Effects of Bifidobacterium animalis Subsp. lactis (BPL1) Supplementation in Children and Adolescents with Prader–Willi Syndrome: A Randomized Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Intervention
2.4. Randomization
2.5. Outcomes
2.6. Subgroup Analyses
2.7. Gut Microbiota
2.8. Statistical Analysis
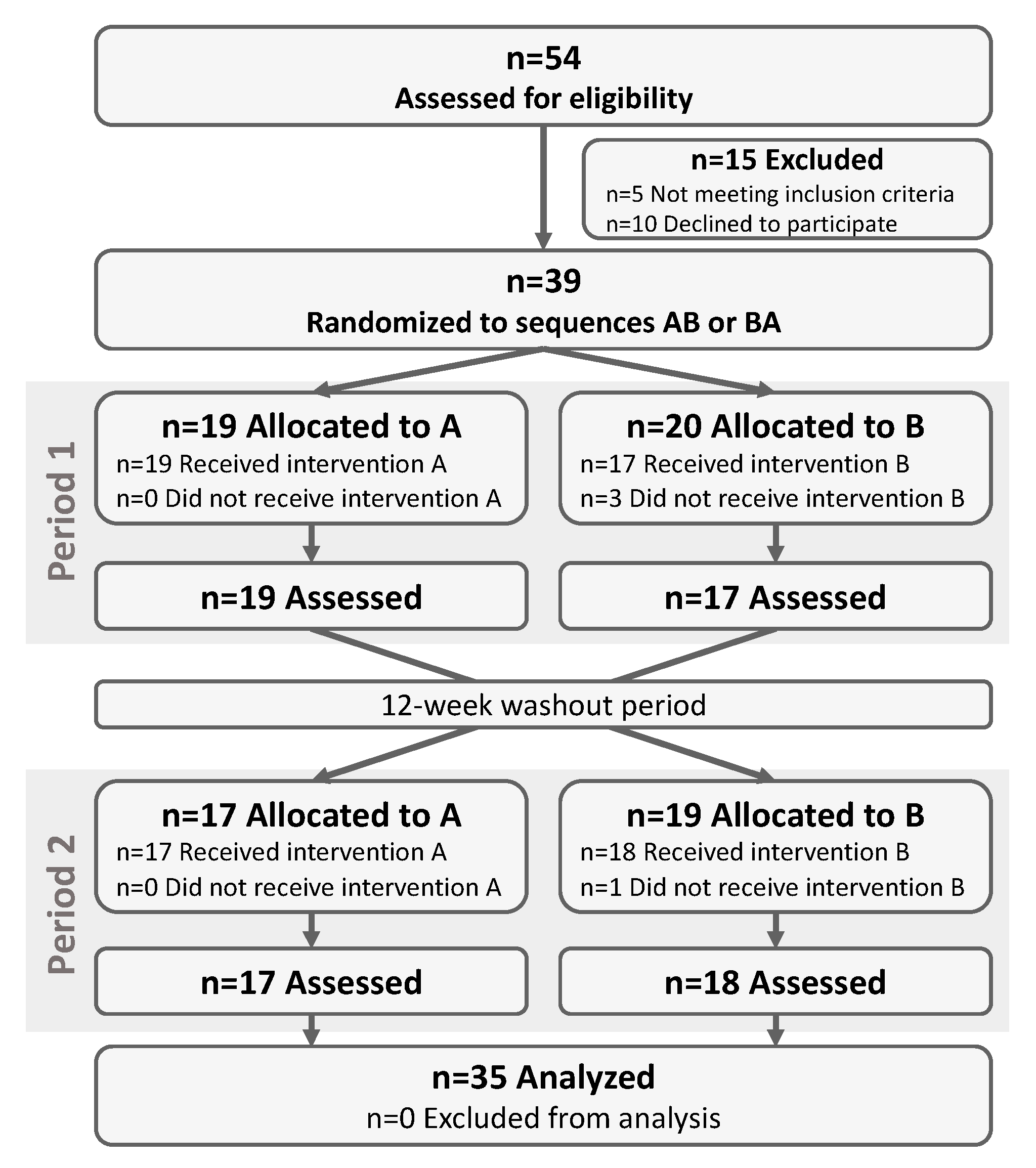
3. Results
3.1. Study Participants
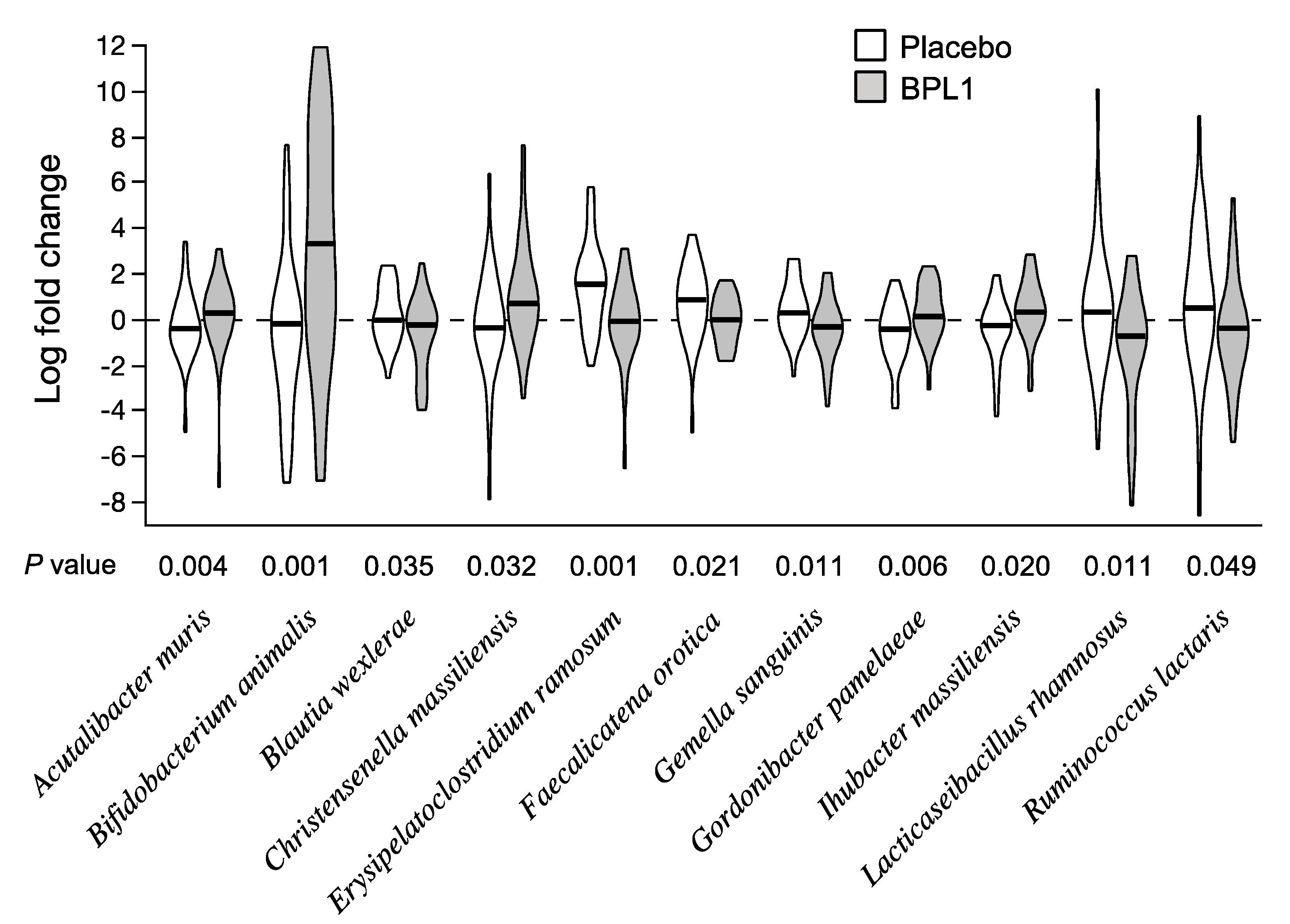
3.2. Effects of BPL1 Probiotic Supplementation on Gut Microbiota Composition
3.3. Effects of BPL1 Probiotic Supplementation on Adiposity
3.4. Effects of BPL1 Probiotic Supplementation on Hyperphagia, Lipid Profile, and Glucose Homeostasis
3.5. Effects of BPL1 Probiotic Supplementation on Mental Health Problems
3.6. Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef]
- Kimonis, V.E.; Tamura, R.; Gold, J.A.; Patel, N.; Surampalli, A.; Manazir, J.; Miller, J.L.; Roof, E.; Dykens, E.; Butler, M.G.; et al. Early Diagnosis in Prader-Willi Syndrome Reduces Obesity and Associated Co-Morbidities. Genes 2019, 10, 898. [Google Scholar] [CrossRef]
- Alsaif, M.; Elliot, S.A.; MacKenzie, M.L.; Prado, C.M.; Field, C.J.; Haqq, A.M. Energy Metabolism Profile in Individuals with Prader-Willi Syndrome and Implications for Clinical Management: A Systematic Review. Adv. Nutr. 2017, 8, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Gerasimidis, K.; Edwards, C.A.; Shaikh, M.G. Mechanisms of obesity in Prader–Willi syndrome. Pediatr. Obes. 2018, 13, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Lynn, C.H.; Driscoll, D.C.; Goldstone, A.P.; Gold, J.-A.; Kimonis, V.; Dykens, E.; Butler, M.G.; Shuster, J.J.; Driscoll, D.J. Nutritional phases in Prader–Willi syndrome. Am. J. Med. Gen. Part A 2011, 155, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Skokauskas, N.; Sweeny, E.; Meehan, J.; Gallagher, L. Mental health problems in children with prader-willi syndrome. J. Can. Acad. Child Adolesc. Psychiatry 2012, 21, 194–203. [Google Scholar] [PubMed]
- Holland, A.J.; Aman, L.C.S.; Whittington, J.E. Defining Mental and Behavioural Disorders in Genetically Determined Neurodevelopmental Syndromes with Particular Reference to Prader-Willi Syndrome. Genes 2019, 10, 1025. [Google Scholar] [CrossRef]
