An Almond-Based Low Carbohydrate Diet Improves Depression and Glycometabolism in Patients with Type 2 Diabetes through Modulating Gut Microbiota and GLP-1: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Sample Size Calculation
2.4. Intervention
2.5. Outcomes
2.5.1. Glycated Hemoglobin (HbA1c)
2.5.2. Depression
2.5.3. Anthropometric Measurements
2.5.4. Dietary Record
2.5.5. Fasting GLP-1 Concentration
2.5.6. Gut Microbiota
2.6. Statistical Analysis
3. Results
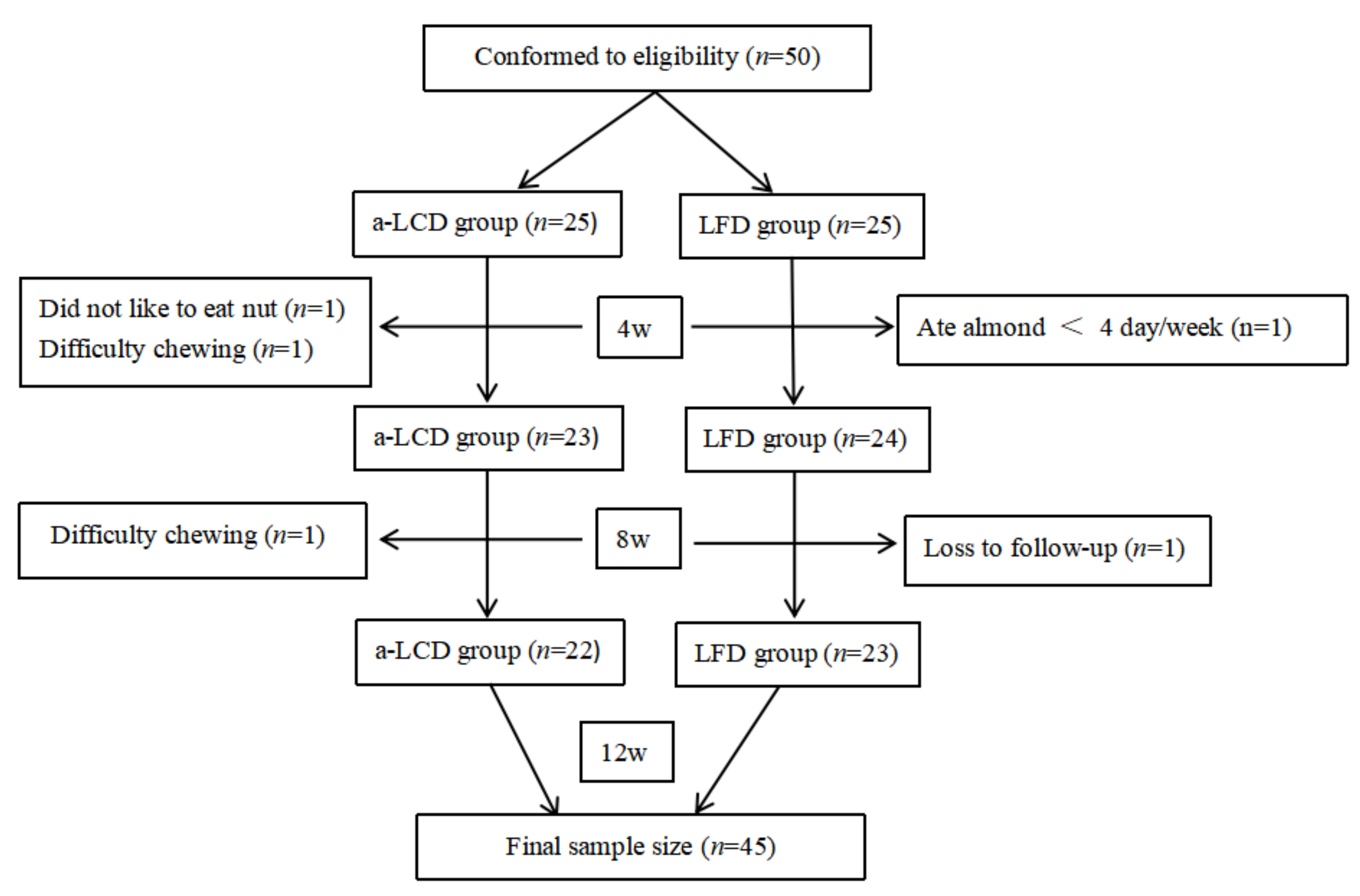
3.1. Study Participants
3.2. Dietary Adherence
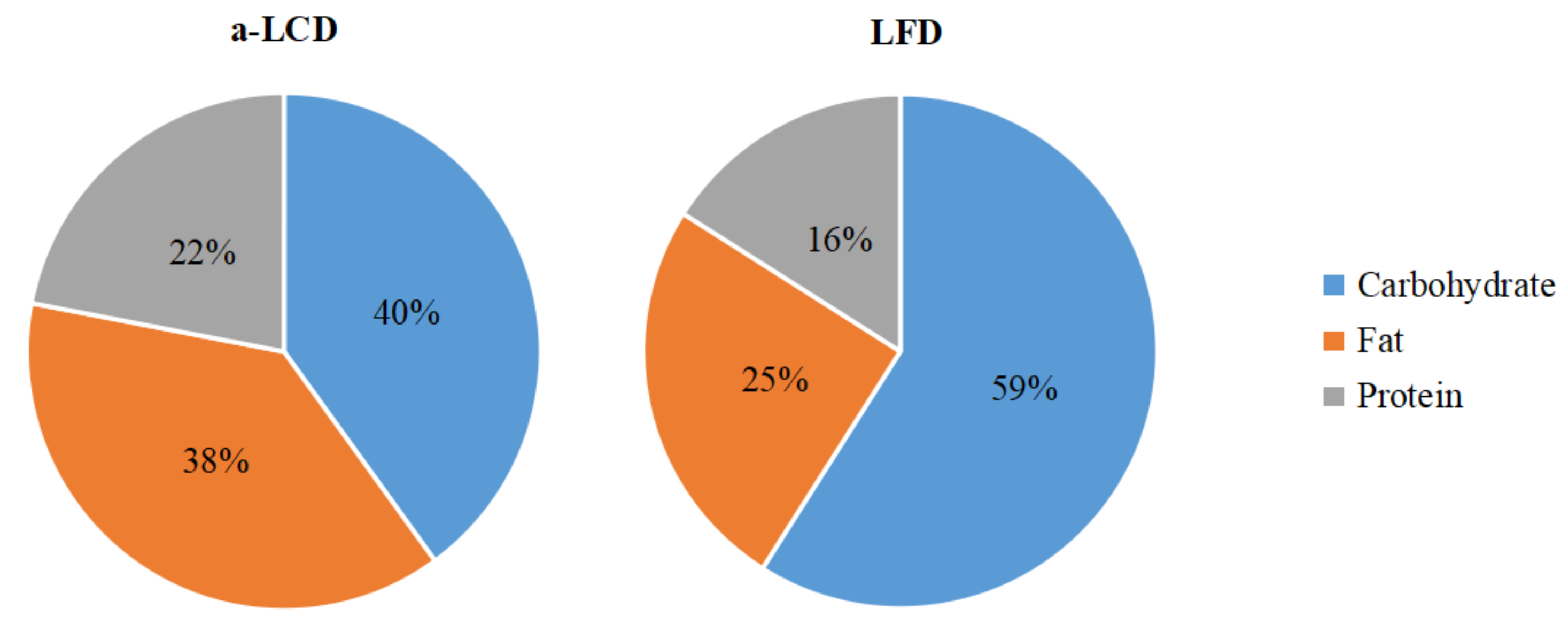
3.2.1. Proportions of Calories from Three Macro-Nutrients the Patients Consumed
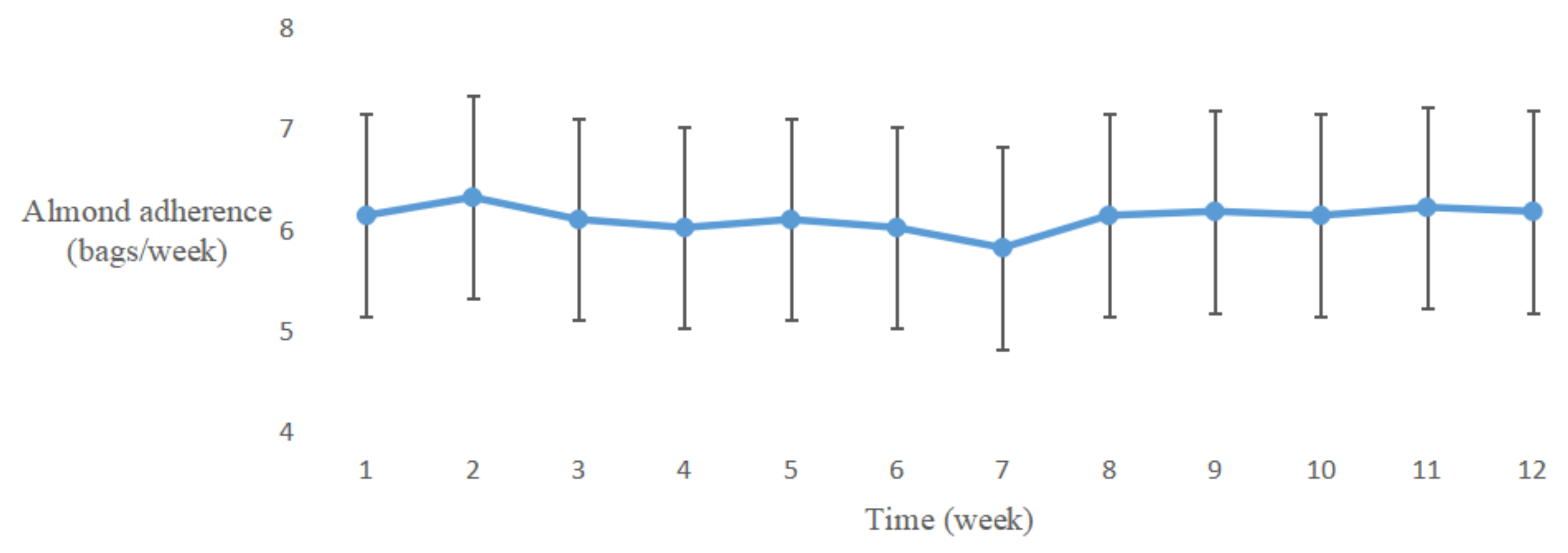
3.2.2. Almond Adherence
3.3. Effect of a-LCD on Glycated Hemoglobin (HbA1c)
3.4. Effect of a-LCD on the Changes of Anti-Diabetics
3.5. Effect of a-LCD on Weight and BMI
3.6. Effect of a-LCD on Depression
3.7. Fasting Plasma GLP-1 Concentration
3.8. Gut Microbiota
3.8.1. Alpha-Adversity
3.8.2. Beta-Diversity
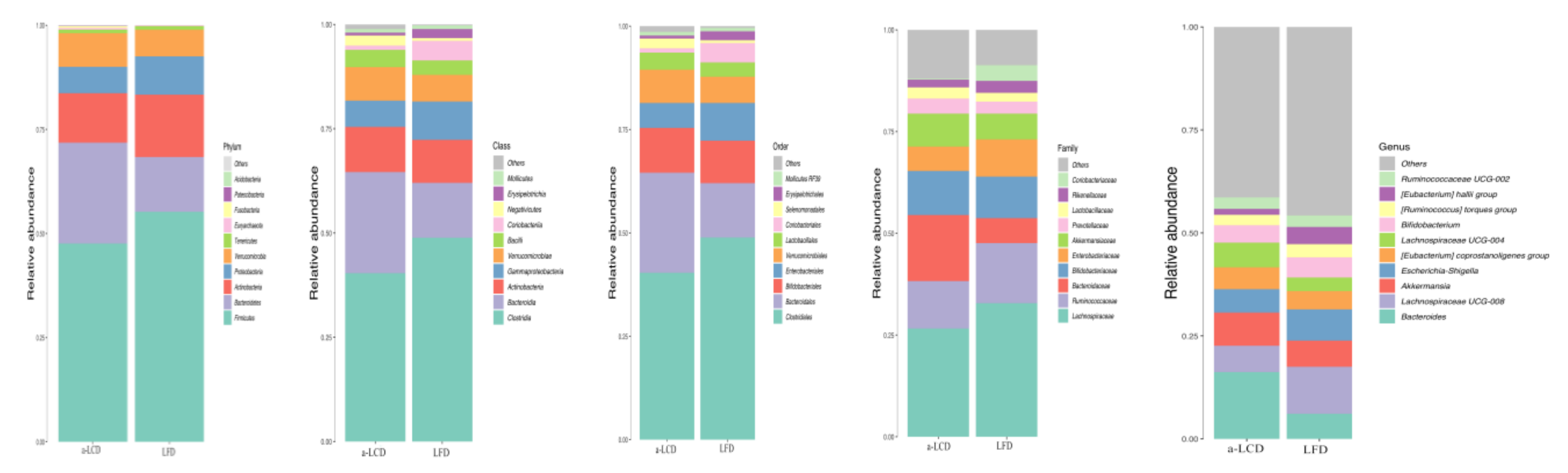
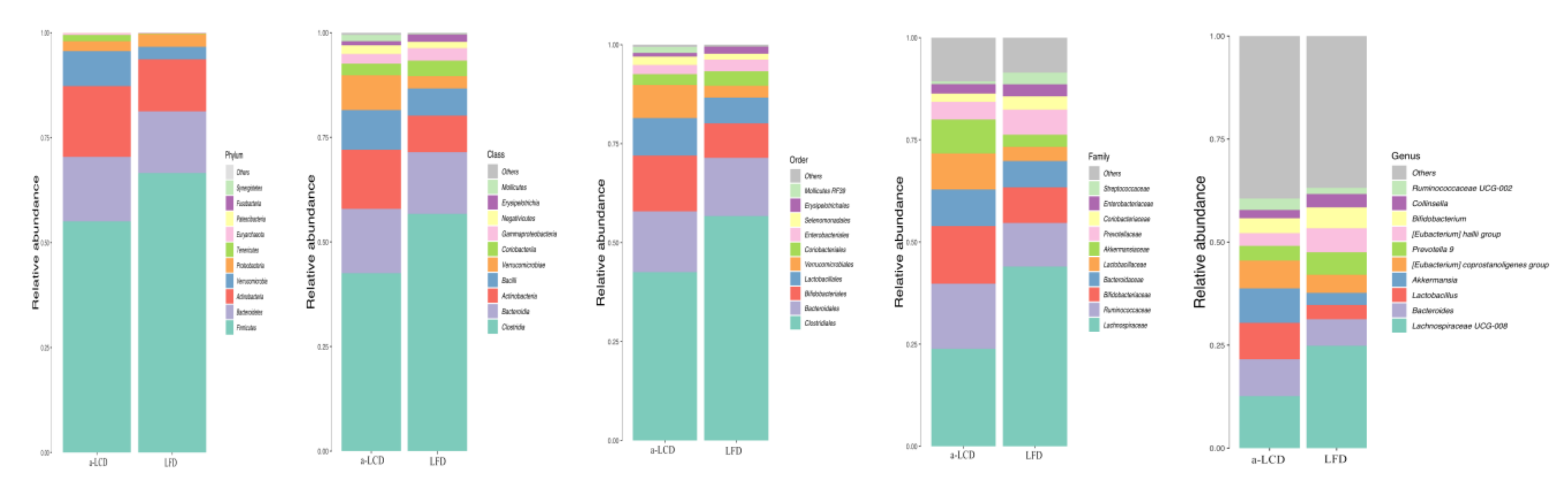
3.8.3. The Comparison of the Composition of Gut Microbiota in the Two Groups
4. Discussion
4.1. Effect of a-LCD on Glycemic Control and Anti-Diabetics
4.2. Effect of a-LCD on Weight and BMI
4.3. Effect of a-LCD on Depression Score
4.4. Effect of a-LCD on Regulation of Gut Microbiota and GLP-1 Expression
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.; Karuranga, S.; Huang, Y.; Fernandes, J.D.D.R.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pr. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Semenkovich, K.; Brown, M.E.; Svrakic, D.M.; Lustman, P.J. Depression in type 2 diabetes mellitus: Prevalence, impact, and treatment. Drugs 2015, 75, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Stone, M.A.; Peters, J.L.; Davies, M.J.; Khunti, K. The prevalence of co-morbid depression in adults with Type 2 diabetes: A systematic review and meta-analysis. Diabet. Med. 2006, 23, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Freedland, K.E.; Clouse, R.E.; Lustman, P.J. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care 2001, 24, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Young-Hyman, D.; De Groot, M.; Hill-Briggs, F.; Gonzalez, J.S.; Hood, K.K.; Peyrot, M. Psychosocial Care for People With Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2126–2140. [Google Scholar] [CrossRef]
