A Diet for Healthy Weight: Why Reaching a Consensus Seems Difficult
Abstract
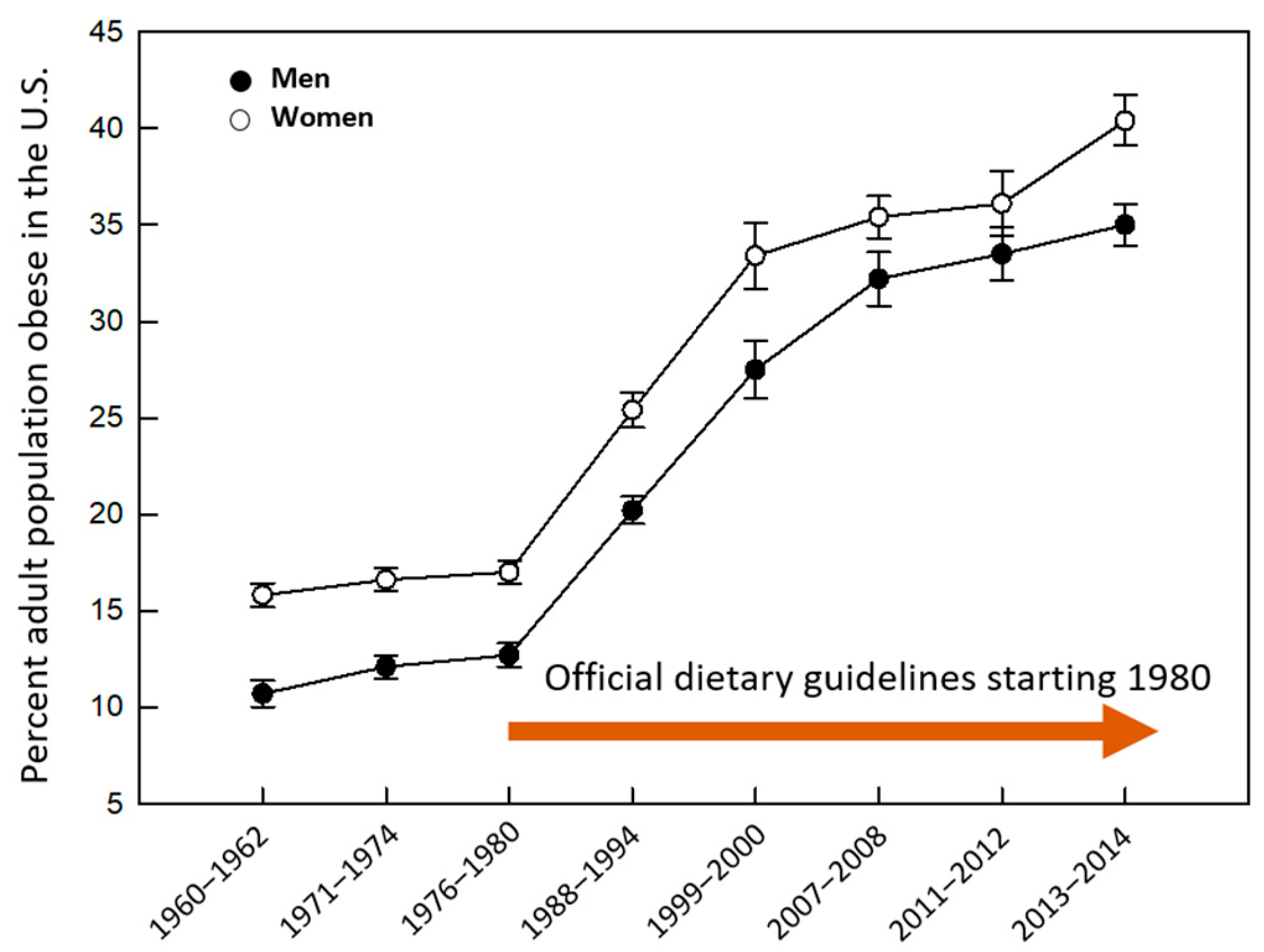
1. The Unmet Goal
2. The Problem
3. Lessons Learned
4. Rethinking Strategies
5. Looking Forward
Author Contributions
Funding
Conflicts of Interest
References
- Mozaffarian, D.; Rosenberg, I.; Uauy, R. History of modern nutrition science-implications for current research, dietary guidelines, and food policy. BMJ 2018, 361, k2392. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Lawman, H.G.; Fryar, C.D.; Kruszon-Moran, D.; Kit, B.K.; Flegal, K.M. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA 2016, 315, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016, 315, 2284–2291. [Google Scholar] [CrossRef]
- Zylke, J.W.; Bauchner, H. The unrelenting challenge of obesity. JAMA 2016, 315, 2277–2278. [Google Scholar] [CrossRef]
- Scully, T. Obesity. Nature 2014, 508, S49. [Google Scholar] [CrossRef]
- Johns, D.M.; Oppenheimer, G.M. Was there ever really a “sugar conspiracy”? Science 2018, 359, 747–750. [Google Scholar] [CrossRef]
- Mozaffarian, D. Food and weight gain: Time to end our fear of fat. Lancet Diabetes Endocrinol. 2016, 4, 633–635. [Google Scholar] [CrossRef]
- Kroemer, G.; Lopez-Otin, C.; Madeo, F.; de Cabo, R. Carbotoxicity-Noxious effects of carbohydrates. Cell 2018, 175, 605–614. [Google Scholar] [CrossRef]
- Hall, K.D.; Guyenet, S.J.; Leibel, R.L. The carbohydrate-insulin model of obesity is difficult to reconcile with current evidence. JAMA Intern. Med. 2018, 178, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Ebbeling, C.B. The Carbohydrate-insulin model of obesity: Beyond “Calories in, calories out”. JAMA Intern. Med. 2018, 178, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S. Fat and heart disease: Challenging the dogma. Lancet 2017, 390, 731. [Google Scholar] [CrossRef]
- Oppenheimer, G.M.; Benrubi, I.D. McGovern‘s senate select committee on nutrition and human needs versus the meat industry on the diet-heart question (1976–1977). Am. J. Public Health 2014, 104, 59–69. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Nutrition and Overweight. In Healthy People 2010; Food and Drug Administration: Hyattsville, MD, USA, 2001; Chapter 19, pp. 1–15.