- Tan, Q.; Orsso, C.E.; Deehan, E.C.; Triador, L.; Field, C.J.; Tun, H.M.; Han, J.C.; Müller, T.D.; Haqq, A.M. Current and emerging therapies for managing hyperphagia and obesity in Prader-Willi syndrome: A narrative review. Obes. Rev. 2020, 21, e12992. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Hsiao Elaine, Y.; McBride Sara, W.; Hsien, S.; Sharon, G.; Hyde Embriette, R.; McCue, T.; Codelli Julian, A.; Chow, J.; Reisman Sarah, E.; Petrosino Joseph, F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Gheorghe, C.E.; Martin, J.A.; Manriquez, F.V.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Focus on the essentials: Tryptophan metabolism and the microbiome-gut-brain axis. Curr. Opin. Pharmacol. 2019, 48, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Anzawa, D.; Takami, K.; Ishizuka, A.; Mawatari, T.; Kamikado, K.; Sugimura, H.; Nishijima, T. Effect of Bifidobacterium animalis ssp. lactis GCL2505 on visceral fat accumulation in healthy Japanese adults: A randomized controlled trial. Biosci. Microbiota Food Health 2016, 35, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Stenman, L.K.; Lehtinen, M.J.; Meland, N.; Christensen, J.E.; Yeung, N.; Saarinen, M.T.; Courtney, M.; Burcelin, R.; Lähdeaho, M.-L.; Linros, J.; et al. Probiotic with or without Fiber Controls Body Fat Mass, Associated with Serum Zonulin, in Overweight and Obese Adults—Randomized Controlled Trial. EBioMedicine 2016, 13, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Pedret, A.; Valls, R.M.; Calderon-Perez, L.; Llaurado, E.; Companys, J.; Pla-Paga, L.; Moragas, A.; Martin-Lujan, F.; Ortega, Y.; Giralt, M.; et al. Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: A randomized controlled trial. Int. J. Obes. 2019, 43, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Martorell, P.; Llopis, S.; González, N.; Chenoll, E.; López-Carreras, N.; Aleixandre, A.; Chen, Y.; Karoly, E.D.; Ramón, D.; Genovés, S. Probiotic Strain Bifidobacterium animalis subsp. lactis CECT 8145 Reduces Fat Content and Modulates Lipid Metabolism and Antioxidant Response in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 3462–3472. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. EBioMedicine 2015, 2, 968–984. [Google Scholar] [CrossRef]
