Abstract
Gut microbiota and its metabolites such as short chain fatty acids (SCFA), lipopolysaccharides (LPS), and trimethylamine-N-oxide (TMAO) impact cardiovascular health. In this review, we discuss how gut microbiota and gut metabolites can affect hypertension and atherosclerosis. Hypertensive patients were shown to have lower alpha diversity, lower abundance of SCFA-producing microbiota, and higher abundance of gram-negative bacteria, which are a source of LPS. Animal studies point towards a direct role for SCFAs in blood pressure regulation and show that LPS has pro-inflammatory effects. Translocation of LPS into the systemic circulation is a consequence of increased gut permeability. Atherosclerosis, a multifactorial disease, is influenced by the gut microbiota through multiple pathways. Many studies have focused on the pro-atherogenic role of TMAO, however, it is not clear if this is a causal factor. In addition, gut microbiota play a key role in bile acid metabolism and some interventions targeting bile acid receptors tend to decrease atherosclerosis. Concluding, gut microbiota affect hypertension and atherosclerosis through many pathways, providing a wide range of potential therapeutic targets. Challenges ahead include translation of findings and mechanisms to humans and development of therapeutic interventions that target cardiovascular risk by modulation of gut microbes and metabolites.
1. Introduction
Cardiovascular diseases, including atherosclerosis and hypertension, are public health care priorities of the World Health Organization (WHO) [1]. Cardiovascular disease is the leading cause of mortality, representing a third of global deaths, and disproportionally affects low- and middle-income countries [2]. Despite current preventive and therapeutic strategies, mortality due to cardiovascular disease is expected to further increase over the next decade [2]. Accumulating evidence describes the role of gut microbiota in cardiovascular disease, potentially providing novel therapeutic targets. The gut microbiome consists of more than 100 trillion micro-organisms, predominantly bacteria and viruses [3]. Due to the development of 16S rRNA gene amplicon sequencing and shotgun metagenomic sequencing, the understanding of the role of the gut microbiota in health and disease has increased tremendously over the past decade [4]. Gut microbiota composition is largely determined by exposure to dietary factors, but conversely, gut microbiota are needed for digestion of macronutrients and production of a wide range of metabolites [5]. Alterations in gut microbiota composition have been observed in a variety of health conditions, including type 2 diabetes, inflammatory bowel disease, asthma, psychiatric disorders, but also in cardiovascular disease [6,7,8,9,10]. In addition, several gut metabolites have been shown to interact with metabolism and the nervous system, affecting insulin sensitivity, energy balance, and appetite regulation [11,12,13].
Low-grade chronic inflammation contributes to the development of both atherosclerosis and hypertension [14,15,16,17]. Gut microbiota can induce systemic inflammation, as has been shown in patients with type 2 diabetes [18]. In addition, gut microbiota could affect cardiovascular risk indirectly, through metabolites such as short chain fatty acids (SCFA) and trimethylamine N-oxide (TMAO). The relation between gut microbiota and its key metabolites in hypertension and atherosclerosis could improve our understanding of differences in susceptibility for cardiovascular disease and provide potential therapeutic targets. In this narrative review, we will focus on the role of gut microbiota in hypertension and atherosclerosis. After summarizing the current evidence, we will discuss future perspectives in this field.
2. Gut Microbiota in Hypertension
2.1. Gut Microbiota Composition in Hypertension
Hypertension is the most important modifiable risk factor for cardiovascular disease [19]. Although hypertension is thought to be driven by a combination of genetic and lifestyle factors, genome-wide association studies showed that only a small (<5%) proportion of the incidence of hypertension can be explained by genetics [20]. In contrast, lifestyle tends to have a much larger influence, with separate lifestyle factors such as body mass index (BMI) and salt intake affecting blood pressure levels with 5 mmHg [21]. Several dietary interventions, including diets such as the Mediterranean diet and the DASH (Dietary Approaches to Stop Hypertension) diet have illustrated that higher intake of fruits, vegetables, and fibers are associated with lower blood pressure [22,23]. The Mediterranean diet has been shown to induce a rise in SCFAs, key metabolites produced by the gut microbiome [24].
Several animal studies have reported compositional differences in the gut microbiota of animal models for hypertension, including Dahl-sensitive rats, spontaneous hypertensive rats, angiotensin-II induced hypertensive rats, and deoxycorticosterone acetate (DOCA)-salt mice, when compared to wild-type animals [25,26,27,28]. These differences include a lower abundance of SCFA-producing bacteria, higher abundance of lactate-producing bacteria [27], lower abundance of Bacteroidetes, and higher abundance of Proteobacteria and Cyanobacteria [28] compared to control animals. Intervention studies in animals showed that blood pressure levels in these animal models for hypertension can be modified by fecal microbiota transplants and antibiotic treatment [27].
In humans, several cross-sectional studies have assessed associations between gut microbiota composition and blood pressure or hypertension (Table 1) [27,29,30,31,32,33,34,35,36,37]. Despite differences in sequencing methods and downstream analyses, some results regarding microbial alpha diversity and microbiota composition are consistent across studies. Higher blood pressure was associated with lower gut microbiota alpha diversity in almost all studies [27,30,32,34,35,36,37]. Low alpha diversity is considered an adverse but nonspecific characteristic, since a decrease in diversity has also been observed in obesity, hyperinsulinemia, and dyslipidemia. In addition, higher abundances of Gram-negative microbiota including Klebsiella, Parabacteroides, Desulfovibrio, and Prevotella were associated with higher blood pressure. Gram-negative bacteria are a source of lipopolysaccharides (LPS), also known as endotoxins, that are pro-inflammatory. In contrast, SCFA-producing bacteria, including Ruminococcaceae, Roseburia, and Faecalibacterium spp. were less abundant in hypertensive compared to normotensive patients [29,31,34,35,37]. Of note, the majority of these studies did not adjust for important confounders such as age, BMI, or dietary factors in their analyses.

Table 1.
Cross-sectional studies on gut microbiota composition in hypertension in humans.
Dietary salt intake affects both the incidence of hypertension as well as gut microbiota composition. Higher salt intake has been associated with a shift in microbiota composition in several animal models, including an increase in Lachnospiraceae, Ruminococcus, and Parasutterella spp. and decrease in Lactobacillus and Oscillibacter [38,39,40]. Lactobacillus abundance has been associated with salt sensitivity in hypertension, since supplementation of Lactobacillus spp. in a mice model has been shown to attenuate salt-sensitive hypertension, presumably by modulation of Th17-cells [40]. The blood pressure lowering effect of Lactobacillus was confirmed by several other animal models [41,42,43,44]. In humans, however, a decrease of Lactobacillus spp. was only reported by one of the cross-sectional studies in hypertensive subjects in Table 1 [29]. A meta-analysis including nine randomized-controlled trials, predominantly with healthy controls, found a blood pressure lowering effect of probiotics with several Lactobacillus spp. [43]. The blood pressure lowering effect tended to be stronger in the only included placebo-controlled intervention study with hypertensive subjects (17/13), although this study did not assess changes in gut microbiota composition [45].
In summary, animal studies suggest a causal link between gut microbiota composition and blood pressure regulation. Cross-sectional studies in human subjects show specific differences in microbiota composition between hypertensive subjects and controls, including lower SCFA-producing bacteria and higher Gram-negative species. These differences point to a role for SCFAs and LPS in hypertension, although the direction of this association is unclear.
2.2. Short Chain Fatty Acids
SCFAs, including acetate, propionate, and butyrate, are produced by specific gut microbes by fermentation of otherwise indigestible dietary fibers [46]. Fecal and plasma levels of SCFA are associated with the abundance of SCFA-producing microbiota in the gut and the intake of dietary fibers [36,47,48]. Butyrate-producing microbiota include bacteria from the families Ruminococcaceae and Lachnospiraceae, but also bacteria such as Anaerobutyricum hallii and Anaerostipes spp. Acetate and propionate are mainly produced by Bifidobacterium spp. and mucin-degrading bacteria such as Akkermansia muciniphila [49]. Most of the produced acetate and propionate is absorbed by the gut, while butyrate is used as a primary energy source by colonocytes and only absorbed in very small proportions [50,51]. As a result, plasma concentrations of acetate and propionate are much higher than circulating butyrate levels.
Human studies on the role of SCFAs in blood pressure regulation are rather scarce. Intriguingly, fecal SCFA concentrations in humans have been associated with higher blood pressure [30], while SCFA-producing microbiota are often associated with lower blood pressure [31,35,37]. Perhaps, increased SCFA availability in the intestines results in upregulation of absorption mechanisms, which could lead to relatively lower fecal concentrations and higher plasma availability, as was supported by a murine model [52]. There are no results from human intervention studies with SCFAs to target blood pressure. However, butyrate tended to lower blood pressure in intervention trials in subjects with metabolic syndrome [53,54]. Moreover, the Mediterranean diet, which induces a rise in SCFA levels, has been reported to have a blood pressure lowering effect [24].
