Obese Older Type 2 Diabetes Mellitus Patients with Muscle Insulin Resistance Benefit from an Enriched Protein Drink during Combined Lifestyle Intervention: The PROBE Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Study Products
2.4. Clinical Chemistry and Glycemic Control
2.5. Subtyping of Type 2 Diabetes
2.6. Physical Measurements
2.7. Statistics
2.8. Data and Resource Availability
3. Results
3.1. Study Population
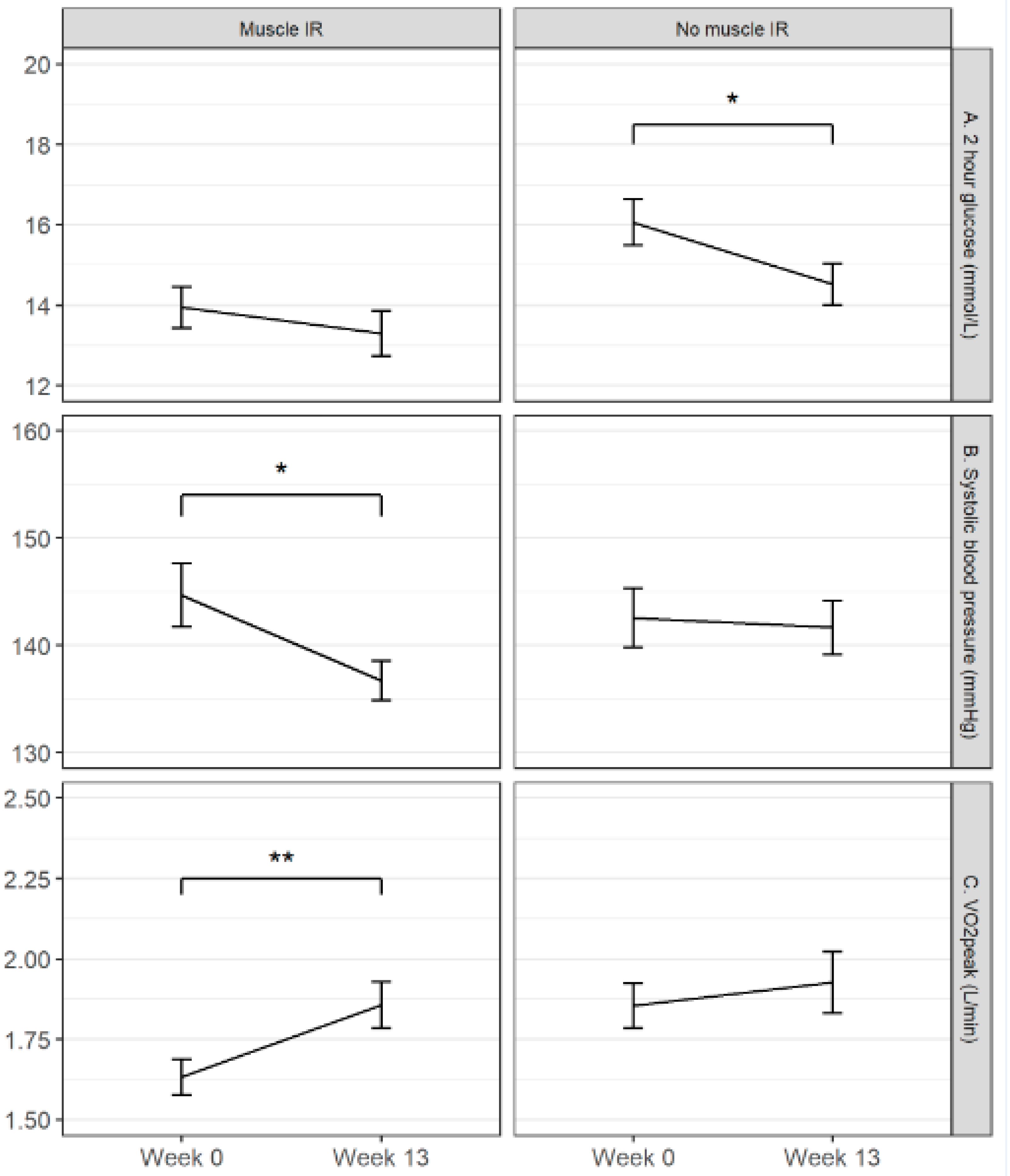
3.2. Protein+ Drink Effect within the Muscle Insulin Resistance Subgroup
3.3. Protein+ Drink Effect within the No-Muscle Insulin Resistance Subgroup
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Component | Test Product Protein+ | Control Product |
|---|---|---|
| Energy (kcal) | 150 | 150 |
| Protein (g) | ||
| Total (g) | 20.7 | - |
| Leucine, total a (g) | 2.8 | - |
| EAA, total a (g) | 10.6 | - |
| Carbohydrates | ||
| Total (g) | 9.4 | 24.5 |
| Sugars b (g) | 4.2 | 15.6 |
| Fat | ||
| Total (g) | 3.0 | 5.8 |
| Saturated (g) | 0.8 | 3.2 |
| Mono-unsaturated (g) | 1.7 | 2.1 |
| Poly-unsaturated (g) | 0.6 | 0.6 |
| Fiber (g) | 1.25 | - |
| Vitamin D3 c (µg) | 20 | - |
| Calcium c (mg) | 500 | 0.7 |
References
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of Type 2 Diabetes: Normalisation of Beta Cell Function in Association with Decreased Pancreas and Liver Triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef]
- Steven, S.; Hollingsworth, K.G.; Small, P.K.; Woodcock, S.A.; Pucci, A.; Aribisala, B.; Al-Mrabeh, A.; Daly, A.K.; Batterham, R.L.; Taylor, R. Weight Loss Decreases Excess Pancreatic Triacylglycerol Specifically in Type 2 Diabetes. Diabetes Care 2016, 39, 158–165. [Google Scholar] [CrossRef]
- Steven, S.; Hollingsworth, K.G.; Al-Mrabeh, A.; Avery, L.; Aribisala, B.; Caslake, M.; Taylor, R. Very Low-Calorie Diet and 6 Months of Weight Stability in Type 2 Diabetes: Pathophysiological Changes in Responders and Nonresponders. Diabetes Care 2016, 39, 808–815. [Google Scholar] [CrossRef]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary Care-Led Weight Management for Remission of Type 2 Diabetes (DiRECT): An Open-Label, Cluster-Randomised Trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Buckley, J.D.; Noakes, M.; Clifton, P.M.; Brinkworth, G.D. Comparison of the Effects of Weight Loss from a High-Protein versus Standard-Protein Energy-Restricted Diet on Strength and Aerobic Capacity in Overweight and Obese Men. Eur. J. Nutr. 2013, 52, 317–325. [Google Scholar] [CrossRef]
- Villareal, D.T.; Chode, S.; Parimi, N.; Sinacore, D.R.; Hilton, T.; Armamento-Villareal, R.; Napoli, N.; Qualls, C.; Shah, K. Weight Loss, Exercise, or Both and Physical Function in Obese Older Adults. N. Engl. J. Med. 2011, 364, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef]