- Olsson, L.M.; Poitou, C.; Tremaroli, V.; Coupaye, M.; Aron-Wisnewsky, J.; Backhed, F.; Clement, K.; Caesar, R. Gut microbiota of obese subjects with Prader-Willi syndrome is linked to metabolic health. Gut 2019, 69, 1229–1238. [Google Scholar] [CrossRef]
- Garcia-Ribera, S.; Amat-Bou, M.; Climent, E.; Llobet, M.; Chenoll, E.; Corripio, R.; Ibáñez, L.; Ramon-Krauel, M.; Lerin, C. Specific Dietary Components and Gut Microbiota Composition are Associated with Obesity in Children and Adolescents with Prader-Willi Syndrome. Nutrients 2020, 12, 1063. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tan, Q.; Afhami, S.; Deehan, E.C.; Liang, S.; Gantz, M.; Triador, L.; Madsen, K.L.; Walter, J.; Tun, H.M.; et al. The Gut Microbiota Profile in Children with Prader-Willi Syndrome. Genes 2020, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Blössner, M.; Siyam, A.; Borghi, E.; Onyango, A.; de Onis, M. WHO AnthroPlus for Personal Computers. Manual: Software for Assessing Growth of the World’s Children and Adolescents; World Health Organization: Geneva, Switzerland, 2009; Available online: http://www.who.int/growthref/tools/en/ (accessed on 14 September 2020).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; López, -S.A.; Andrés, P.; Requejo, A.M.; Aparicio, A.; Molinero, L.M. DIAL Software for Assessing Diets and Food Calculations (for Windows, version 3.5.0.3). Available online: http://www.alceingenieria.net/nutricion/descarga.htm (accessed on 19 February 2020).
- Dekker, M.C.; Koot, H.M.; Van Der Ende, J.; Verhulst, F.C. Emotional and behavioral problems in children and adolescents with and without intellectual disability. J. Child Psychol. Psychiatry 2002, 43, 1087–1098. [Google Scholar] [CrossRef]
- Veltman, M.W.; Thompson, R.J.; Roberts, S.E.; Thomas, N.S.; Whittington, J.; Bolton, P.F. Prader-Willi syndrome—A study comparing deletion and uniparental disomy cases with reference to autism spectrum disorders. Eur. Child. Adolesc. Psychiatry 2004, 13, 42–50. [Google Scholar] [CrossRef]