- Global Guideline for Type 2 Diabetes: Recommendations for standard, comprehensive, and minimal care. Diabet. Med. 2006, 23, 579–593. [CrossRef] [PubMed]
- Antoine, M.-H.; Gall, D.; Schiffmann, S.N.; Lebrun, P. Tricyclic antidepressant imipramine reduces the insulin secretory rate in islet cells of Wistar albino rats through a calcium antagonistic action. Diabetologia 2004, 47, 909–916. [Google Scholar] [CrossRef][Green Version]
- Society, C.D. Guidelines for the prevention and treatment of type 2 diabetes in China. Chin. Med. J. 2017, 10, 4–67. [Google Scholar]
- Meng, Y.; Bai, H.; Wang, S.; Li, Z.; Wang, Q.; Chen, L. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2017, 131, 124–131. [Google Scholar] [CrossRef]
- Hite, A.H.; Berkowitz, V.G.; Berkowitz, K. Low-carbohydrate diet review: Shifting the paradigm. Nutr. Clin. Pract. 2011, 26, 300–308. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [PubMed]
- Pellegrin, K.L.; O’Neil, P.M.; Stellefson, E.J.; Fossey, M.D.; Ballenger, J.C.; Cochrane, C.E.; Currey, H.S. Average daily nutrient intake and mood among obese women. Nutr. Res. 1998, 7, 18. [Google Scholar] [CrossRef]
- Deijen, J.B.; Heemstra, M.L.; Orlebeke, J.F. Dietary effects on mood and performance. J. Psychiatr. Res. 1989, 23, 275–283. [Google Scholar] [CrossRef]
- Rosenthal, N.E.; Genhart, M.J.; Caballero, B.; Jacobsen, F.M.; Skwerer, R.G.; Coursey, R.D.; Rogers, S.; Spring, B. Psychobiological effects of carbohydrate- and protein-rich meals in patients with seasonal affective disorder and normal controls. Biol. Psychiatry 1989, 25, 1029–1040. [Google Scholar] [CrossRef]
- Wang, L.-L.; Wang, Q.; Hong, Y.; Ojo, O.; Jiang, Q.; Hou, Y.-Y.; Huang, Y.-H.; Wang, X. The Effect of Low-Carbohydrate Diet on Glycemic Control in Patients with Type 2 Diabetes Mellitus. Nutrients 2018, 10, 661. [Google Scholar] [CrossRef]
- Pribis, P.; Shukitt-Hale, B. Cognition: The new frontier for nuts and berries. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 347S–352S. [Google Scholar] [CrossRef]
- Mischoulon, D.; Nierenberg, A.; Schettler, P.J.; Kinkead, B.L.; Fehling, K.; Martinson, M.A.; Rapaport, M.H. A double-blind, randomized controlled clinical trial comparing eicosapentaenoic acid versus docosahexaenoic acid for depression. J. Clin. Psychiatry 2014, 76, 54–61. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Covas, M.I.; Arós, F.; Romaguera, D.; Gomez-Gracia, E.; Lapetra, J.; et al. Mediterranean dietary pattern and depression: The PREDIMED randomized trial. BMC Med. 2013, 11, 208. [Google Scholar] [CrossRef]