- Seok, W.P.; Goodpaster, B.H.; Jung, S.L.; Kuller, L.H.; Boudreau, R.; De Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. Excessive Loss of Skeletal Muscle Mass in Older Adults with Type 2 Diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef]
- Thiebaud, D.; Jacot, E.; DeFronzo, R.A.; Maeder, E.; Jequier, E.; Felber, J.-P.P. The Effect of Graded Doses of Insulin on Total Glucose Uptake, Glucose Oxidation, and Glucose Storage in Man. Diabetes 1982, 31, 957–963. [Google Scholar] [CrossRef]
- Willey, K.A.; Fiatarone Singh, M.A. Battling Insulin Resistance in Elderly Obese People with Type 2 Diabetes: Bring on the Heavy Weights. Diabetes Care 2003, 26, 1580–1588. [Google Scholar] [CrossRef]
- Trouwborst, I.; Verreijen, A.; Memelink, R.; Massanet, P.; Boirie, Y.; Weijs, P.; Tieland, M. Exercise and Nutrition Strategies to Counteract Sarcopenic Obesity. Nutrients 2018, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Frimel, T.N.; Sinacore, D.R.; Villareal, D.T. Exercise Attenuates the Weight-Loss-Induced Reduction in Muscle Mass in Frail Obese Older Adults. Med. Sci. Sports Exerc. 2008, 40, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Verreijen, A.M.; Verlaan, S.; Engberink, M.F.; Swinkels, S.; de Vogel-van den Bosch, J.; Weijs, P.J.M. A High Whey Protein-, Leucine-, and Vitamin D-Enriched Supplement Preserves Muscle Mass during Intentional Weight Loss in Obese Older Adults: A Double-Blind Randomized Controlled Trial. Am. J. Clin. Nutr. 2015, 101, 279–286. [Google Scholar] [CrossRef]
- Memelink, R.; Pasman, W.; Bongers, A.; Tump, A.; van Ginkel, A.; Tromp, W.; Wopereis, S.; Verlaan, S.; de Vogel-van den Bosch, J.; Weijs, P. Effect of a Whey Protein Drink Enriched with Leucine and Vitamin D on Lean Mass and Glycemic Control during a Lifestyle Intervention in Obese Older Adults with (Pre-)Diabetes Type 2: A Double-Blind RCT. Clin. Nutr. 2018, 37, S216–S217. [Google Scholar] [CrossRef]
- Van Ommen, B.; van der Greef, J.; Ordovas, J.M.; Daniel, H. Phenotypic Flexibility as Key Factor in the Human Nutrition and Health Relationship. Genes Nutr. 2014, 9, 1–9. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Insulin Resistance, Lipotoxicity, Type 2 Diabetes and Atherosclerosis: The Missing Links. The Claude Bernard Lecture 2009. Diabetologia 2010, 53, 1270–1287. [Google Scholar] [CrossRef]