- Yuan, S.; Cohen, D.B.; Ravel, J.; Abdo, Z.; Forney, L.J. Evaluation of methods for the extraction and purification of DNA from the human microbiome. PLoS ONE 2012, 7, e33865. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2012, 41, e1. [Google Scholar] [CrossRef]
- Bushnell, B.; Rood, J.; Singer, E. BBMerge—Accurate paired shotgun read merging via overlap. PLoS ONE 2017, 12, e0185056. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 3. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Uusitupa, H.-M.; Rasinkangas, P.; Lehtinen, M.J.; Mäkelä, S.M.; Airaksinen, K.; Anglenius, H.; Ouwehand, A.C.; Maukonen, J. Bifidobacterium animalis subsp. lactis 420 for Metabolic Health: Review of the Research. Nutrients 2020, 12, 892. [Google Scholar] [CrossRef] [PubMed]
- Stenman, L.K.; Waget, A.; Garret, C.; Klopp, P.; Burcelin, R.; Lahtinen, S. Potential probiotic Bifidobacterium animalis ssp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Benef. Microbes 2014, 5, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Stenman, L.K.; Waget, A.; Garret, C.; Briand, F.; Burcelin, R.; Sulpice, T.; Lahtinen, S. Probiotic B420 and prebiotic polydextrose improve efficacy of antidiabetic drugs in mice. Diabetol. Metab. Syndr. 2015, 7, 75. [Google Scholar] [CrossRef]
- Carreras, N.L.; Martorell, P.; Chenoll, E.; Genoves, S.; Ramon, D.; Aleixandre, A. Anti-obesity properties of the strain Bifidobacterium animalis subsp. lactis CECT 8145 in Zucker fatty rats. Benef. Microbes 2018, 9, 629–641. [Google Scholar] [CrossRef]
- Van de Wouw, M.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. J. Nutr. 2017, 147, 727–745. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Yde, C.C.; Ziegler, M.L.; Honoré, A.H.; Saarinen, M.T.; Lahtinen, S.; Stahl, B.; Jensen, H.M.; Stenman, L.K. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Balaguer, F.; Enrique, M.; Llopis, S.; Barrena, M.; Álvarez, B.; Chenoll, E.; Ramón, D.; Tortajada, M.; Martorell, P. Lipoteichoic acid from Bifidobacterium animalis BPL1: A novel postbiotic that reduces fat deposition via IGF-1 pathway. Manuscript in preparation.