- Pribis, P. Effects of Walnut Consumption on Mood in Young Adults—A Randomized Controlled Trial. Nutrients 2016, 8, 668. [Google Scholar] [CrossRef]
- Arab, L.; Guo, R.; Elashoff, D.A. Lower Depression Scores among Walnut Consumers in NHANES. Nutrients 2019, 11, 275. [Google Scholar] [CrossRef]
- Su, Q.; Yu, B.; He, H.; Zhang, Q.; Meng, G.; Wu, H.; Du, H.; Liu, L.; Shi, H.; Xia, Y.; et al. NUT CONSUMPTION IS ASSOCIATED WITH DEPRESSIVE SYMPTOMS AMONG CHINESE ADULTS. Depress. Anxiety 2016, 33, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Nueno-Palop, C.; Bisignano, G.; Wickham, M.S.J.; Narbad, A. Potential prebiotic properties of almond (Amygdalus communis L.) seeds. Appl. Environ. Microbiol. 2008, 74, 4264–4270. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, X.; Huang, G.; Zhang, W.; Rao, P.; Ni, L. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 2014, 26, 1–6. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G.S. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2014, 39, 424–429. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2017, 15, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-Y.; Ojo, O.; Wang, L.-L.; Wang, Q.; Jiang, Q.; Shao, X.-Y.; Wang, X. A Randomized Controlled Trial to Compare the Effect of Peanuts and Almonds on the Cardio-Metabolic and Inflammatory Parameters in Patients with Type 2 Diabetes Mellitus. Nutrients 2018, 10, 1565. [Google Scholar] [CrossRef]
- Asghari, G.; Ghorbani, Z.; Mirmiran, P.; Azizi, F. Nut consumption is associated with lower incidence of type 2 diabetes: The Tehran Lipid and Glucose Study. Diabetes Metab. 2017, 43, 18–24. [Google Scholar] [CrossRef]
- Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Keage, H.A.D.; Murphy, K.J. A Mediterranean diet supplemented with dairy foods improves mood and processing speed in an Australian sample: Results from the MedDairy randomized controlled trial. Nutr. Neurosci. 2020, 23, 646–658. [Google Scholar] [CrossRef]
- Li, S.-C.; Liu, Y.-H.; Liu, J.-F.; Chang, W.-H.; Chen, C.-M. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 474–479. [Google Scholar] [CrossRef]
- Galletly, C.; Moran, L.; Noakes, M.; Clifton, P.M.; Tomlinson, L.; Norman, R.J. Psychological benefits of a high-protein, low-carbohydrate diet in obese women with polycystic ovary syndrome—A pilot study. Appetite 2007, 49, 590–593. [Google Scholar] [CrossRef]
- Collier, G.; O’Dea, K. The effect of coingestion of fat on the glucose, insulin, and gastric inhibitory polypeptide responses to carbohydrate and protein. Am. J. Clin. Nutr. 1983, 37, 941–944. [Google Scholar] [CrossRef]