- Blanco-Rojo, R.; Alcala-Diaz, J.F.; Wopereis, S.; Perez-Martinez, P.; Quintana-Navarro, G.M.; Marin, C.; Ordovas, J.M.; van Ommen, B.; Perez-Jimenez, F.; Delgado-Lista, J.; et al. The Insulin Resistance Phenotype (Muscle or Liver) Interacts with the Type of Diet to Determine Changes in Disposition Index after 2 Years of Intervention: The CORDIOPREV-DIAB Randomised Clinical Trial. Diabetologia 2016, 59, 67–76. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Matsuda, M.; Balas, B.; DeFronzo, R.A. Muscle and Liver Insulin Resistance Indexes Derived from the Oral Glucose Tolerance Test. Diabetes Care 2007, 30, 89–94. [Google Scholar] [CrossRef]
- Matsuda, M.; Defronzo, R.A. Insulin Sensitivity Indices Obtained From Comparison with the Euglycemic Insulin Clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar]
- Kahn, S.E.; Prigeon, R.L.; Mcculloch, D.K.; Boyko, E.J.; Bergman, R.N.; Schwartz, M.W.; Neifing, J.L.; Ward, W.K.; Beard, J.C.; Palmer, J.P.; et al. Quantification of the Relationship Between Insulin Sensitivity and P-Cell Function in Human Subjects Evidence for a Hyperbolic Function with a Regulated Feedback Loop Control System Such That for Any Difference in S, a Proportionate Reciprocal Difference. Diabetes 1993, 42, 1663–1672. [Google Scholar]
- Ter Horst, K.W.; Van Galen, K.A.; Gilijamse, P.W.; Hartstra, A.V.; De Groot, P.F.; Van Der Valk, F.M.; Ackermans, M.T.; Nieuwdorp, M.; Romijn, J.A.; Serlie, M.J. Methods for Quantifying Adipose Tissue Insulin Resistance in Overweight/Obese Humans. Int. J. Obes. 2017, 41, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.M.; Dalskov, S.; Van Baak, M.; Jebb, S.; Kafatos, A.; Pfeiffer, A.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunešová, M.; Holst, C.; et al. The Diet, Obesity and Genes (Diogenes) Dietary Study in Eight European Countries—A Comprehensive Design for Long-Term Intervention. Obes. Rev. 2010, 11, 76–91. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Perez-Martinez, P.; Garcia-Rios, A.; Alcala-Diaz, J.F.; Perez-Caballero, A.I.; Gomez-Delgado, F.; Fuentes, F.; Quintana-Navarro, G.; Lopez-Segura, F.; Ortiz-Morales, A.M.; et al. CORonary Diet Intervention with Olive Oil and Cardiovascular PREVention Study (the CORDIOPREV Study): Rationale, Methods, and Baseline Characteristics A Clinical Trial Comparing the Efficacy of a Mediterranean Diet Rich in Olive Oil versus a Low-Fat Diet. Am. Heart J. 2016, 177, 42–50. [Google Scholar] [CrossRef]
- Wopereis, S.; Stroeve, J.H.M.; Stafleu, A.; Bakker, G.C.M.; Burggraaf, J.; van Erk, M.J.; Pellis, L.; Boessen, R.; Kardinaal, A.A.F.; van Ommen, B. Multi-Parameter Comparison of a Standardized Mixed Meal Tolerance Test in Healthy and Type 2 Diabetic Subjects: The PhenFlex Challenge. Genes Nutr. 2017, 12, 1–14. [Google Scholar] [CrossRef]
- Van Den Broek, T.J.; Bakker, G.C.M.M.; Rubingh, C.M.; Bijlsma, S.; Stroeve, J.H.M.M.; Van Ommen, B.; Van Erk, M.J.; Wopereis, S. Ranges of Phenotypic Flexibility in Healthy Subjects. Genes Nutr. 2017, 12, 1–14. [Google Scholar] [CrossRef]