In animal models, SCFAs were associated with both higher and lower blood pressure, which might be explained by the differential effects of SCFA receptors [55]. Several SCFA receptors have been identified, including fatty acid receptor (FFAR)-2 and FFAR3 (formerly known as GPR43 and GPR41) [56]. Animal studies have shown that SCFAs can have differential effects on blood pressure depending on the receptors involved. FFAR2 is expressed in a variety of tissues, including renal arteries, and causes vasodilation in response to SCFAs. In contrast, a blood pressure elevating effect is mediated by Olfr78 in mice through renin release from granules in the renal juxtaglomerular apparatus [57,58]. The potency of SCFAs is much lower for Olfr78 and the human analogue, OR51E2, than for FFAR2, and therefore, it was suggested that Olfr78 serves as a negative feedback loop for the blood pressure lowering effects of FFAR2 [59].
In addition, SCFAs, in particular butyrate, have anti-inflammatory effects that are presumed to be mediated by inhibition of histone deacetylase (HDAC) [60,61]. Butyrate suppresses the production of pro-inflammatory cytokines, such as tumor-necrosis factor-α (TNF-α), interleukin-12 (IL-12), and interferon-γ (IF-γ), and upregulates the production of anti-inflammatory interleukin-10 (IL-10) by monocytes in vitro [62]. In addition, SCFAs have anti-inflammatory effects on epithelial cells that are partly mediated through HDAC [63]. In spontaneously hypertensive rats, HDAC activation has been associated with hypertension [64]. Conversely, butyrate administration to mice resulted in decreased blood pressure levels and reduced renal inflammation by HDAC inhibition [65].
SCFAs have also been suggested to be implicated in gut–brain communication. Vagal afferents express receptors that can sense SCFAs, which provides another pathway for the blood pressure modulating effects of SCFAs [66]. Animal studies showed that higher colonic levels of acetate could result in blood pressure lowering through parasympathetic activation. In addition, the blood pressure lowering effects of butyrate in rats were shown to be significantly reduced by vagotomy [67]. Another study with spontaneous hypertensive rats described a reduced central responsiveness to butyrate, as a result from reduced expression of butyrate receptors in the hypothalamus [52]. Thus, SCFAs could affect blood pressure through direct vascular and renal receptors, through HDAC inhibition, but also through colonic nerve signaling.
2.3. Gut Permeability and Lipopolysaccharides
Gut microbiota can also affect gut permeability and therefore influence the extent to which metabolites and endotoxins are absorbed (Figure 1). The barrier of the intestinal epithelium consists primarily of enterocyte brush borders and is more permeable for hydrophobic than for water soluble compounds. However, intercellular junctions on the enterocyte’s lateral margins provide an alternative paracellular absorption route [68]. These intercellular junctions are dynamic structures that regulate paracellular permeability, and consist of tight junctions on the luminal side and adherens junctions on the laminal side. The level of permeability can be influenced by dietary factors, but also by the zonulin pathway. Zonulin is secreted by the basal lamina of the intestinal epithelium and binds enterocytes to initiate a complex intracellular signaling pathway that eventually phosphorylates the tight junction, resulting in permeability of the paracellular route [69]. Gut microbiota such as Vibrio cholerae appear to exploit this physiological pathway by excreting zona occludens toxin, a zonulin homologue that has similar effects [70].
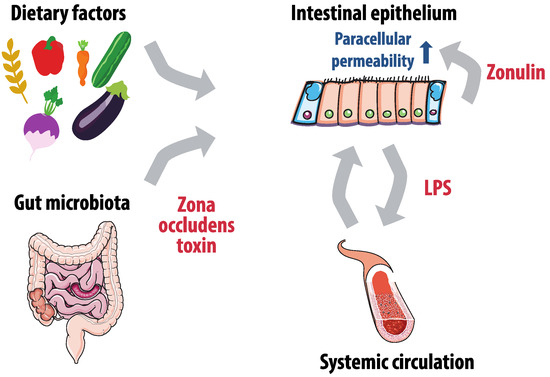
Figure 1.
Gut microbiota, gut permeability and lipopolysaccharides (LPS) absorption. Paracellular permeability of the intestinal epithelium is affected by zonulin production of the basal lamina, dietary factors and gut microbiota that produce zone occludens toxin. Increased permeability leads to more LPS translocation to the systemic circulation, which has a pro-inflammatory effect and further increases gut permeability.
Animal models suggest that gut permeability is higher in the hypertensive state. Hypertensive rats had lower levels of mRNA of gap junction proteins, indicating higher gut permeability, which was restored after fecal microbiota transplantation from controls [71]. In a similar model, an increase in blood pressure in spontaneous hypertensive rats was associated with more permeability and lower levels of tight junction proteins [72].
A consequence of higher gut permeability is increased translocation of certain metabolites and endotoxins in the portal and systemic circulation, which could cause further amplification of gut permeability [73]. Lipopolysaccharides (LPS), also known as endotoxins, can be found in the outer membrane of Gram-negative bacteria, the most abundant bacteria in the gut microbiome [74]. The lipid A component of LPS is the main pathogen-associated molecular pattern (PAMP) that can interact with Toll-like receptor 4 (TLR4) [75,76]. When translocated from the gut into the circulation, LPS forms a complex with LPS-binding protein (LBP) which can bind to CD14 on mononuclear cells [77]. This could lead to production of pro-inflammatory cytokines, such as TNF-α, interleukin-1 (IL-1), and interleukin-6 (IL-6), mediated by the MD2/TLR4 receptor complex [76,78]. Butyrate was shown to attenuate the pro-inflammatory effects of LPS-stimulation [79].
LPS is known to induce systemic inflammation and has been shown to have both metabolic and cardiovascular effects. In mice, infusion of LPS to 2- to 3-fold higher plasma levels resulted in higher glucose and insulin levels and weight gain comparable to mice on a 4-week high-fat diet [73]. LPS-administration to rats increased heart rate and norepinephrine levels, decreased baroreflex sensitivity, and increased neuroinflammation, as indicated by increased TLR and TNF-alfa expression in the paraventricular nucleus (PVN) that plays a key role in blood pressure regulation [80]. The same effects were observed in a small (n = 8) group of human subjects that showed a significant decrease in systolic and diastolic blood pressure after administration of LPS. Moreover, in this study, LPS increased brain microglial activation on positron emission tomography (PET)-scans [81]. Summarizing, there is a limited number of studies suggesting that systemic LPS could have pro-inflammatory, sympathetic activating, and neuroinflammatory effects, all of which are relevant in hypertension pathogenesis.
2.4. Gut-Brain Interactions and Sympathetic Activation
Increased sympathetic activation is considered one of the causal factors in the development of hypertension, and can already be observed in early stages [82]. The sympathetic nervous system modulates blood pressure levels through vasoconstriction in peripheral blood vessels, renal regulation of water and sodium balance, and release of renin by juxtaglomerular cells [83]. Regions in the central nervous system that are involved in sympathetic activation include the PVN, the nucleus of the solitary tract (NTS), and the rostral ventrolateral medulla (RVLM) [84]. Hypertension is associated with neuroinflammation in these regions, which might be mediated by the renin-angiotensin aldosterone system, since prorenin was shown to cause microglial activation in mice and spontaneously hypertensive rats (SHR) [85,86].
Gut–brain communication could stimulate sympathetic activation and therefore play a role in the hypertension pathogenesis. The gut is innervated by the autonomic nervous system that signals physiological conditions such as acidity, osmolarity, and pain [87]. Intrinsically, the enteric nervous system (ENS), consisting of the myenteric plexus and the submucosal plexus, controls intestinal motor and sensory functions [88]. The ENS is a complex system that is sometimes referred to as the ‘second brain’, because of the structural and functional similarities [89]. It communicates with the brain via the vagal nerve, which projects to the NTS, that is involved in sympathetic regulation. Gut microbiota interfere in ENS–brain interactions by stimulating enterochromaffin cells to produce serotonin, a neurotransmitter that affects gut secretion, motility, and local nerve reflexes [90]. Conversely, central sympathetic activation can, through a cascade of events, lead to increased gut permeability and increased translocation of metabolites into the systemic circulation [91].
Elevated sympathetic drive shifts bone marrow hemopoietic stem cells to a pro-inflammatory state, and the release of these immune cells contributes to further hypertension development [92,93]. An animal study with SHR showed that microbiota affect inflammation in brain regions crucial to sympathetic outflow. Microbiota composition in these rats was associated with reactive oxygen species (ROS) and proinflammatory cytokines in the PVN [71]. In addition, fecal transplantation in rat models from Wistar Kyoto (WKY) rats to SHR led to higher sympathetic activity, independent of renin levels [71,72]. Taken together, this suggests that gut microbiota can stimulate sympathetic drive, possibly by direct ENS–brain interactions or by promoting neuroinflammation. This increased sympathetic activity can contribute to hypertension development directly or indirectly, by stimulating low-grade systemic inflammation.