- Cook, K.F.; Jensen, S.E.; Schalet, B.D.; Beaumont, J.L.; Amtmann, D.; Czajkowski, S.; DeWalt, D.A.; Fries, J.F.; Pilkonis, P.A.; Reeve, B.B.; et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J. Clin. Epidemiol. 2016, 73, 89–102. [Google Scholar] [CrossRef]
- Kuczynski, J.; Lauber, C.L.; Walters, W.A.; Parfrey, L.W.; Clemente, J.C.; Gevers, D.; Knight, R. Experimental and analytical tools for studying the human microbiome. Nat. Rev. Genet. 2011, 13, 47–58. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef]
- Vadivel, V.; Kunyanga, C.N.; Biesalski, H.K. Health benefits of nut consumption with special reference to body weight control. Nutrition 2012, 28, 1089–1097. [Google Scholar] [CrossRef]
- Tan, S.-Y.; Dhillon, J.; Mattes, R.D. A review of the effects of nuts on appetite, food intake, metabolism, and body weight. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 412S–422S. [Google Scholar] [CrossRef]
- Brinkworth, G.D.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.; Wilson, C.J. Long-term effects of very low-carbohydrate and high-carbohydrate weight-loss diets on psychological health in obese adults with type 2 diabetes: Randomized controlled trial. J. Intern. Med. 2016, 280, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Saslow, L.R.; Kim, S.; Daubenmier, J.J.; Moskowitz, J.T.; Phinney, S.D.; Goldman, V.; Murphy, E.J.; Cox, R.M.; Morán, P.; Hecht, F.M. A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PLoS ONE 2014, 9, e91027. [Google Scholar] [CrossRef] [PubMed]
- Sugizaki, C.S.A.; Naves, M.M.V. Potential Prebiotic Properties of Nuts and Edible Seeds and Their Relationship to Obesity. Nutrients 2018, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2019, 78, 394–411. [Google Scholar] [CrossRef]
- Sureda, A.; Tejada, S. Polyphenols and depression: From chemistry to medicine. Curr. Pharm. Biotechnol. 2015, 16, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Deacon, G.; Kettle, C.; Hayes, D.; Dennis, C.; Tucci, J. Omega 3 polyunsaturated fatty acids and the treatment of depression. Crit. Rev. Food Sci. Nutr. 2015, 57, 212–223. [Google Scholar] [CrossRef]
- Yachi, S.; Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1463–1468. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Dhillon, J.; Li, Z.; Ortiz, R.M. Almond Snacking for 8 wk Increases Alpha-Diversity of the Gastrointestinal Microbiome and Decreases Bacteroides fragilis Abundance Compared with an Isocaloric Snack in College Freshmen. Curr. Dev. Nutr. 2019, 3, nzz079. [Google Scholar] [CrossRef]