- Redesigning the Process for Establishing the Dietary Guidelines for Americans; National Academies of Sciences, Engineering, and Medicine: Washington, DC, USA, 2017.
- Krishnan, S.; Adams, S.H.; Allen, L.H.; Laugero, K.D.; Newman, J.W.; Stephensen, C.B.; Burnett, D.J.; Witbracht, M.; Welch, L.C.; Que, E.S.; et al. A randomized controlled-feeding trial based on the Dietary Guidelines for Americans on cardiometabolic health indexes. Am. J. Clin. Nutr. 2018, 108, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Bero, L. Developing reliable dietary guidelines. BMJ 2017, 359, j4845. [Google Scholar] [CrossRef] [PubMed]
- Optimizing the Process for Establishing the Dietary Guidelines for Americans: The Selection Process; National Academies of Sciences, Engineering, and Medicine: Washington, DC, USA, 2017.
- Taubes, G. Nutrition. The soft science of dietary fat. Science 2001, 291, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Soares, H.; Raveh-Sadka, T.; Azulay, S.; Edens, K.; Ben-Shlomo, Y.; Cohen, Y.; Ofek, T.; Bachrach, D.; Stevens, J.; Colibaseanu, D.; et al. Assessment of a personalized approach to predicting postprandial glycemic responses to food among individuals without diabetes. JAMA Netw. Open 2019, 2, e188102. [Google Scholar] [CrossRef] [PubMed]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized nutrition by prediction of glycemic responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Dhakal, S.; McCormack, L.; Dey, M. Association of the gut microbiota with weight-loss response within a retail weight-management program. Microorganisms 2020, 8, 1246. [Google Scholar] [CrossRef]
- Mars, R.A.T.; Yang, Y.; Ward, T.; Houtti, M.; Priya, S.; Lekatz, H.R.; Tang, X.; Sun, Z.; Kalari, K.R.; Korem, T.; et al. Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell 2020, 182, 1460–1473.e17. [Google Scholar] [CrossRef]
- Upadhyaya, B.; McCormack, L.; Fardin-Kia, A.R.; Juenemann, R.; Clapper, J.; Specker, B.; Dey, M. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci. Rep. 2016, 6, 28797. [Google Scholar] [CrossRef]
- The National Institute of Diabetes and Digestive and Kidney Diseases. NIH Nutrition Research Report, 2015 & 2016; Department of Health and Human Services, National Institutes of Health: Bethesda, MD, USA, 2017.