- Neeter, C.; Gustavsson, A.; Thomeé, P.; Augustsson, J.; Thomeé, R.; Karlsson, J. Development of a Strength Test Battery for Evaluating Leg Muscle Power after Anterior Cruciate Ligament Injury and Reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 571–580. [Google Scholar] [CrossRef]
- Praet, S.F.E.; Van Loon, L.J.C. Optimizing the Therapeutic Benefits of Exercise in Type 2 Diabetes. J. Appl. Physiol. 2007, 103, 1113–1120. [Google Scholar] [CrossRef]
- Blaak, E.E. Metabolic Fluxes in Skeletal Muscle in Relation to Obesity and Insulin Resistance. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 391–403. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Seelaender, M.; Sotiropoulos, A.; Coletti, D.; Lancha, A.H. Vitamin D, Muscle Recovery, Sarcopenia, Cachexia, and Muscle Atrophy. Nutrition 2019, 60, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, A.; Dave, P.; Lokshin, V.; Bahtiyar, G. Type 2 Diabetes Mellitus, Insulin Resistance, and Vitamin D. Curr. Diabetes Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pramono, A.; Jocken, J.W.E.; Blaak, E.E.; van Baak, M.A. The Effect of Vitamin D Supplementation on Insulin Sensitivity: A Systematic Review and Meta-Analysis. Diabetes Care 2020, 43, 1659–1669. [Google Scholar] [CrossRef]
- Rhoads, R.P.; Baumgard, L.H.; El-Kadi, S.W.; Zhao, L.D. Roles for Insulin-Supported Skeletal Muscle Growth. J. Anim. Sci. 2016, 94, 1791–1802. [Google Scholar] [CrossRef]
- Stokes, T.; Hector, A.J.; Morton, R.W.; McGlory, C.; Phillips, S.M. Recent Perspectives Regarding the Role of Dietary Protein for the Promotion of Muscle Hypertrophy with Resistance Exercise Training. Nutrients 2018, 10, 180. [Google Scholar] [CrossRef]
- Abdulla, H.; Smith, K.; Atherton, P.J.; Idris, I. Role of Insulin in the Regulation of Human Skeletal Muscle Protein Synthesis and Breakdown: A Systematic Review and Meta-Analysis. Diabetologia 2016, 59, 44–55. [Google Scholar] [CrossRef]
- James, H.A.; O’Neill, B.T.; Nair, K.S. Insulin Regulation of Proteostasis and Clinical Implications. Cell Metab. 2017, 26, 310–323. [Google Scholar] [CrossRef]
- Krebs, J.; Hall, R.; Parry Strong, A. Importance of Low Carbohydrate Diets in Diabetes Management. Nutr. Diet. Suppl. 2016, 8, 9. [Google Scholar] [CrossRef]
- Van Zuuren, E.J.; Fedorowicz, Z.; Kuijpers, T.; Pijl, H. Effects of Low-Carbohydrate-Compared with Low-Fat-Diet Interventions on Metabolic Control in People with Type 2 Diabetes: A Systematic Review Including GRADE Assessments. Am. J. Clin. Nutr. 2018, 108, 300–331. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D.; Serra-Majem, L.; Wärnberg, J.; Romaguera, D.; Estruch, R.; et al. Effect of a Lifestyle Intervention Program with Energy-Restricted Mediterranean Diet and Exercise on Weight Loss and Cardiovascular Risk Factors: One-Year Results of the PREDIMED-Plus Trial. Diabetes Care 2019, 42, 777–788. [Google Scholar] [CrossRef]