- Dash, S.; Clarke, G.; Berk, M.; Jacka, F.N. The gut microbiome and diet in psychiatry: Focus on depression. Curr. Opin. Psychiatry 2015, 28, 1–6. [Google Scholar] [CrossRef]
- Weina, H.; Yuhu, N.; Christian, H.; Birong, L.; Feiyu, S.; Le, W. Liraglutide attenuates the depressive- and anxiety-like behaviour in the corticosterone induced depression model via improving hippocampal neural plasticity. Brain Res. 2018, 1694, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Gault, V.A.; Hölscher, C. GLP-1 receptor agonists show neuroprotective effects in animal models of diabetes. Peptides 2018, 100, 101–107. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Fang, F.; Qin, T.; Xiao, W.; Wang, Z.; Ma, S.-P. Geniposide improves repeated restraint stress-induced depression-like behavior in mice by ameliorating neuronal apoptosis via regulating GLP-1R/AKT signaling pathway. Neurosci. Lett. 2018, 676, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.; Lipscomb, D.; Quin, J. Psychological and quality of life changes in patients using GLP-1 analogues. J. Diabetes Complicat. 2011, 25, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Taylor, A.M.; Swanson, K.S.; Novotny, J.A.; Baer, D.J. Almond Consumption and Processing Affects the Composition of the Gastrointestinal Microbiota of Healthy Adult Men and Women: A Randomized Controlled Trial. Nutrients 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.-W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef]
- Senyigit, A.; Tabak, O.; Orhanoglu, T.; Karadag, A.; Ugurlu, S.; Uzun, H.; Konukoglu, D. Glucagon-like peptide-1 levels and dipeptidyl peptidase-4 activity in type 2 diabetes. Clin. Investig. Med. 2017, 40, E188–E199. [Google Scholar] [CrossRef]
- Cohen, A.E.; Johnston, C.S. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A1c in individuals with well-controlled type 2 diabetes mellitus. Metabolism 2011, 60, 1312–1317. [Google Scholar] [CrossRef]
| Variables | a-LCD (n = 22) | LFD (n = 23) | t/χ2/Z | p | |
|---|---|---|---|---|---|
| x ± SD/n(%)/ M (P25,P50) | x ± SD/n(%)/ M (P25,P50) | ||||
| Demographic data | |||||
| Age (years) | 73.55 ± 4.99 | 70.48 ± 5.91 | −1.877 a | 0.067 | |
| Gender-male | 9 (40.9%) | 11 (47.8%) | 0.218 b | 0.641 | |
| Marital status (married) | 20 (90.9%) | 21 (91.3%) | 0.002 c | 0.963 | |
| Education | Primary and below | 2 (9.1%) | 2 (8.7%) | 4.037 b | 0.258 |
| Journal high school | 4 (18.2%) | 10 (43.5%) | |||