- Corrias, A.; Bellone, J.; Beccaria, L.; Bosio, L.; Trifiro, G.; Livieri, C.; Ragusa, L.; Salvatoni, A.; Andreo, M.; Ciampalini, P.; et al. GH/IGF-I axis in Prader-Willi syndrome: Evaluation of IGF-I levels and of the somatotroph responsiveness to various provocative stimuli. Genetic Obesity Study Group of Italian Society of Pediatric Endocrinology and Diabetology. J. Endocrinol. Investig. 2000, 23, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Aycan, Z.; Baş, V.N. Prader-Willi syndrome and growth hormone deficiency. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Dykens, E.M.; Lee, E.; Roof, E. Prader-Willi syndrome and autism spectrum disorders: An evolving story. J. Neurodev. Disord. 2011, 3, 225–237. [Google Scholar] [CrossRef] [PubMed]
| All (n = 35) | AB (n = 18) | BA (n = 17) | p Value | |
|---|---|---|---|---|
| Sex (female) | 21 (60) | 12 (67) | 9 (53) | 0.407 |
| Age (years) | 10.4 (5.0) | 10.3 (5.1) | 10.6 (4.9) | 0.862 |
| Genotype (deletions) | 18 (59) | 12 (67) | 6 (35) | 0.061 |
| Growth hormone use | 34 (97) | 18 (100) | 16 (94) | 0.225 |
| Metformin use | 8 (23) | 6 (33) | 2 (12) | 0.122 |
| Weight (kg) | 46.9 (27.7) | 47.0 (26.0) | 46.7 (30.2) | 0.979 |
| Height (cm) | 137.6 (26.1) | 137.9 (26.6) | 137.3 (26.4) | 0.941 |
| BMI-SDS | 1.22 (1.45) | 1.35 (1.39) | 1.07 (1.55) | 0.577 |
| Body fat mass (g) | 20,380 (15,952) | 20,037 (14,017) | 20,743 (18,213) | 0.899 |
| Body fat mass (%) | 40.8 (9.7) | 40.5 (9.7) | 41.1 (10) | 0.845 |
| Abdominal fat mass (g) | 1449 (1423) | 1408 (1240) | 1491 (1632) | 0.867 |
| Abdominal fat mass (%) | 6.0 (1.8) | 5.8 (2.1) | 6.2 (1.5) | 0.578 |
| HQ-CT (Score) | 6.0 (5.6) | 6.1 (6) | 5.9 (5.3) | 0.929 |
| Daily energy intake (kcal) | 1465 (413) | 1455 (463) | 1475 (366) | 0.891 |
| Systolic pressure (mmHg) | 108.8 (11.9) | 108.4 (12.1) | 109.1 (11.9) | 0.870 |
| Diastolic pressure (mmHg) | 70.6 (8.7) | 68.9 (8.9) | 72.4 (8.3) | 0.238 |
| Triglycerides (mg/dL) | 71 (24) | 68 (21) | 74 (26) | 0.452 |
| Total cholesterol (mg/dL) | 171 (37) | 176 (40) | 166 (34) | 0.456 |
| LDL-cholesterol (mg/dL) | 104 (32) | 107 (33) | 101 (31) | 0.537 |
| HDL-cholesterol (mg/dL) | 56 (13) | 55 (13) | 56 (14) | 0.778 |
| Glucose (mg/dL) | 86 (9) | 82 (8) | 89 (9) | 0.023 |
| HbA1c (%) | 5.2 (0.2) | 5.2 (0.2) | 5.2 (0.3) | 0.579 |
| Insulin (mU/L) | 11.2 (9.6) | 9.9 (9.5) | 12.7 (9.9) | 0.508 |
| HOMA-IR | 2.49 (2.26) | 2.14 (2.15) | 2.89 (2.38) | 0.423 |
| AB Group | BA Group | |||||
|---|---|---|---|---|---|---|
| Period 1 | Period 2 | Period 1 | Period 2 | Treatment Effect | ||
| All Subjects (n = 35) | Placebo | BPL1 | BPL1 | Placebo | B (CI 95%) | p Value |