- Pot, G.K.; Ce Battjes-Fries, M.; Patijn, O.N.; Pijl, H.; Witkamp, R.F.; De Visser, M.; Van Der Zijl, N.; De Vries, M.; Voshol, P.J.; Bolk, L. Nutrition and Lifestyle Intervention in Type 2 Diabetes: Pilot Study in the Netherlands Showing Improved Glucose Control and Reduction in Glucose Lowering Medication. BMJ Nutr. Prev. Health 2019. [Google Scholar] [CrossRef]
- Bouchonville, M.; Armamento-Villareal, R.; Shah, K.; Napoli, N.; Sinacore, D.R.; Qualls, C.; Villareal, D.T. Weight Loss, Exercise or Both and Cardiometabolic Risk Factors in Obese Older Adults: Results of a Randomized Controlled Trial. Int. J. Obes. 2014, 38, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, V.A.; Smart, N.A. Exercise Training for Blood Pressure: A Systematic Review and Meta-Analysis. Journal of the American Heart Association. J. Am. Heart Assoc. 2013. [Google Scholar] [CrossRef]
- Vona, M.; Codeluppi, G.M.; Iannino, T.; Ferrari, E.; Bogousslavsky, J.; Von Segesser, L.K. Effects of Different Types of Exercise Training Followed by Detraining on Endothelium-Dependent Dilation in Patients with Recent Myocardial Infarction. Circulation 2009, 119, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of Exercise to Improve Cardiovascular Health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef]
- Keske, M.A.; Dwyer, R.M.; Russell, R.D.; Blackwood, S.J.; Brown, A.A.; Hu, D.; Premilovac, D.; Richards, S.M.; Rattigan, S. Regulation of Microvascular Flow and Metabolism: An Overview. Clin. Exp. Pharm. Physiol. 2017, 44, 143–149. [Google Scholar] [CrossRef]
- Di Meo, S.; Iossa, S.; Venditti, P. Skeletal Muscle Insulin Resistance: Role of Mitochondria and Other ROS Sources. J. Endocrinol. 2017, 233, R15–R42. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Turner, N. Mitochondrial Dysfunction and Insulin Resistance: An Update. Endocr. Connect. 2015, 4. [Google Scholar] [CrossRef]
- Broskey, N.T.; Greggio, C.; Boss, A.; Boutant, M.; Dwyer, A.; Schlueter, L.; Hans, D.; Gremion, G.; Kreis, R.; Boesch, C.; et al. Skeletal Muscle Mitochondria in the Elderly: Effects of Physical Fitness and Exercise Training. J. Clin. Endocrinol. Metab. 2014, 99, 1852–1861. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Defronzo, R.A. Pathogenesis of Insulin Resistance in Skeletal Muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef]