| Technical and senior high school | 11 (50.0%) | 9 (39.1%) | |||
| Journal college school and above | 5 (22.7%) | 2 (8.7%) | |||
| Payment | Medical insurance | 22 (100%) | 21 (91.3%) | 2.002 c | 0.157 |
| Monthly income (thousand yuan) | <2 | 0 (0%) | 3 (13.0%) | 3.950 b | 0.139 |
| 2~5 | 19 (86.4%) | 15 (65.2%) | |||
| ≥5 | 3 (13.6%) | 5 (21.7%) | |||
| Occupation status | Retire | 22 (100%) | 22 (95.7%) | 0.978 c | 0.323 |
| On the job | 0 (0%) | 1 (4.3%) | |||
| Residential status | Living by oneself | 2 (9%) | 2 (8%) | 0.311 b | 0.856 |
| Living with spouse | 19 (86%) | 19 (83%) | |||
| Living with children | 1 (5%) | 2 (9%) | |||
| Exercise intensity (d) | Low intensity | 19 (86.4%) | 18 (78.3%) | 0.505 b | 0.477 |
| Moderate intensity | 3 (13.6%) | 5 (21.7%) | |||
| Exercise time/(minute) | 315.00(210.00,420.00) | 360.00(210.00,420.00) | −0.537 d | 0.591 | |
| Clinical data | |||||
| Smoking (Yes) | 1 (4.5%) | 2 (8.7%) | 0.311 c | 0.577 | |
| Drinking (Yes) | 4 (18.2%) | 3 (13.0%) | 0.226 c | 0.634 | |
| Diabetes duration (years) | 14.18 ± 7.06 | 15.65 ± 7.02 | 0.700 a | 0.487 | |
| Family history of diabetes (Yes) | 12 (54.5%) | 11 (47.8%) | 0.203 b | 0.652 | |
| Diabetic complication (Yes) | 9 (40.9%) | 11 (59.3%) | 0.573 b | 0.449 | |
| Accompanying disease (Yes) | 16 (72.7%) | 17 (73.9%) | 0.008 b | 0.928 | |
| Therapy method | None | 1 (4.5%) | 1 (4.3%) | 1.825 b | 0.610 |
| Only Hypoglycemic drugs | 12 (54.5%) | 14 (60.9%) | |||
| Only Insulin | 1 (4.5%) | 3 (13.0%) | |||
| Hypoglycemic drugs + insulin | 8 (36.4%) | 5 (21.7%) | |||
| Number of combination medication | 0 | 2 (8.7%) | 4 (14.8%) | 5.022 b | 0.170 |
| 1 | 8 (34.8%) | 4 (14.8%) | |||
| 2 | 13 (56.5%) | 16 (59.3%) | |||
| 3 | 0 (0%) | 3 (11.1%) | |||
| Variables | a-LCD (n =22) | LFD (n =23) | t | p | |
|---|---|---|---|---|---|
| Baseline | Total calorie intake/day | 1686.34 ± 231.25 | 1781.91 ± 280.91 | −1.232 | 0.184 |
| Carbohydrate-calorie (Kcal) | 974.95 ± 148.22 | 1007.61 ± 136.32 | −0.761 | 0.504 | |
| Fat-calorie (Kcal) | 406.76 ± 143.88 | 478.29 ± 149.97 | −1.614 | 0.085 | |
| Protein-calorie (Kcal) | 318.45 ± 63.19 | 292.38 ± 65.12 | 1.348 | 0.524 | |
| Third month | Total calorie intake/day | 1642.08 ± 227.74 | 1764.77 ± 297.40 | −1.536 | 0.114 |
| Carbohydrate-calorie (Kcal) | 673.14 ± 91.80 | 1042.10 ± 195.41 | −8.016 | <0.01 ** | |
| Fat-calorie (Kcal) | 648.19 ± 128.93 | 433.01 ± 137.39 | 5.357 | <0.01 ** | |
| Protein-calorie (Kcal) | 372.03 ± 64.45 | 288.94 ± 64.34 | 1.962 | 0.067 |