- Nestle, M. Food Industry Funding of Nutrition Research: The Relevance of History for Current Debates. JAMA Intern. Med. 2016, 176, 1685–1686. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Anderson, J.T.; Grande, F. Serum cholesterol response to changes in the diet: II. The effect of cholesterol in the diet. Metabolism 1965, 14, 759–765. [Google Scholar] [CrossRef]
- Keys, A.; Anderson, J.T.; Grande, F. Serum cholesterol response to changes in the diet: IV. Particular saturated fatty acids in the diet. Metabolism 1965, 14, 776–787. [Google Scholar] [CrossRef]
- National Research Council Food and Nutrition Board. Toward Healthful Diets; National Academy of Sciences: Washington, DC, USA, 1980. [Google Scholar]
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Saslow, L.R.; Daubenmier, J.J.; Moskowitz, J.T.; Kim, S.; Murphy, E.J.; Phinney, S.D.; Ploutz-Snyder, R.; Goldman, V.; Cox, R.M.; Mason, A.E.; et al. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr. Diabetes 2017, 7, 304. [Google Scholar] [CrossRef]
- Furmli, S.; Elmasry, R.; Ramos, M.; Fung, J. Therapeutic use of intermittent fasting for people with type 2 diabetes as an alternative to insulin. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- McKenzie, A.L.; Hallberg, S.J.; Creighton, B.C.; Volk, B.M.; Link, T.M.; Abner, M.K.; Glon, R.M.; McCarter, J.P.; Volek, J.S.; Phinney, S.D. A novel intervention including individualized nutritional recommendations reduces hemoglobin A1c level, medication use, and weight in type 2 diabetes. JMIR Diabetes 2017, 2, e5. [Google Scholar] [CrossRef]
- Bhanpuri, N.H.; Hallberg, S.J.; Williams, P.T.; McKenzie, A.L.; Ballard, K.D.; Campbell, W.W.; McCarter, J.P.; Phinney, S.D.; Volek, J.S. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: An open label, non-randomized, controlled study. Cardiovasc. Diabetol. 2018, 17, 56. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Misra, A.; Mohan, V.; Taylor, R.; Yancy, W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ 2018, 361, k2234. [Google Scholar] [CrossRef]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef]
- Lucini, D.; Vigo, C.; Malacarne, M.; Gatzemeier, W.; Pagani, M. Lifestyle changes as internal medicine. Eur. J. Intern. Med. 2017, 43, e40–e42. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Trogdon, J.G.; Cohen, J.W.; Dietz, W. Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Aff. (Millwood) 2009, 28, w822–w831. [Google Scholar] [CrossRef]
- Adams, K.M.; Lindell, K.C.; Kohlmeier, M.; Zeisel, S.H. Status of nutrition education in medical schools. Am. J. Clin. Nutr. 2006, 83, 941S–944S. [Google Scholar] [CrossRef] [PubMed]
- Mogre, V.; Stevens, F.C.J.; Aryee, P.A.; Amalba, A.; Scherpbier, A. Why nutrition education is inadequate in the medical curriculum: A qualitative study of students’ perspectives on barriers and strategies. BMC Med. Educ. 2018, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Finley, C.E.; Barlow, C.E.; Greenway, F.L.; Rock, C.L.; Rolls, B.J.; Blair, S.N. Retention rates and weight loss in a commercial weight loss program. Int. J. Obes. 2007, 31, 292–298. [Google Scholar] [CrossRef]
- Alexander, E.; Tseng, E.; Durkin, N.; Jerome, G.J.; Dalcin, A.; Appel, L.J.; Clark, J.M.; Gudzune, K.A. Factors associated with early dropout in an employer-based commercial weight-loss program. Obes. Sci. Pract. 2018, 4, 545–553. [Google Scholar] [CrossRef]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter, J.P.; Phinney, S.D.; et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: An open-label, non-randomized, controlled study. Diabetes Ther. 2018, 9, 583–612. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, M.; Kashyap, P.C. A Diet for Healthy Weight: Why Reaching a Consensus Seems Difficult. Nutrients 2020, 12, 2997. https://doi.org/10.3390/nu12102997
Dey M, Kashyap PC. A Diet for Healthy Weight: Why Reaching a Consensus Seems Difficult. Nutrients. 2020; 12(10):2997. https://doi.org/10.3390/nu12102997
Chicago/Turabian StyleDey, Moul, and Purna C. Kashyap. 2020. "A Diet for Healthy Weight: Why Reaching a Consensus Seems Difficult" Nutrients 12, no. 10: 2997. https://doi.org/10.3390/nu12102997
APA StyleDey, M., & Kashyap, P. C. (2020). A Diet for Healthy Weight: Why Reaching a Consensus Seems Difficult. Nutrients, 12(10), 2997. https://doi.org/10.3390/nu12102997