3. Gut Microbiota in Atherosclerosis
3.1. Atherosclerosis and Gut Microbiota
Atherosclerosis is a multifactorial process, with lipid metabolism, inflammation, vascular ageing, and blood pressure as key players. Atherosclerosis is closely related to arterial stiffness, which is caused by a loss of elastic fibers and thickening of arteriole walls. Arterial stiffness tends to increase with age and results in a less compliant arterial system and higher pulse wave velocity. The resulting increased shear stress has an aggravating effect on the formation of subsequent atherosclerotic plaques [94,95]. In this process, cholesterol accumulation in vessel walls leads to transformation of macrophages to foam cells after phagocytic uptake of lipid particles. Oxidation of lipids results in cholesterol crystallization, inflammasome activation, and production of proinflammatory cytokines such as TNF-alpha and IL-1B. Statins have been proven effective in preventing atherosclerotic events, not only by lowering low-density lipoprotein (LDL) cholesterol, but also through anti-inflammatory effects [96]. The Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS)-trial underlined the role of inflammation in atherosclerosis by demonstrating that treatment with canakinumab, a monoclonal inhibitor of IL-1B, lowers the incidence of cardiovascular events [97].
An atherosclerotic plaque was shown to be a microbial environment on itself, containing microbes such as Streptococcus, Pseudomonas, Klebsiella, Veillonella spp., and Chlamydia pneumoniae [98,99,100]. Most studies could not relate plaque microbiota composition to outcomes such as plaque vulnerability, rupture, or cardiovascular events [101,102]. It was suggested that pathogenic bacteria originating from oral or gut microbiomes make vessel walls more prone to plaque formation, either by direct infection of the vessel wall or by distant infections eliciting an auto-immune inflammatory reaction through molecular mimicry [103,104]. Interventions with antibiotic treatment as secondary prevention, targeted at eliminating plaque microbiota, did not result in lower incidence of cardiovascular events [105,106]. Therefore, these studies did not provide evidence for direct vessel wall infection as a causal factor, although some argue that not all microbes were targeted by the antibiotics used and that interventions were too short [104,107].
In humans, cross-sectional studies showed that higher abundance of the Collinsella genus, Enterobacteriaceae, Streptococcaceae, and Klebsiella spp., and lower abundance of SCFA-producing bacteria Eubacterium, Roseburia, and Ruminococcaceae spp. in the gut microbiota of patients with symptomatic atherosclerosis compared to healthy controls [108,109,110]. Pulse wave velocity, a marker of arterial stiffness, was associated with a lower alpha diversity and lower number of SCFA-producing bacteria such as Ruminococcaceae spp. in middle aged women in the TwinUK cohort [111]. Hence, the compositional differences in atherosclerosis overlap with findings in hypertensive patients, which is not surprising considering the shared risk factors and pathogenesis. Causal evidence of gut microbiota composition in atherosclerosis is based on fecal microbiota transplantation (FMT) in animal studies. For example, mice transplanted with a more pro-inflammatory gut microbiota composition from Caspase1-/- mice had 29% larger plaque sizes than controls [112]. Alternatively, gut microbiota could have indirect proatherogenic effects, by production of pro-atherogenic metabolites. These metabolites could also very well include the metabolites that are described for hypertension, including SCFAs. For the scope of this review, we chose to focus on the role of trimethylaminoxide (TMAO) and bile acids in atherosclerosis.
3.2. Trimethylamine-N-Oxide
The role of trimethylamine (TMA) and TMAO in the development of atherosclerosis is an extensively researched topic. The role of gut microbiota in TMAO production is illustrated by Figure 2. TMA is produced by gut microbes, primarily those from the families Clostridia and Enterobacteriaceae, in the degradation of nutrients such as carnitine, choline, and lecithin, that can be found in dietary products including meat and eggs [113]. After absorption, TMA is oxidized into trimethylamine-N-oxide (TMAO) by the hepatic enzyme flavin mono-oxygenase (FMO)-3 [114]. Plasma levels of TMAO have both a high within-individual and inter-individual variability, which hampers comparison of studies [115]. In addition, TMAO levels are higher in women, presumably due to different expression of the converting enzyme FMO3 and higher excretion rates in men [114]. TMAO is primarily excreted by the kidneys through both glomerular filtration and tubular secretion, which is a reason for increasing TMAO levels with decreasing renal function [116].
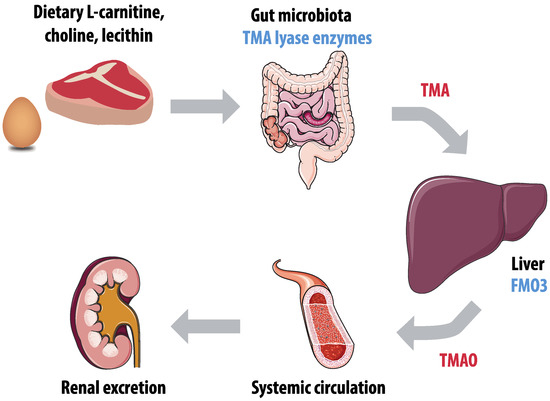
Figure 2.
Production of trimethylamine-N-oxide (TMAO). Gut microbiota enzymes, including trimethylamine (TMA) lyase, convert dietary L-carnitine, choline, and lecithin into TMA. The hepatic enzyme flavin mono-oxygenase 3 (FMO3) converts TMA into TMAO, and TMAO is primarily excreted by the kidneys.
Several mechanisms for the role of TMAO in atherosclerosis have been proposed, including the effects TMAO has on inflammation, cholesterol metabolism, and thrombosis. TMAO was shown to increase the production of pro-inflammatory cytokines such as TNF-alpha and IL-1B, and decrease anti-inflammatory cytokines such as IL-10 [117]. In addition, the hepatic enzyme FMO3 appeared to have a regulating function in lipid metabolism. FMO3 knockdown in mice on a high cholesterol diet lowered intestinal lipid absorption and hepatic cholesterol production and stimulated reverse cholesterol transport, thereby restoring cholesterol balance [118]. Lastly, TMAO was reported to induce platelet hyperreactivity, which can facilitate thrombosis, thus causing atherosclerotic thrombotic events [119].
Administration of TMAO indeed promoted atherosclerosis in several mouse models [120,121]. However, there are also several animal studies that could not confirm this association, or even found a protective effect of TMAO [122,123,124,125]. In humans, higher levels of TMAO have been associated with cardiovascular disease incidence in several prospective studies [123,126,127]. Two meta-analyses concluded that elevated TMAO levels were associated with a higher risk of cardiovascular events and a higher all-cause mortality with relative risks ranging between 55% and 62% [122,128].
Nevertheless, a causal effect of TMAO on atherosclerosis has not yet been proven. An elegant way to assess causality is Mendelian randomization, using genetic variants known to modify the exposure to examine the effect on disease [129]. In this case, the prevalence of cardiovascular disease in individuals with single nucleotide polymorphisms (SNPs) known to cause higher levels of TMAO was compared to individuals without these SNPs [130]. Interestingly, in this study, atherosclerotic cardiovascular disease was not more prevalent in the group with genetically predicted higher TMAO levels. Another way to prove causality is to lower TMAO levels with interventions, such as with TMA lyases that lower TMAO by degrading TMA before oxidization [131]. However, results of human intervention studies have not yet been published.
3.3. Bile Acids
Bile acid metabolism is dependent on microbial modifications in the gut (Figure 3) and this interaction was previously shown to affect inflammatory bowel disease and hyperinsulinemia [132,133]. Primary bile acids are synthesized by the liver, which converts hydrophobic cholesterol to hydrophilic primary bile acids [134]. These bile acids are excreted by the gall bladder and reabsorbed in the terminal ileum by sodium-dependent bile acid transporters [135]. Bile acids affect gut microbiota composition and inhibit microbial growth in the small intestines [136]. A small proportion of bile acids reaches the colon, where microbiota convert primary bile acids to secondary bile acids by several modifications, including deconjugation, 7α-dehydroxylation, and 7α-hydrogenation [137]. Secondary bile acids are hydrophobic and therefore easily absorbed by colonocytes and taken up into the systemic circulation. Only an estimated proportion of 5% of bile acids escape the enterohepatic cycle and are excreted [138]. Bile acids also affect diverse metabolic pathways through Takeda G-protein coupled receptor 5 (TGR5) and the nuclear farnesoid X receptor (FXR), both of which have a preference for secondary bile acids. The composition of the microbiota and the microbial community’s enzymatic repertoire determine the secondary bile acid profile [139]. The impact of gut microbiota on the bile acid pool was illustrated by a study showing that germ-free mice had a 71% decreased bile acid pool compared to controls [140]. Interestingly, the bile acid metabolism interacts with the TMAO pathway, as FXR has been shown to regulate FMO3, the hepatic enzyme that converts TMA in TMAO [114].