- van der Kolk, B.W.; Goossens, G.H.; Jocken, J.W.; Blaak, E.E. Altered Skeletal Muscle Fatty Acid Handling Is Associated with the Degree of Insulin Resistance in Overweight and Obese Humans. Diabetologia 2016, 59, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, B.W.; Vogelzangs, N.; Jocken, J.W.E.; Valsesia, A.; Hankemeier, T.; Astrup, A.; Saris, W.H.M.; Arts, I.C.W.; van Greevenbroek, M.M.J.; Blaak, E.E. Plasma Lipid Profiling of Tissue-Specific Insulin Resistance in Human Obesity. Int. J. Obes. 2019, 43, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Trouwborst, I.; Bowser, S.M.; Goossens, G.H.; Blaak, E.E. Ectopic Fat Accumulation in Distinct Insulin Resistant Phenotypes; Targets for Personalized Nutritional Interventions. Front. Nutr. 2018, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E. Characterisation of Fatty Acid Metabolism in Different Insulin-Resistant Phenotypes by Means of Stable Isotopes. Proc. Nutr. Soc. 2017, 76, 419–424. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Blaak, E.E.; van Loon, L.J.C. Lipotoxicity Plays a Key Role in the Development of Both Insulin Resistance and Muscle Atrophy in Patients with Type 2 Diabetes. Obes. Rev. 2019, 20, 1205–1217. [Google Scholar] [CrossRef]
- Taylor, R.; Al-Mrabeh, A.; Zhyzhneuskaya, S.; Peters, C.; Barnes, A.C.; Aribisala, B.S.; Hollingsworth, K.G.; Mathers, J.C.; Sattar, N.; Lean, M.E.J. Erratum: Remission of Human Type 2 Diabetes Requires Decrease in Liver and Pancreas Fat Content but Is Dependent upon Capacity for β Cell Recovery. Cell Metab. 2018, 28, 667. [Google Scholar] [CrossRef]
- Blaak, E.E. Current metabolic perspective on malnutrition in obesity: Towards more subgroup-based nutritional approaches? Proc. Nutr. Soc. 2020, 1–7. [Google Scholar] [CrossRef]


| Index | Formula | Indicator of |
|---|---|---|
| Matsuda Index [19] | 10000/√(fG × fI)(mG x mI) | Systemic insulin sensitivity |
| Disposition Index (DI) [20] | [AUC30min insulin/AUC30min glucose] × Matsuda | β-cell function |
| Hepatic Insulin Resistance Index (HIRI) [18] | fG × fI | Hepatic insulin resistance |
| Muscle Insulin Sensitivity Index (MISI) [18] | (∆G/∆t)/mI | Muscle insulin resistance |
| Adipose Tissue Insulin Resistance Index (Adipo-IR) [21] | Fasting FFA × fI | Adipose tissue insulin resistance |
| MIR + | no-MIR | |||
|---|---|---|---|---|
| Test | Control | Test | Control | |
| Number | 20 | 22 | 20 | 20 |
| Gender (male/female) | 12/8 | 11/11 | 14/6 | 16/4 |
| Age (years) (mean ± SD ++) | 67.3 ± 5.4 | 67.1 ± 5.7 | 68.3 ± 5.6 | 63.6 ± 5.9 |
| T2D @ duration (months); median (IQR #) | 56 (40–100) | 32 (15–51) | 100 (59–162) | 110 (69–132) |
| Diabetes status (T2DM/pre-diabetes) * | 13/7 | 18/4 | 19/1 | 19/1 |
| Use of Metformin (yes/no) | 11/9 | 16/6 | 19/1 | 19/1 |
| Use of SU derivatives (yes/no) | 7/13 | 6/16 | 5/15 | 9/11 |
| Test (n = 20) | Control (n = 22) | Time Effect | Interaction Effect (Time × Treatment) | |||
