q.; Yu, L.; Wang, T.T.Y. Reversible toxic effects of the dietary supplement Indole-3-carbinol in an immune compromised rodent model: Intestine as the main target. J. Diet. Suppl. 2017, 14, 303–322. [Google Scholar] [CrossRef]
- Reed, G.A.; Arneson, D.W.; Putnam, W.C.; Smith, H.J.; Gray, J.C.; Sullivan, D.K.; Mayo, M.S.; Crowell, J.A.; Hurwitz, A. Single-dose and multiple-dose administration of indole-3-carbinol to women: Pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiol. Prev. Biomark. 2006, 15, 2477–2481. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Cheung, L.; Botero, S. Long-term selenium deficiency increases the pathogenicity of a Citrobacter rodentium infection in mice. Biol. Trace Elem. Res. 2011, 144, 965–982. [Google Scholar] [CrossRef]
- Smith, A.; Bhagwat, A.A. Hypervirulent-host-associated Citrobacter rodentium cells have poor acid tolerance. Curr. Microbiol. 2013, 66, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Cerceo, E.; Deitelzweig, S.B.; Sherman, B.M.; Amin, A.N. Multidrug-resistant gram-negative bacterial infections in the hospital setting: Overview, implications for clinical practice, and emerging treatment options. Microb. Drug Resist. 2016, 22, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.S.; Lee, D.G. In vitro antimicrobial activity and the mode of action of indole-3-carbinol against human pathogenic microorganisms. Biol. Pharm. Bull. 2007, 30, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Bradlow, H.L.; Michnovicz, J.J.; Halper, M.; Miller, D.G.; Wong, G.Y.; Osborne, M.P. Long-term responses of women to indole-3-carbinol or a high fiber diet. Cancer Epidemiol. Prev. Biomark. 1994, 3, 591–595. [Google Scholar]
- Barthold, S.W.; Coleman, G.L.; Bhatt, P.N.; Osbaldiston, G.W.; Jonas, A.M. The etiology of transmissible murine colonic hyperplasia. Lab. Anim. Sci. 1976, 26, 889–894. [Google Scholar]
- Maaser, C.; Housley, M.P.; Iimura, M.; Smith, J.R.; Vallance, B.A.; Finlay, B.B.; Schreiber, J.R.; Varki, N.M.; Kagnoff, M.F.; Eckmann, L. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect. Immun. 2004, 72, 3315–3324. [Google Scholar] [CrossRef]
- Schauer, D.B.; Falkow, S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect. Immun. 1993, 61, 2486–2492. [Google Scholar] [CrossRef]
- Liang, S.C.; Tan, X.-Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef]
- Zheng, Y.; Valdez, P.A.; Danilenko, D.M.; Hu, Y.; Sa, S.M.; Gong, Q.; Abbas, A.R.; Modrusan, Z.; Ghilardi, N.; De Sauvage, F.J. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008, 14, 282–289. [Google Scholar] [CrossRef]
- Valeri, M.; Raffatellu, M. Cytokines IL-17 and IL-22 in the host response to infection. Pathog. Dis. 2016, 74, ftw111. [Google Scholar] [CrossRef]
- Veldhoen, M.; Hirota, K.; Christensen, J.; O’Garra, A.; Stockinger, B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J. Exp. Med. 2009, 206, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Guo, X.; Zong-ming, E.C.; He, L.; Sonnenberg, G.F.; Artis, D.; Fu, Y.-X.; Zhou, L. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity 2013, 39, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Busbee, P.B.; Menzel, L.; Alrafas, H.R.; Dopkins, N.; Becker, W.; Miranda, K.; Tang, C.; Chatterjee, S.; Singh, U.P.; Nagarkatti, M. Indole-3-carbinol prevents colitis and associated microbial dysbiosis in an IL-22–dependent manner. JCI Insight 2020, 5, e127551. [Google Scholar] [CrossRef] [PubMed]
- Bohnsack, J.F.; Brown, E.J. The role of the spleen in resistance to infection. Annu. Rev. Med. 1986, 37, 49–59. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Primers | Catalog Number | Primers | Catalog Number |
|---|---|---|---|
| TBP | Mm00446971_m1 | IL-1β | Mm00434228_m1 |
| Cyp1a1 | Mm00487217_m1 | TNF-α | Mm00443258_m1 |
| IL-17A | Mm00439618_m1 | IFN-γ | Mm01168134_m1 |
| IL-22 | Mm01226722_g1 | Cd8a | Mm01182108_m1 |
| IL-6 | Mm00446190_m1 | Cd8b | Mm00438116_m1 |
| Foxp3 | Mm00475162_m1 | FasL | Mm00438864_m1 |
| Cd4 | Mm00442754_m1 | Cd5 | Mm00432417_m1 |
| Cd19 | Mm00515420_m1 | Cd45 | Mm01293577_m1 |
| Itgam | Mm00434455_m1 | F4/80 | Mm00802529_m1 |
| PD-1 | Mm00435532_m1 | PD-L1 | Mm03048248_m1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, J.; He, Q.; Yu, L.; Pham, Q.; Cheung, L.; Zhang, Z.; Kim, Y.S.; Smith, A.D.; Wang, T.T.Y. Dietary Indole-3-Carbinol Alleviated Spleen Enlargement, Enhanced IgG Response in C3H/HeN Mice Infected with Citrobacter rodentium. Nutrients 2020, 12, 3148. https://doi.org/10.3390/nu12103148
Wu Y, Wang J, He Q, Yu L, Pham Q, Cheung L, Zhang Z, Kim YS, Smith AD, Wang TTY. Dietary Indole-3-Carbinol Alleviated Spleen Enlargement, Enhanced IgG Response in C3H/HeN Mice Infected with Citrobacter rodentium. Nutrients. 2020; 12(10):3148. https://doi.org/10.3390/nu12103148
Chicago/Turabian StyleWu, Yanbei, Jing Wang, Qiang He, Liangli Yu, Quynhchi Pham, Lumei Cheung, Zhi Zhang, Young S. Kim, Allen D. Smith, and Thomas T. Y. Wang. 2020. "Dietary Indole-3-Carbinol Alleviated Spleen Enlargement, Enhanced IgG Response in C3H/HeN Mice Infected with Citrobacter rodentium" Nutrients 12, no. 10: 3148. https://doi.org/10.3390/nu12103148
APA StyleWu, Y., Wang, J., He, Q., Yu, L., Pham, Q., Cheung, L., Zhang, Z., Kim, Y. S., Smith, A. D., & Wang, T. T. Y. (2020). Dietary Indole-3-Carbinol Alleviated Spleen Enlargement, Enhanced IgG Response in C3H/HeN Mice Infected with Citrobacter rodentium. Nutrients, 12(10), 3148. https://doi.org/10.3390/nu12103148