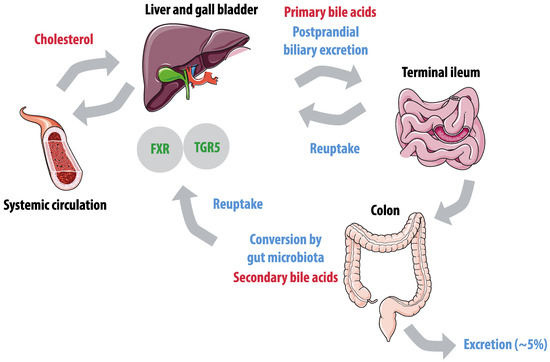
Figure 3.
Enterohepatic cycle of bile acids. Hepatic conversion of cholesterol results in primary bile acids, that are excreted postprandially by the gallbladder. Active reuptake takes place in the terminal ileum. In the colon, primary bile acids are converted to secondary bile acids by gut microbiota, and passively reabsorbed. Farnesoid X receptor (FXR) and Takeda G-protein coupled receptor 5 (TGR5) have a preference for secondary bile acids.
TGR5 is expressed in a variety of tissues, including liver, gall bladder, intestines, kidneys, pancreas, muscle, and adipose tissue, but can also be found on leukocytes, macrophages, and endothelial cells [141]. A TGR5 agonist (INT-777) was shown to have immunosuppressive effects, including reduced pro-inflammatory cytokine production by macrophages and attenuation of atherosclerotic plaque formation in LDL−/− mice [142,143]. Translation of findings from animal studies on TGR5 to humans in other contexts has not always been successful. Despite beneficial metabolic effects of TGR5 agonists in mice, including lower glucose levels and improved lipid profiles, the TGR5 agonist SB-756050 increased fasting glucose levels compared to placebo in human subjects with type 2 diabetes [144]. TGR5 agonists had limited adverse effects in this trial, which is surprising considering the number of tissues that express this receptor. In animal models, TGR5 agonists have been associated with increased gastrointestinal motility, a potential higher incidence of biliary stones, lower vascular tone and blood pressure, and itching [145].
Atherogenic mice models with FXR knock-out showed conflicting findings, with both increased and decreased atherosclerosis [146,147,148]. However, administration of synthetic FXR agonists to atherogenic mice prevented plaque formation in three studies, presumably by lipid-lowering and anti-inflammatory effects [149,150,151]. Although the FXR agonist obeticholic acid (OCA) lowered hepatic fat in human subjects with non-alcoholic steatohepatitis (NASH), it had paradoxical effects on cholesterol levels, increasing LDL and decreasing high-density lipoprotein (HDL) cholesterol [152].
Dual agents that target both TGR5 and FXR might have more therapeutic potential. Animal studies on the effect of dual agonists reported beneficial effects on metabolic syndrome, NASH, cholangiopathy, progression of diabetic nephropathy, and atherosclerosis [153,154,155,156,157]. In a mouse model for atherosclerosis, dual targeting with INT-767 seemed to be more effective in attenuating atherosclerosis than separate effects on TGR5 and FXR [153]. All in all, although findings in animal studies are promising, it remains to be seen whether these results can be translated to humans, especially considering the substantial differences in atherosclerosis pathogenesis and bile acid metabolism between men and mice.
4. Therapeutic Strategies
The changes in gut microbiota composition and gut metabolites discussed in this review could all be potential therapeutic targets in the treatment of atherosclerosis and hypertension. The most direct ways of altering gut microbiota composition are oral supplementation of specific microbial strains and fecal microbiota transplantation (FMT).
Probiotics containing SCFA-producing microbes including Bifidobacterium, Enterococcus, and Lactobacillus were suggested to have a variety of health benefits including anti-inflammatory and beneficial metabolic effects [158]. In addition, oral treatment with specific Bifidobacterium, Lactobacillus, and SCFA-producing Anaerobutyricum soehngenii species had modest blood pressure lowering effects in humans [43,159]. However, our understanding of mechanisms is based on animal research. Evidence in humans is limited and inconclusive due to heterogeneity in investigational products and study designs [160]. Therefore, the effect of specific strains is often unclear, which is one of the reasons that probiotics are marketed as nutritional supplements rather than medication [160].
Probiotic efficacy is both disease-specific and strain-specific [161], underlining the need for well-designed trials that survey gut microbiota composition before and after the intervention. Preferably, this should be measured with metagenomic sequencing (as opposed to 16S rRNA sequencing) in order to provide species-level resolution to compositional data. Another advantage of this technique is the potential to assess differences in gut microbiota functionality, as differences in microbiota composition do not always match differences in function. In addition, the gut microbiome has a spatial dimension, with composition gradients along the different parts of the intestinal tract, yet due to sampling difficulties, fecal samples are used as a proxy for the entire extent of the intestinal tract lumen. Localized sampling would aid in deciphering the actual biology in the intestine.
Alternatively, FMT could be used to optimize microbiota composition in individuals at risk for cardiovascular disease. FMT has been shown to be efficacious with limited adverse effects [162]. However, optimal FMT approaches, including donor selection, screening and preparation, have yet to be defined [163,164]. In addition, the long-term effects of FMT are not clear, since the follow-up in most studies is less than a year. As our understanding of the gut microbiome progresses, so does our knowledge of potential risks of FMT. To illustrate, bacteriophages—long understudied yet now known to play an important role in the microbiome—were shown to be transferred from donor to host by FMT, with uncertain implications [165].
To date, only one FMT trial targeted cardiovascular risk by transplantation from lean vegan donors to meat-consuming subjects with metabolic syndrome in order to lower TMAO levels [166]. Despite alterations in gut microbiota composition, TMAO levels did not change upon this intervention. Other FMT trials in obesity and metabolic syndrome also showed that effects on microbiota composition and glucose metabolism are small and transient, underlining the importance of pre-screening in order to select recipients most likely to respond [167,168]. To that end, a better understanding of the structural and functional aspects of the microbiota that affect hypertension and atherosclerosis incidence is needed.
Prebiotics and dietary interventions target gut microbiota composition indirectly. Prebiotics selectively stimulate specific microbes in the colon. Prebiotics are often fibers, although not all fibers are prebiotics [169]. Prebiotics were shown to stimulate growth of SCFA-producing microbes such as Bifidobacterium and Lactobacillus. Diet also has a substantial influence on gut microbiota composition. Dietary interventions such as the DASH and the Mediterranean diet were shown to lower cardiovascular risk [170,171]. However, since dietary interventions are multifaceted, it is difficult to point out what mechanisms explain the beneficial effects.
In summary, multiple interventions could target gut microbiota composition and its associated metabolites, ranging from targeted approaches to more accessible but non-specific interventions. However, translation of findings from animal studies to humans is needed, preferably by prospective cohort studies using metagenomic sequencing that can also assess microbiome functionality. In addition, adjusting for confounders when assessing associations between microbiota and cardiovascular disease is vital, since microbiota composition is shaped by a combination of lifestyle factors, health conditions, and medication use.
5. Conclusions
The pathways by which gut microbiota affect hypertension and atherosclerosis are diverse and often interact, as shown in Figure 4. Gut microbiota produce or convert metabolites, produce substrates needed for production of metabolites elsewhere, and are involved in regulating local intestinal homeostasis, resulting in a wide range of potential therapeutic targets. However, our understanding of mechanisms is mainly based on animal research and translation to humans remains challenging, as illustrated by developments in bile acid receptors research. Longitudinal studies in human subjects are needed to identify beneficial or adverse characteristics of gut microbiota structure and functionality, in order to better target potential therapeutic strategies.
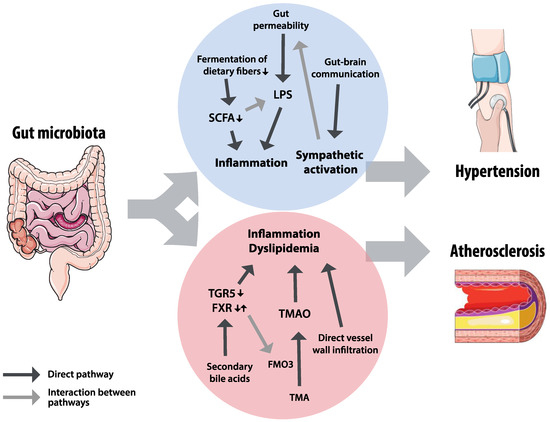
Figure 4.