| Study Period | a-LCD (n =22) | LFD (n =23) | t/F | p |
|---|---|---|---|---|
| Baseline | 7.67 ± 1.60 | 7.54 ± 1.31 | −0.287 a | 0.776 |
| Third month | 6.85 ± 1.02 (adjusted:6.77 ± 0.13) | 7.37 ± 1.29 (adjusted:7.44 ± 0.12) | 14.111 b | <0.01 ** |
| t | 4.081 c | 2.614 c | ||
| p | <0.01 ** | 0.016 * |
| a-LCD (n = 22) | LFD (n = 23) | χ2 | p | |
|---|---|---|---|---|
| Reduction | 3 (14%) | 5 (22%) | 0.019 | 0.889 |
| No change | 19 (86%) | 18 (78%) |
| Variables | a-LCD (n = 22) | LFD (n = 23) | t | p | |
|---|---|---|---|---|---|
| Weight (Kg) | Baseline | 66.60 ± 8.81 | 63.07 ± 12.88 | 0.784 a | 0.459 |
| Third month | 59.34 ± 8.90 | 62.58 ± 13.12 | 0.967 a | 0.339 | |
| t | 2.164 b | 1.397b | |||
| p | 0.042 * | 0.176 | |||
| BMI (Kg/m2) | Baseline | 23.53 ± 2.33 | 23.69 ± 2.83 | 0.216 | 0.830 |
| Third month | 23.02 ± 2.45 | 23.53 ± 3.04 | 0.641 | 0.524 | |
| t | −2.261 | −1.283 | |||
| p | 0.034 * | 0.211 |
| Study Period | a-LCD (n = 22) | LFD (n = 23) | t/F | p |
|---|---|---|---|---|
| Baseline | 48.41 ± 8.05 | 49.57 ± 8.46 | 0.471 a | 0.640 |
| Third month | 42.07 ± 5.80(adjusted:42.58 ± 0.89) | 48.65 ± 7.69(adjusted:48.16 ± 0.87) | 19.308 b | <0.01 ** |
| t | 6.196 c | 0.838 c | ||
| p | <0.01 ** | 0.411 |
| Study Period | a-LCD (n =22) | LFD (n =23) | Z | p |
|---|---|---|---|---|
| Baseline | 1.381 (0.697,3.157) | 1.190 (0.804,1.896) | −0.409 | 0.683 |
| Third month | 1.092 (0.886,2.671) | 0.630 (0.261,1.997) | −2.396 | 0.017 * |
| Z | −0.221 | −1.339 | ||
| p | 0.833 | 0.162 |
| Phylum | Genus | Study Period | a-LCD (n =22) | LFD (n =23) | Z | p(adj. val.) |
|---|---|---|---|---|---|---|
| Firmicutes | Baseline | 0.389 (0.283,0.729) | 0.544 (0.455,0.671) | −1.317 | 0.188 (0.194) | |
| Third month | 0.580 (0.371,0.672) | 0.684 (0.561,0.778) | −2.317 | 0.021 * (0.026) | ||
| Z | −1.282 | −2.281 | ||||
| p(adj. val.) | 0.200 (0.213) | 0.023 * (0.038) | ||||
| Roseburia | Baseline | 0.002 (0.000,0.005) | 0.009 (0.005,0.024) | −1.892 | <0.01 ** (<0.01) | |
| Third month | 0.005 (0.000,0.006) | 0.000 (0.000,0.001) | −2.626 | <0.01 ** (<0.01) | ||
| Z | −2.193 | −4.075 | ||||
| p(adj. val.) | 0.028 * (0.021) | <0.01 ** (<0.01) | ||||
| Eubacterium | Baseline | 0.008 (0.004,0.0220) | 0.037 (0.018,0.070) | −3.747 | <0.01 ** (<0.01) | |
| Third month | 0.026 (0.004,0.057) | 0.042 (0.024,0.099) | −2.082 | 0.037 * (0.073) | ||
| Z | −2.678 | −1.734 | ||||
| p(adj. val.) | <0.01 ** (0.013) | 0.083 (0.052) | ||||