| Body fat mass (g) | 73 (1060) | 58 (1166) | 158 (1457) | 624 (1236) | −173 (−907, 562) | 0.635 |
| Body fat mass (%) | −0.5 (1.5) | −1.3 (1.3) | −0.4 (2.2) | −0.5 (1.8) | −0.18 (−1.08, 0.73) | 0.691 |
| Abdominal fat mass (g) | −18 (177) | −28 (98) | −48 (121) | 69 (140) | −62 (−133, 8) | 0.082 |
| Abdominal fat mass (%) | 0.1 (0.5) | −0.1 (0.5) | −0.2 (0.6) | 0.0 (0.5) | −0.23 (−0.53, 0.06) | 0.121 |
| >4.5 Years (n = 28) | ||||||
| Body fat mass (g) | 59 (1209) | 27 (1328) | 20 (1571) | 489 (1312) | −156 (−1081, 768) | 0.729 |
| Body fat mass (%) | −0.5 (1.6) | −1.5 (1.3) | −1.1 (1.7) | −1.0 (1.4) | −0.30 (−1.31, 0.71) | 0.548 |
| Abdominal fat mass (g) | −26 (202) | −40 (106) | −72 (119) | 72 (152) | −74 (−157, 8) | 0.076 |
| Abdominal fat mass (%) | 0.0 (0.5) | −0.2 (0.4) | −0.3 (0.5) | 0.1 (0.4) | −0.33 (−0.59, −0.06) | 0.017 |
| AB Group | BA Group | |||||
|---|---|---|---|---|---|---|
| Period 1 | Period 2 | Period 1 | Period 2 | Treatment Effect | ||
| Placebo | BPL1 | BPL1 | Placebo | B (95% CI) | p Value | |
| HQ-CT (Score) | −0.50 (2.12) | −1.50 (3.98) | 0.65 (4.18) | −0.93 (3.38) | 0.05 (−1.79, 1.89) | 0.956 |
| E.I. (kcal/day) | −2 (397) | −20 (149) | 125 (199) | −31 (231) | 43 (−93, 179) | 0.521 |
| SP (mmHg) | 0.06 (9.55) | −1.00 (11.26) | 5.07 (7.99) | 2.60 (12.72) | 0.95 (−4.67, 6.56) | 0.730 |
| DP (mmHg) | −0.06 (8.9) | −0.13 (12.35) | 0.29 (7.89) | 0.2 (8.01) | 0.63 (−4.79, 6.05) | 0.812 |
| TG (mg/dL) | 1.1 (28.3) | −3.4 (32.4) | −2.2 (17.1) | −7.1 (18.3) | −0.49 (−15.52, 14.53) | 0.947 |
| Total cho (mg/dL) | −5.3 (19.4) | −4.1 (28.8) | −6.1 (18.2) | −7.6 (20.2) | 2.46 (−8.79, 13.71) | 0.658 |
| LDL-cho (mg/dL) | −1.2 (16.6) | 3.6 (14.4) | −3.8 (16.9) | −2.1 (16.7) | 1.61 (−7.25, 10.47) | 0.712 |
| HDL-cho (mg/dL) | −4.3 (10.0) | −1.8 (5.1) | −1.6 (5.8) | −4.5 (8.2) | 2.31 (−2.08, 6.71) | 0.290 |
| Glucose (mg/dL) | 2.4 (8.9) | −0.3 (8.9) | −1.4 (9.9) | −0.1 (10.7) | −3.21 (−8.50, 2.08) | 0.225 |
| HbA1c (%) | −0.01 (0.15) | 0.06 (0.12) | −0.05 (0.12) | 0.02 (0.14) | −0.01 (−0.09, 0.06) | 0.690 |
| Insulin (mU/L) | 0.41 (8.34) | −2.02 (6.05) | −2.78 (9.11) | 1.94 (9.26) | −4.44 (−8.51, −0.38) | 0.033 |
| HOMA-IR | 0.06 (1.95) | −0.52 (1.45) | −0.70 (2.17) | 0.45 (2.12) | −1.07 (−2.04, −0.10) | 0.031 |
| AB Group | BA Group | |||||
|---|---|---|---|---|---|---|
| Period 1 | Period 2 | Period 1 | Period 2 | Treatment Effect | ||
| Placebo | BPL1 | BPL1 | Placebo | B (95% CI) | p Value | |
| Anxious/Depressed | −2.4 (7.3) | 0.5 (1.9) | −0.3 (6.4) | −0.7 (6.3) | 1.2 (−4.2, 6.7) | 0.641 |
| Withdrawn/Depressed | 1.1 (5.9) | −3.0 (5.3) | −2.0 (6.0) | 2.7 (3.9) | −4.7 (−9.0, −0.3) | 0.037 |