Summary of hypothesized pathways for the effects of gut microbiota on hypertension and atherosclerosis. Gut microbiota could affect hypertension through inflammatory factors, influenced by short chain fatty acids (SCFAs) and lipopolysaccharides (LPS), and through sympathetic activation by gut–brain interactions. The effects on inflammation and dyslipidemia in atherosclerosis could be mediated by bile acid receptors Takeda G-protein-coupled receptor 5 (TGR5) and farnesoid X receptor (FXR), trimethylamine-N-oxide (TMAO) and trimethylamine (TMA), and direct vessel infiltration of microbiota. The grey arrows indicate interactions between pathways: FXR regulates the TMAO-converting enzyme flavin mono-oxygenase 3 (FMO3), sympathetic activation increases gut permeability, and short chain fatty acids can attenuate the inflammatory effects of LPS.
Author Contributions
B.J.H.V. drafted the manuscript; A.P., M.N. and M.M. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
B.J.H.V. is appointed on an Amsterdam Cardiovascular Sciences grant (ACSPhD2019P003) and an Alzheimer Nederland grant (WE.03-2017-12). M.N. is supported by a CVON in control grant. The illustrations in the figures are from Servier Medical Art (smart.servier.com).
Conflicts of Interest
MN is in the Scientific Advisory Board of Caelus Pharmaceuticals, the Netherlands and Kaleido, USA. None of these are directly relevant to the current paper. The other authors declare no conflict of interest.
References
- WHO. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. In Follow-up to the Political Declaration of the High-level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases; World Health Organization: Geneva, Switzerland, 2013; pp. 68–69. ISBN 978-92-4-1506236. Available online: https://www.who.int/nmh/events/ncd_action_plan/en/ (accessed on 28 September 2020).
- World Health Organization Cardiovascular Diseases (CVDs) Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 28 September 2020).
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.-C.; Khelaifia, S.; Alou, M.T.; Ndongo, S.; Dione, N.; Hugon, P.; Caputo, A.; Cadoret, F.; Traore, S.I.; Seck, E.H.; et al. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat. Microbiol. 2016, 1, 16203. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.P.; Gratz, S.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Zuo, T.; Ng, S.C. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar] [CrossRef]
- Wang, Q.; Li, F.; Liang, B.; Liang, Y.; Chen, S.; Mo, X.; Ju, Y.; Zhao, H.; Jia, H.; Spector, T.D.; et al. A metagenome-wide association study of gut microbiota in asthma in UK adults. BMC Microbiol. 2018, 18, 114. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Tang, W.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; Heuvel, J.K.V.D.; Meijer, O.C.; Berbée, J.F.P.; et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2017, 67, 1269–1279. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Inflammation, atherosclerosis, and cardiovascular risk: An epidemiologic view. Blood Coagul. Fibrinolysis 1999, 10 (Suppl. 1), S9–S12. [Google Scholar]
- Sesso, H.D.; Buring, J.; Rifai, N.; Blake, G.J.; Gaziano, J.M.; Ridker, P.M. C-Reactive Protein and the Risk of Developing Hypertension. J. Am. Med. Assoc. 2003, 290, 2945–2951. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Ioakeimidis, N.; Aznaouridis, K.; Bratsas, A.; Baou, K.; Xaplanteris, P.; Lazaros, G.; Stefanadis, C. Association of Interleukin-18 Levels With Global Arterial Function and Early Structural Changes in Men Without Cardiovascular Disease. Am. J. Hypertens. 2010, 23, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.; Feely, J. Arterial Stiffness Is Related to Systemic Inflammation in Essential Hypertension. Hypertension 2005, 46, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- Giri, A.; Hellwege, J.N.; Keaton, J.M.; Park, J.; Qiu, C.; Warren, H.R.; Torstenson, E.S.; Kovesdy, C.P.; Sun, Y.V.; Wilson, O.D.; et al. Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat. Genet. 2018, 51, 51–62. [Google Scholar] [CrossRef]
- Pazoki, R.; Dehghan, A.; Evangelou, E.; Warren, H.; Gao, H.; Caulfield, M.; Elliott, P.; Tzoulaki, I. Genetic predisposition to high blood pressure and lifestyle factors: Associations with midlife blood pressure levels and cardiovascular events. Circulation 2018, 137, 653–661. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A Clinical Trial of the Effects of Dietary Patterns on Blood Pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Mell, B.; Jala, V.R.; Mathew, A.V.; Byun, J.; Waghulde, H.; Zhang, Y.; Haribabu, B.; Vijay-Kumar, M.; Pennathur, S.; Joe, B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol. Genom. 2015, 47, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Adnan, S.; Nelson, J.W.; Ajami, N.J.; Venna, V.R.; Petrosino, J.F.; Bryan, R.M.; Durgan, D.J. Alterations in the gut microbiota can elicit hypertension in rats. Physiol. Genom. 2017, 49, 96–104. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertensioin 2015, 65, 1331–1340. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.-Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Dan, X.; Mushi, Z.; Baili, W.; Han, L.; Enqi, W.; Huanhu, Z.; Shuchun, L. Differential Analysis of Hypertension-Associated Intestinal Microbiota. Int. J. Med. Sci. 2019, 16, 872–881. [Google Scholar] [CrossRef]
- De La Cuesta-Zuluaga, J.; Mueller, N.T.; Alvarez, R.Q.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef]
- Huart, J.; Leenders, J.; Taminiau, B.; Descy, J.; Saint-Remy, A.; Daube, G.; Krzesinski, J.-M.; Melin, P.; De Tullio, P.; Jouret, F. Gut Microbiota and Fecal Levels of Short-Chain Fatty Acids Differ Upon 24-Hour Blood Pressure Levels in Men. Hypertension 2019, 74, 1005–1013. [Google Scholar] [CrossRef]
- Jackson, M.A.; Verdi, S.; Maxan, M.-E.; Shin, C.M.; Zierer, J.; Bowyer, R.C.E.; Martin, T.; Williams, F.M.; Menni, C.; Bell, J.T.; et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat. Commun. 2018, 9, 2655. [Google Scholar] [CrossRef]
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.; Richards, E.M.; Pepine, C.J.; et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin. Sci. 2018, 132, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Lulla, A.; Sioda, M.; Winglee, K.; Wu, M.C.; Jr, D.R.J.; Shikany, J.M.; Lloyd-Jones, D.M.; Launer, L.J.; Fodor, A.A.; et al. Gut microbiota composition and blood pressure: The CARDIA study. Hypertension 2019, 73, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.J.H.; Collard, D.; Prodan, A.; Levels, J.H.M.; Zwinderman, A.H.; Bäckhed, F.; Vogt, L.; Peters, M.J.L.; Muller, M.; Nieuwdorp, M.; et al. Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: The HELIUS study. Eur. Heart J. 2020, ehaa704. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Gu, Y.; Li, X.; Yang, W.; Jia, L.; Chen, C.; Han, X.; Huang, Y.; Zhao, L.; Li, P.; et al. Alterations of the Gut Microbiome in Hypertension. Front. Cell. Infect. Microbiol. 2017, 7, 381. [Google Scholar] [CrossRef]
- Bier, A.; Braun, T.; Khasbab, R.; Di Segni, A.; Grossman, E.; Haberman, Y.; Leibowitz, A. A High Salt Diet Modulates the Gut Microbiota and Short Chain Fatty Acids Production in a Salt-Sensitive Hypertension Rat Model. Nutrients 2018, 10, 1154. [Google Scholar] [CrossRef]
- Miranda, P.; Serkis, V.; De Palma, G.; Pigrau, M.; Lu, J.; Collins, S.; Bercík, P. High salt diet increases susceptibility to experimental colitis: A putative role of gut microbiota. Gastroenterology 2016, 150, S583. [Google Scholar] [CrossRef]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef]
- Tanida, M.; Yamano, T.; Maeda, K.; Okumura, N.; Fukushima, Y.; Nagai, K. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci. Lett. 2005, 389, 109–114. [Google Scholar] [CrossRef]
- Gomez-Guzman, M.; Toral, M.; Romero, M.; Jiménez, R.; Galindo, P.; Sanchez, M.; Zarzuelo, M.J.; Olivares, M.; Gálvez, J.; Duarte, J. Antihypertensive effects of probioticsLactobacillusstrains in spontaneously hypertensive rats. Mol. Nutr. Food Res. 2015, 59, 2326–2336. [Google Scholar] [CrossRef]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension 2014, 64, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; Hashimoto, H.; Hosoda, M.; Morita, H.; Hosono, A. Effect of Administration of Fermented Milk Containing Whey Protein Concentrate to Rats and Healthy Men on Serum Lipids and Blood Pressure. J. Dairy Sci. 2000, 83, 255–263. [Google Scholar] [CrossRef]