| Ruminococcus | Baseline | 0.017 (0.011,0.033) | 0.020 (0.005,0.037) | −0.829 | 0.470 (0.407) | |
| Third month | 0.026 (0.005,0.044) | 0.005 (0.000,0.019) | −2.015 | 0.044 * (0.073) | ||
| Z | −0.341 | −2.312 | ||||
| p | 0.733 (0.308) | 0.021 * (0.020) | ||||
| Lactobacillus | Baseline | 0.007 (0.003,0.049) | 0.005 (0.002,0.012) | −1.420 | 0.156 (0.223) | |
| Third month | 0.007 (0.000,0.068) | 0.000 (0.000,0.047) | −1.666 | 0.096 (0.245) | ||
| Z | −1.150 | −0.973 | ||||
| p(adj. val.) | 0.130 (0.073) | 0.330 (0.167) | ||||
| Bacteroidetes | Baseline | 0.249 (0.120,0.323) | 0.110 (0.072,0.180) | −2.793 | <0.01 ** (0.011) | |
| Third month | 0.151 (0.061,0.256) | 0.108 (0.042,0.236) | −0.591 | 0.555 (0.415) | ||
| Z | −2.451 | −1.004 | ||||
| p(adj. val.) | 0.014 * (0.016) | 0.361 (0.188) | ||||
| Bacteroides | Baseline | 0.144 (0.057,0.256) | 0.047 (0.023,0.119) | −3.244 | <0.01 ** (<0.01) | |
| Third month | 0.064 (0.027,0.106) | 0.057 (0.009,0.085) | −0.978 | 0.328 (0.364) | ||
| Z | −2.354 | −0.335 | ||||
| p(adj. val.) | 0.019 * (0.013) | 0.735 (0.308) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, M.; Zhang, H.; Qi, J.; Hu, A.; Jiang, Q.; Hou, Y.; Feng, Q.; Ojo, O.; Wang, X. An Almond-Based Low Carbohydrate Diet Improves Depression and Glycometabolism in Patients with Type 2 Diabetes through Modulating Gut Microbiota and GLP-1: A Randomized Controlled Trial. Nutrients 2020, 12, 3036. https://doi.org/10.3390/nu12103036
Ren M, Zhang H, Qi J, Hu A, Jiang Q, Hou Y, Feng Q, Ojo O, Wang X. An Almond-Based Low Carbohydrate Diet Improves Depression and Glycometabolism in Patients with Type 2 Diabetes through Modulating Gut Microbiota and GLP-1: A Randomized Controlled Trial. Nutrients. 2020; 12(10):3036. https://doi.org/10.3390/nu12103036
Chicago/Turabian StyleRen, Mengxiao, Huaiyu Zhang, Jindan Qi, Anni Hu, Qing Jiang, Yunying Hou, Qianqian Feng, Omorogieva Ojo, and Xiaohua Wang. 2020. "An Almond-Based Low Carbohydrate Diet Improves Depression and Glycometabolism in Patients with Type 2 Diabetes through Modulating Gut Microbiota and GLP-1: A Randomized Controlled Trial" Nutrients 12, no. 10: 3036. https://doi.org/10.3390/nu12103036
APA StyleRen, M., Zhang, H., Qi, J., Hu, A., Jiang, Q., Hou, Y., Feng, Q., Ojo, O., & Wang, X. (2020). An Almond-Based Low Carbohydrate Diet Improves Depression and Glycometabolism in Patients with Type 2 Diabetes through Modulating Gut Microbiota and GLP-1: A Randomized Controlled Trial. Nutrients, 12(10), 3036. https://doi.org/10.3390/nu12103036