| Somatic Complaints | 0.6 (3.3) | −0.1 (3.8) | −3.3 (6.2) | −0.4 (6.8) | −1.7 (−6.6, 3.2) | 0.460 |
| Social Problems | −3.3 (8.4) | 0.0 (5.3) | 0.6 (6.3) | 1.3 (6.9) | −0.4 (−5.7, 4.8) | 0.869 |
| Thought Problems | −5.5 (7.9) | −1.3 (2.7) | −2.1 (7.4) | 2.1 (5.2) | 0.3 (−4.2, 4.9) | 0.877 |
| Attention Problems | −0.4 (4.2) | −2.0 (4.9) | −1.0 (3.0) | 2.3 (4.9) | −2.9 (−6.5, 0.6) | 0.097 |
| Rule-Breaking Behavior | 0.5 (3.5) | −2.0 (3.3) | 0.7 (5.6) | 0.6 (7.9) | −2.0 (−5.2, 1.2) | 0.208 |
| Aggressive Behavior | −2.0 (4.3) | −1.4 (2.5) | −0.8 (6.2) | 0.3 (5.4) | 0.2 (−3.7, 4.0) | 0.933 |
| Internalizing Behavior | −1.1 (6.1) | −1.9 (3.2) | −2.5 (5.9) | 2.0 (5.3) | −3.0 (−8.3, 2.3) | 0.249 |
| Externalizing Behavior | −0.3 (3.6) | −2.4 (3.3) | −0.2 (4.2) | 0.6 (6.1) | −1.7 (−4.4, 1.0) | 0.203 |
| Total Score | −1.4 (4.6) | −2.5 (4.8) | −1.6 (4.3) | 2.3 (5) | −3.2 (−7.3, 0.9) | 0.120 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amat-Bou, M.; Garcia-Ribera, S.; Climent, E.; Piquer-Garcia, I.; Corripio, R.; Sanchez-Infantes, D.; Villalta, L.; Elias, M.; Jiménez-Chillarón, J.C.; Chenoll, E.; et al. Effects of Bifidobacterium animalis Subsp. lactis (BPL1) Supplementation in Children and Adolescents with Prader–Willi Syndrome: A Randomized Crossover Trial. Nutrients 2020, 12, 3123. https://doi.org/10.3390/nu12103123
Amat-Bou M, Garcia-Ribera S, Climent E, Piquer-Garcia I, Corripio R, Sanchez-Infantes D, Villalta L, Elias M, Jiménez-Chillarón JC, Chenoll E, et al. Effects of Bifidobacterium animalis Subsp. lactis (BPL1) Supplementation in Children and Adolescents with Prader–Willi Syndrome: A Randomized Crossover Trial. Nutrients. 2020; 12(10):3123. https://doi.org/10.3390/nu12103123
Chicago/Turabian StyleAmat-Bou, Montse, Sonika Garcia-Ribera, Eric Climent, Irene Piquer-Garcia, Raquel Corripio, David Sanchez-Infantes, Laia Villalta, Maria Elias, Josep C. Jiménez-Chillarón, Empar Chenoll, and et al. 2020. "Effects of Bifidobacterium animalis Subsp. lactis (BPL1) Supplementation in Children and Adolescents with Prader–Willi Syndrome: A Randomized Crossover Trial" Nutrients 12, no. 10: 3123. https://doi.org/10.3390/nu12103123
APA StyleAmat-Bou, M., Garcia-Ribera, S., Climent, E., Piquer-Garcia, I., Corripio, R., Sanchez-Infantes, D., Villalta, L., Elias, M., Jiménez-Chillarón, J. C., Chenoll, E., Ramón, D., Ibañez, L., Ramon-Krauel, M., & Lerin, C. (2020). Effects of Bifidobacterium animalis Subsp. lactis (BPL1) Supplementation in Children and Adolescents with Prader–Willi Syndrome: A Randomized Crossover Trial. Nutrients, 12(10), 3123. https://doi.org/10.3390/nu12103123