- Hata, Y.; Yamamoto, M.; Ohni, M.; Nakajima, K.; Nakamura, Y.; Takano, T. A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Am. J. Clin. Nutr. 1996, 64, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Calderón-Pérez, L.; Gosalbes, M.J.; Yuste, S.; Valls, R.M.; Pedret, A.; Llauradó, E.; Jimenez-Hernandez, N.; Artacho, A.; Pla-Pagà, L.; Companys, J.; et al. Gut metagenomic and short chain fatty acids signature in hypertension: A cross-sectional study. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. Fiber from a regular diet is directly associated with fecal short-chain fatty acid concentrations in the elderly. Nutr. Res. 2013, 33, 811–816. [Google Scholar] [CrossRef]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Mooter, G.V.D.; De Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef]
- Roediger, W.E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 1980, 21, 793–798. [Google Scholar] [CrossRef]
- Yang, T.; Magee, K.L.; Colon-Perez, L.M.; Larkin, R.; Liao, Y.-S.; Balazic, E.; Cowart, J.R.; Arocha, R.; Redler, T.; Febo, M.; et al. Impaired butyrate absorption in the proximal colon, low serum butyrate and diminished central effects of butyrate on blood pressure in spontaneously hypertensive rats. Acta Physiol. 2019, 226, e13256. [Google Scholar] [CrossRef]
- Bouter, K.E.; Bakker, G.J.; Levin, E.; Hartstra, A.V.; Kootte, R.S.; Udayappan, S.D.; Katiraei, S.; Bähler, L.; Gilijamse, P.W.; Tremaroli, V.; et al. Differential metabolic effects of oral butyrate treatment in lean versus metabolic syndrome subjects. Clin. Transl. Gastroenterol. 2018, 9, e155-10. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, N.; Jafarabadi, M.A.; Ghavami, A.; Alipour, S.; Alamdari, N.M.; Barati, M.; Namin, S.A.M.; Mahdavi, R.; Alizadeh, E.; Hedayati, M. Effect of Butyrate and Inulin Supplementation on Glycemic Status, Lipid Profile and Glucagon-Like Peptide 1 Level in Patients with Type 2 Diabetes: A Randomized Double-Blind, Placebo-Controlled Trial. Horm. Metab. Res. 2017, 49, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. Gut microbiota in renal physiology: Focus on short-chain fatty acids and their receptors. Kidney Int. 2016, 90, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brézillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.-X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Q.; Lu, A.; Liu, X.; Zhang, L.; Xu, C.; Liu, X.; Li, H.; Yang, T. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin–angiotensin system. J. Hypertens. 2017, 35, 1899–1908. [Google Scholar] [CrossRef]
- Pluznick, J.L. Microbial Short-Chain Fatty Acids and Blood Pressure Regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Aguilar, E.; Leonel, A.; Teixeira, L.; Silva, A.; Silva, J.; Pelaez, J.; Capettini, L.; Lemos, V.S.; Santos, R.; Alvarez-Leite, J.I. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar] [CrossRef]
- Saemann, M.D.; Böhmig, G.A.; Österreicher, C.H.; Burtscher, H.; Parolini, O.; Diakos, C.; Stockl, J.; Hörl, W.H.; Zlabinger, G.J. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000, 14, 2380–2382. [Google Scholar] [CrossRef]
- Li, M.; Van Esch, B.C.A.M.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The Anti-inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α-Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, J.P.; Sriramula, S.; Pariaut, R.; Guggilam, A.; Mariappan, N.; Elks, C.M.; Francis, J. HDAC Inhibition Attenuates Inflammatory, Hypertrophic, and Hypertensive Responses in Spontaneously Hypertensive Rats. Hypertension 2010, 56, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Gogulamudi, V.R.; Periasamy, R.; Raghavaraju, G.; Subramanian, U.; Pandey, K.N. Inhibition of HDAC enhances STAT acetylation, blocks NF-κB, and suppresses the renal inflammation and fibrosis in Npr1 haplotype male mice. Am. J. Physiol. Renal Physiol. 2017, 313, F781–F795. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Kirkup, A.J.; Brunsden, A.M.; Thompson, D.G.; Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Liver Physiol. 2001, 281, G907–G915. [Google Scholar] [CrossRef]
- Onyszkiewicz, M.; Gawrys-Kopczynska, M.; Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Koźniewska, E.; Samborowska, E.; Ufnal, M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch. Eur. J. Physiol. 2019, 471, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Bistritz, L.; Meddings, J. Alterations in intestinal permeability. Gut 2006, 55, 1512–1520. [Google Scholar] [CrossRef]
- Wang, W.; Uzzau, S.; Goldblum, S.E.; Fasano, A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 2000, 113, 4435–4440. [Google Scholar]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Toral, M.; Robles-Vera, I.; De La Visitacion, N.; Romero, M.; Yang, T.; Sánchez, M.; Gomez-Guzman, M.; Jiménez, R.; Raizada, M.K.; Duarte, J. Critical Role of the Interaction Gut Microbiota—Sympathetic Nervous System in the Regulation of Blood Pressure. Front. Physiol. 2019, 10, 231. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Qi, Y.F.; Zubcevic, J.; Kim, S.; Yang, T.; Shenoy, V.; Cole-Jeffrey, C.T.; Lobaton, G.; Stewart, D.C.; Rubiano, A.; et al. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ. Res. 2017, 120, 312–323. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide Endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Aderem, A.; Underhill, D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Tobias, P.S.; Soldau, K.; Gegner, J.A.; Mintz, D.; Ulevitch, R.J. Lipopolysaccharide Binding Protein-mediated Complexation of Lipopolysaccharide with Soluble CD14. J. Biol. Chem. 1995, 270, 10482–10488. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.; Ramos, R.; Tobias, P.; Ulevitch, R.; Mathison, J.; Nishibe, S.; Wahl, M.; Hernandez-Sotomayor, S.; Tonks, N.; Rhee, S.; et al. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990, 249, 1431–1433. [Google Scholar] [CrossRef]
- Maa, M.-C.; Chang, M.Y.; Hsieh, M.-Y.; Chen, Y.-J.; Yang, C.-J.; Chen, Z.-C.; Li, Y.K.; Yen, C.-K.; Wu, R.-R.; Leu, T.-H. Butyrate reduced lipopolysaccharide-mediated macrophage migration by suppression of Src enhancement and focal adhesion kinase activity. J. Nutr. Biochem. 2010, 21, 1186–1192. [Google Scholar] [CrossRef]
- Masson, G.S.; Nair, A.R.; Dange, R.B.; Silva-Soares, P.P.; Michelini, L.C.; Francis, J. Toll-Like Receptor 4 Promotes Autonomic Dysfunction, Inflammation and Microglia Activation in the Hypothalamic Paraventricular Nucleus: Role of Endoplasmic Reticulum Stress. PLoS ONE 2015, 10, e0122850. [Google Scholar] [CrossRef]
- Sandiego, C.M.; Gallezot, J.-D.; Pittman, B.; Nabulsi, N.; Lim, K.; Lin, S.-F.; Matuskey, D.; Lee, J.-Y.; O’Connor, K.C.; Huang, Y.; et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc. Natl. Acad. Sci. USA 2015, 112, 12468–12473. [Google Scholar] [CrossRef]
- Mancia, G.; Grassi, G. The Autonomic Nervous System and Hypertension. Circ. Res. 2014, 114, 1804–1814. [Google Scholar] [CrossRef]
- Burnstock, G.; Loesch, A. Sympathetic innervation of the kidney in health and disease: Emphasis on the role of purinergic cotransmission. Auton. Neurosci. Basic Clin. 2017, 204, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Young, C.N.; Fadel, P.J. Central sympathetic overactivity: Maladies and mechanisms. Auton. Neurosci. Basic Clin. 2009, 148, 5–15. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, A.; Liu, M.; Rodríguez, V.; Krause, E.G.; Sumners, C. Role of neurons and glia in the CNS actions of the renin-angiotensin system in cardiovascular control. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R444–R458. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Grobe, J.L.; Desland, F.A.; Zhou, G.; Shen, X.Z.; Shan, Z.; Liu, M.; Raizada, M.K.; Sumners, C. Direct Pro-Inflammatory Effects of Prorenin on Microglia. PLoS ONE 2014, 9, e92937. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Blackshaw, L.A.; Brookes, S.J.H.; Grundy, D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol. Motil. 2004, 16, 28–33. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar] [CrossRef]
- Gershon, M.D. The enteric nervous system: A second brain. Hosp. Pract. 1999, 34, 31–52. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Schäper, J.; Wagner, A.; Enigk, F.; Brell, B.; Mousa, H.A.; Habazettl, H.; Schäfer, M. Regional Sympathetic Blockade Attenuates Activation of Intestinal Macrophages and Reduces Gut Barrier Failure. Anesthesiology 2013, 118, 134–142. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Ahmari, N.; Carvajal, J.M.; Zingler, M.B.; Qi, Y.; Kim, S.; Joseph, J.; Garcia-Pereira, F.; Johnson, R.D.; Shenoy, V.; et al. Involvement of Bone Marrow Cells and Neuroinflammation in Hypertension. Circ. Res. 2015, 117, 178–191. [Google Scholar] [CrossRef]
- Zubcevic, J.; Jun, J.Y.; Kim, S.; Perez, P.D.; Afzal, A.; Shan, Z.; Li, W.; Santisteban, M.M.; Yuan, W.; Febo, M.; et al. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension 2014, 63, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-L.; Kim, S.-H. Pulse Wave Velocity in Atherosclerosis. Front. Cardiovasc. Med. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Van Popele, N.M.; Grobbee, D.E.; Bots, M.L.; Asmar, R.; Topouchian, J.; Reneman, R.S.; Hoeks, A.P.G.; Van Der Kuip, D.A.M.; Hofman, A.; Witteman, J.C.M. Association Between Arterial Stiffness and Atherosclerosis. Stroke 2001, 32, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cannon, C.P.; Morrow, D.; Rifai, N.; Rose, L.M.; McCabe, C.H.; Pfeffer, M.A.; Braunwald, E. C-Reactive Protein Levels and Outcomes after Statin Therapy. N. Engl. J. Med. 2005, 352, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ott, S.J.; El-Mokhtari, N.E.; Musfeldt, M.; Hellmig, S.; Freitag, S.; Rehman, A.; Kühbacher, T.; Nikolaus, S.; Namsolleck, P.; Blaut, M.; et al. Detection of Diverse Bacterial Signatures in Atherosclerotic Lesions of Patients with Coronary Heart Disease. Circulation 2006, 113, 929–937. [Google Scholar] [CrossRef]
- Koren, O.; Spor, A.; Felin, J.; Fåk, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA 2010, 108, 4592–4598. [Google Scholar] [CrossRef]
- Lanter, B.B.; Sauer, K.; Davies, D.G. Bacteria Present in Carotid Arterial Plaques Are Found as Biofilm Deposits Which May Contribute to Enhanced Risk of Plaque Rupture. mBio 2014, 5, e01206-14. [Google Scholar] [CrossRef]
- Mitra, S.; Drautz-Moses, D.I.; Alhede, M.; Maw, M.T.; Liu, Y.; Purbojati, R.W.; Hwee, Y.Z.; Kushwaha, K.K.; Gheorghe, A.G.; Bjarnsholt, T.; et al. In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome 2015, 3, 38. [Google Scholar] [CrossRef]
- Jonsson, A.L.; Fåk, F.; Akrami, R.; Johansson, E.; Wester, P.; Arnerlöv, C.; Bäckhed, F.; Bergström, G. Bacterial profile in human atherosclerotic plaques. Atherosclerosis 2017, 263, 177–183. [Google Scholar] [CrossRef]
- Epstein, S.E.; Zhu, J.; Burnett, M.S.; Zhou, Y.F.; Vercellotti, G.; Hajjar, D. Infection and atherosclerosis: Potential roles of pathogen burden and molecular mimicry. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.A.; Rosenfeld, M. Pathogens and atherosclerosis: Update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb. Haemost. 2011, 106, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Grayston, J.T. Antibiotic Treatment of Atherosclerotic Cardiovascular Disease. Circulation 2003, 107, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Brassard, P.; Brophy, J.M. A meta-analysis of antibiotic use for the secondary prevention of cardiovascular diseases. Can. J. Cardiol. 2008, 24, 391–395. [Google Scholar] [CrossRef]
- Jonsson, A.L.; Bäckhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 79–87. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Hu, X.; Niu, H.; Tian, R.; Wang, H.; Pang, H.; Jiang, L.; Qiu, B.; Chen, X.; et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome 2019, 7, 68. [Google Scholar] [CrossRef]
- Menni, C.; Lin, C.; Cecelja, M.; Mangino, M.; Hernandez, M.M.; Keehn, L.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Kuo, C.-F.; et al. Gut microbial diversity is associated with lower arterial stiffness in women. Eur. Hear. J. 2018, 39, 2390–2397. [Google Scholar] [CrossRef]
- Brandsma, E.; Kloosterhuis, N.J.; Koster, M.; Dekker, D.C.; Gijbels, M.J.; Van Der Velden, S.; Ríos-Morales, M.; Van Faassen, M.J.; Loreti, M.G.; De Bruin, A.; et al. A Proinflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis. Circ. Res. 2019, 124, 94–100. [Google Scholar] [CrossRef]
- Rath, S.; Heidrich, B.; Pieper, D.H.; Vital, M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.; Vallim, T.Q.D.A.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; Moré, M.; Bellamine, A. Trimethylamine N-Oxide in Relation to Cardiometabolic Health—Cause or Effect? Nutrients 2020, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2014, 116, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zheng, X.; Feng, M.; Li, D.; Zhang, H. Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide Contributes to Cardiac Dysfunction in Western Diet-Induced Obese Mice. Front. Physiol. 2017, 8, 139. [Google Scholar] [CrossRef]
- Warrier, M.; Shih, D.M.; Burrows, A.C.; Ferguson, D.; Gromovsky, A.D.; Brown, A.L.; Marshall, S.; McDaniel, A.; Schugar, R.C.; Wang, Z.; et al. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep. 2015, 10, 326–338. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Hear. Assoc. 2017, 6, e004947. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Johansen, K.L.; Chertow, G.M.; Dalrymple, L.S.; Kornak, J.; Grimes, B.; Dwyer, T.; Chassy, A.W.; Fiehn, O. Associations of Trimethylamine N-Oxide With Nutritional and Inflammatory Biomarkers and Cardiovascular Outcomes in Patients New to Dialysis. J. Ren. Nutr. 2015, 25, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Aldana-Hernández, P.; Leonard, K.-A.; Zhao, Y.-Y.; Curtis, J.M.; Field, C.J.; Jacobs, R.L. Dietary Choline or Trimethylamine N-oxide Supplementation Does Not Influence Atherosclerosis Development in Ldlr-/- and Apoe-/- Male Mice. J. Nutr. 2019, 150, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.M.; Allenspach, M.; Othman, A.; Saely, C.H.; Muendlein, A.; Vonbank, A.; Drexel, H.; Von Eckardstein, A. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis 2015, 243, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Wang, Z.; Levison, B.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Lever, M.; George, P.M.; Slow, S.; Bellamy, D.; Young, J.M.; Ho, M.; McEntyre, C.J.; Elmslie, J.L.; Atkinson, W.; Molyneux, S.L.; et al. Betaine and Trimethylamine-N-Oxide as Predictors of Cardiovascular Outcomes Show Different Patterns in Diabetes Mellitus: An Observational Study. PLoS ONE 2014, 9, e114969. [Google Scholar] [CrossRef]
- Qi, J.; You, T.; Li, J.; Pan, T.; Xiang, L.; Han, Y.; Zhu, L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: A systematic review and meta-analysis of 11 prospective cohort studies. J. Cell. Mol. Med. 2017, 22, 185–194. [Google Scholar] [CrossRef]
- Smith, G.D.; Ebrahim, S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef]
- Jia, J.; Dou, P.; Gao, M.; Kong, X.; Li, C.; Liu, Z.; Huang, T. Assessment of Causal Direction Between Gut Microbiota–Dependent Metabolites and Cardiometabolic Health: A Bidirectional Mendelian Randomization Analysis. Diabetes 2019, 68, 1747–1755. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, A.; Buffa, J.A.; Levison, B.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef]
- Vrieze, A.; Out, C.; Fuentes, S.; Jonker, L.; Reuling, I.J.; Kootte, R.S.; Van Nood, E.; Holleman, F.; Knaapen, M.; Romijn, J.A.; et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 2014, 60, 824–831. [Google Scholar] [CrossRef]
- Baars, A.; Oosting, A.; Knol, J.; Garssen, J.; Van Bergenhenegouwen, J. The Gut Microbiota as a Therapeutic Target in IBD and Metabolic Disease: A Role for the Bile Acid Receptors FXR and TGR5. Microorganisms 2015, 3, 641–666. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, F.; Bloks, V.W.; Groen, A.K. Beyond intestinal soap-bile acids in metabolic control. Nat. Publ. Gr. 2014, 10, 488–498. [Google Scholar] [CrossRef]
- Hagenbuch, B.; Dawson, P. The sodium bile salt cotransport family SLC10. Pflugers Arch. Eur. J. Physiol. 2004, 447, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F.; Eckmann, L. How bile acids confer gut mucosal protection against bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 4333–4334. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Gahan, C.G.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [PubMed]
- Brufau, G.; Groen, A.K.; Kuipers, F. Reverse cholesterol transport revisited: Contribution of biliary versus intestinal cholesterol excretion. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1726–1733. [Google Scholar] [CrossRef]
- Selwyn, F.P.; Csanaky, I.L.; Zhang, Y.; Klaassen, C.D. Importance of Large Intestine in Regulating Bile Acids and Glucagon-Like Peptide-1 in Germ-Free Mice. Drug Metab. Dispos. 2015, 43, 1544–1556. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-beta-muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Hodge, R.J.; Nunez, D.J. Therapeutic potential of Takeda-G-protein-receptor-5 (TGR5) agonists. Hope or hype? Diabetes Obes. Metab. 2016, 18, 439–443. [Google Scholar] [CrossRef]
- Yoneno, K.; Hisamatsu, T.; Shimamura, K.; Kamada, N.; Ichikawa, R.; Kitazume, M.T.; Mori, M.; Uo, M.; Namikawa, Y.; Matsuoka, K.; et al. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn’s disease. Immunology 2013, 139, 19–29. [Google Scholar] [CrossRef]
- Pols, T.W.; Nomura, M.; Harach, T.; Sasso, G.L.; Oosterveer, M.H.; Thomas, C.; Rizzo, G.; Gioiello, A.; Adorini, L.; Pellicciari, R.; et al. TGR5 Activation Inhibits Atherosclerosis by Reducing Macrophage Inflammation and Lipid Loading. Cell Metab. 2011, 14, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Hodge, R.J.; Lin, J.; Johnson, L.S.V.; Gould, E.P.; Bowers, G.D.; Nunez, D.J.; on behalf of the SB-756050 Project Team. Safety, Pharmacokinetics, and Pharmacodynamic Effects of a Selective TGR5 Agonist, SB-756050, in Type 2 Diabetes. Clin. Pharmacol. Drug Dev. 2013, 2, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Pols, T.; Eggink, H.; Soeters, M. TGR5 ligands as potential therapeutics in inflammatory diseases. Int. J. Interf. Cytokine Mediat. Res. 2014, 6, 27–38. [Google Scholar] [CrossRef][Green Version]
- Hanniman, E.A.; Lambert, G.; McCarthy, T.C.; Sinal, C.J. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J. Lipid Res. 2005, 46, 2595–2604. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Vales, C.; Lee, F.Y.; Lee, H.; Lusis, A.J.; Edwards, P.A. FXR Deficiency Causes Reduced Atherosclerosis in Ldlr −/− Mice. Thromb. Vasc. Biol. 2006, 26, 2316–2321. [Google Scholar] [CrossRef]
- Guo, G.L.; Santamarina-Fojo, S.; Akiyama, T.E.; Amar, M.J.; Paigen, B.J.; Brewer, B.; Gonzalez, F.J. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2006, 1761, 1401–1409. [Google Scholar] [CrossRef]
- Hartman, H.B.; Gardell, S.J.; Petucci, C.J.; Wang, S.; Krueger, J.A.; Evans, M.J. Activation of farnesoid X receptor prevents atherosclerotic lesion formation in LDLR−/− and apoE−/− mice. J. Lipid Res. 2009, 50, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; Renga, B.; Distrutti, E.; Fiorucci, S. Antiatherosclerotic effect of farnesoid X receptor. Am. J. Physiol. Hear. Circ. Physiol. 2009, 296, H272–H281. [Google Scholar] [CrossRef]
- Hambruch, E.; Miyazaki-Anzai, S.; Hahn, U.; Matysik, S.; Boettcher, A.; Perović-Ottstadt, S.; Schlüter, T.; Kinzel, O.; Krol, H.D.; Deuschle, U.; et al. Synthetic Farnesoid X Receptor Agonists Induce High-Density Lipoprotein-Mediated Transhepatic Cholesterol Efflux in Mice and Monkeys and Prevent Atherosclerosis in Cholesteryl Ester Transfer Protein Transgenic Low-Density Lipoprotein Receptor (−/−) Mice. J. Pharmacol. Exp. Ther. 2012, 343, 556–567. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Van Natta, M.L.; Connelly, M.A.; Vuppalanchi, R.; Neuschwander-Tetri, B.A.; Tonascia, J.; Guy, C.; Loomba, R.; Dasarathy, S.; Wattacheril, J.; et al. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. J. Hepatol. 2019, 72, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki-Anzai, S.; Masuda, M.; Levi, M.; Keenan, A.L.; Miyazaki, M. Dual Activation of the Bile Acid Nuclear Receptor FXR and G-Protein-Coupled Receptor TGR5 Protects Mice against Atherosclerosis. PLoS ONE 2014, 9, e108270. [Google Scholar] [CrossRef] [PubMed]
- Baghdasaryan, A.; Claudel, T.; Gumhold, J.; Silbert, D.; Adorini, L.; Roda, A.; Vecchiotti, S.; Gonzalez, F.J.; Schoonjans, K.; Strazzabosco, M.; et al. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2 −/− (Abcb4 −/−) mouse cholangiopathy model by promoting biliary HCO 3− output. Hepatology 2011, 54, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, K.; Xu, Y.; Xu, Y.; Li, Y.; Xu, J.; Zhu, Y.; Adorini, L.; Lee, Y.K.; Kasumov, T.; Yin, L.; et al. Reversal of metabolic disorders by pharmacological activation of bile acid receptors TGR5 and FXR. Mol. Metab. 2018, 9, 131–140. [Google Scholar] [CrossRef]
- Iracheta-Vellve, A.; Calenda, C.D.; Petrasek, J.; Ambade, A.; Kodys, K.; Adorini, L.; Szabo, G. FXR and TGR5 Agonists Ameliorate Liver Injury, Steatosis, and Inflammation After Binge or Prolonged Alcohol Feeding in Mice. Hepatol. Commun. 2018, 2, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Wang, N.; Luo, Y.; Myakala, K.; Dobrinskikh, E.; Rosenberg, A.Z.; Levi, J.; Kopp, J.B.; Field, A.; Hill, A.; et al. FXR/TGR5 Dual Agonist Prevents Progression of Nephropathy in Diabetes and Obesity. J. Am. Soc. Nephrol. 2017, 29, 118–137. [Google Scholar] [CrossRef]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef]
- Gilijamse, P.W.; Hartstra, A.V.; Levin, E.; Wortelboer, K.; Serlie, M.J.; Ackermans, M.T.; Herrema, H.; Nederveen, A.J.; Imangaliyev, S.; Aalvink, S.; et al. Treatment with Anaerobutyricum soehngenii: A pilot study of safety and dose-response effects on glucose metabolism in human subjects with metabolic syndrome. npj Biofilms Microbiomes 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Quigley, E.M. Prebiotics and Probiotics in Digestive Health. Clin. Gastroenterol. Hepatol. 2019, 17, 333–344. [Google Scholar] [CrossRef]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef]
- Dailey, F.E.; Turse, E.P.; Daglilar, E.; Tahan, V. The dirty aspects of fecal microbiota transplantation: A review of its adverse effects and complications. Curr. Opin. Pharmacol. 2019, 49, 29–33. [Google Scholar] [CrossRef]
- Hecht, G.A.; Blaser, M.J.; Gordon, J.; Kaplan, L.M.; Knight, R.; Laine, L.; Peek, R.; Sanders, M.E.; Sartor, B.; Wu, G.D.; et al. What is the value of a food and drug administration investigational new drug application for fecal microbiota transplantation to treat clostridium difficile infection? Clin. Gastroenterol. Hepatol. 2014, 12, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Sung, J.; Cheng, F.; Tang, W.; Wong, S.H.; Chan, P.K.; Kamm, M.A.; Sung, J.J.; Kaplan, G.G.; Chan, F.K.; et al. Systematic review with meta-analysis: Review of donor features, procedures and outcomes in 168 clinical studies of faecal microbiota transplantation. Aliment. Pharmacol. Ther. 2019, 49, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Draper, L.A.; Ryan, F.J.; Smith, M.K.; Jalanka, J.; Mattila, E.; Arkkila, P.A.; Ross, R.P.; Satokari, R.; Hill, C. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome 2018, 6, 220. [Google Scholar] [CrossRef] [PubMed]
- Smits, L.P.; Kootte, R.S.; Levin, E.; Prodan, A.; Fuentes, S.; Zoetendal, E.G.; Wang, Z.; Levison, B.S.; Cleophas, M.C.P.; Kemper, E.M.; et al. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients With Metabolic Syndrome. J. Am. Hear. Assoc. 2018, 7, e008342. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga–Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health: A Critical Review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Siervo, M.; Lara, J.; Chowdhury, S.; Ashor, A.W.; Oggioni, C.; Mathers, J.C. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: A systematic review and meta-analysis. Br. J. Nutr. 2014, 113, 1–15. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).