|---|---|---|---|---|---|---|
| Week 0 | Week 13 | Week 0 | Week 13 | p-value | p-value | |
| Weight (kg) | 98.8 (14.8) | 96.7 (14.4) | 98.3 (16.2) | 95.7 (16.2) | <0.001 | 0.67 |
| Waist circumference (cm) | 115.8 (8.0) | 113.3 (8.6) | 117.2 (10.7) | 112.4 (8.9) | <0.001 | 0.35 |
| BMI (kg/m2) | 34.0 (4.4) | 33.2 (4.2) | 33.6 (4.6) | 32.8 (4.9) | <0.001 | 0.71 |
| Systolic blood pressure (mm Hg) | 144 (20) | 136 (13) | 145 (18) | 138 (11) | 0.02 | 0.87 |
| Diastolic blood pressure (mm Hg) | 86 (10) | 80 (9) | 89 (8) | 85 (7) | 0.05 | 0.62 |
| Fasting plasma glucose (mmol/L) | 7.8 (1.3) | 7.1 (1.1) | 7.4 (1.1) | 7.3 (1.2) | 0.02 ^ | |
| 2hr plasma glucose (mmol/L) | 14.0 (3.4) | 13.5 (3.1) | 13.9 (3.3) | 13.1 (4.1) | 0.10 | 0.64 |
| HbA1c (mmol/mol) | 47.84 (5.77) | 44.60 (4.78) | 46.72 (6.43) | 44.22 (4.87) | 0.04 | 0.24 |
| Fasting plasma insulin (pmol/L) | 135.9 (78.1) | 115.1 (55.9) | 116.6 (36.9) | 126.3 (57.8) | 0.03 ^ | |
| Total cholesterol (mmol/L) | 4.1 (1.0) | 4.1 (1.1) | 4.2 (0.8) | 4.0 (0.7) | 0.24 | 0.56 |
| HDL cholesterol (mmol/L) | 1.14 (0.31) | 1.21 (0.27) | 1.15 (0.23) | 1.19 (0.18) | 0.11 | 0.54 |
| LDL cholesterol (mmol/L) | 2.2 (0.8) | 2.1 (0.8) | 2.3 (0.6) | 2.2 (0.6) | 0.15 | 0.72 |
| Triglycerides (mmol/L) | 1.5 (0.4) | 1.4 (0.3) | 1.5 (0.5) | 1.5 (0.4) | 0.66 | 0.32 |
| Calcidiol (nmol/L) | 56.1 (22.1) | 73.5 (13.8) | 58.1 (17.3) | 55.2 (21.1) | <0.001 ^ | |
| Leucine | 0.20 (0.04) | 0.20 (0.03) | 0.19 (0.04) | 0.19 (0.04) | 0.19 | 0.74 |
| Fat mass (kg) | 36.0 (10.0) | 32.7 (9.0) | 35.3 (8.0) | 32.5 (8.5) | <0.001 | 0.28 |
| VAT (cm2) | 197 (52) | 172 (41) | 189 (51) | 166 (46) | <0.01 | 0.61 |
| ASMM (kg) | 25.4 (5.1) | 26.3 (5.3) | 25.5 (5.5) | 25.5 (5.6) | 0.04 ^ | |
| Matsuda index | 1.63 (0.59) | 2.10 (0.81) | 1.82 (0.69) | 1.71 (0.83) | <0.01 ^ | |
| Disposition index | 1.23 (1.43) | 1.31 (1.44) | 1.44 (2.28) | 1.43 (2.12) | 0.60 | 0.17 |
| Hepatic insulin resistance index | 2868.5 (2113.5) | 2168.8 (1324.8) | 2237.7 (743.3) | 2421.12 (1193.1) | 0.02 ^ | |
| Muscle insulin sensitivity index | −0.41 (0.34) | −1.01 (0.80) | −0.39 (0.32) | −0.83 (0.79) | 0.08 | 0.47 |
| Adipose tissue insulin resistance index | 92.90 (59.20) | 69.13 (39.99) | 75.96 (30.15) | 77.17 (39.70) | 0.04 ^ | |
| 10-RM leg press (kg) | 159 (78) | 213 (73) | 119 (45) | 161 (66) | <0.001 | 0.73 |
| Knee extension power (Watt) | 186 (84) | 210 (69) | 172 (50) | 184 (56) | 0.01 | 0.90 |
| VO2peak (L/min) | 1.68 (0.37) | 1.89 (0.39) | 1.58 (0.33) | 1.83 (0.51) | <0.001 | 0.45 |
| Test (n = 20) | Control (n = 20) | Time Effect | Interaction Effect (Time × Treatment) | |||
|---|---|---|---|---|---|---|
| Week 0 | Week 13 | Week 0 | Week 13 | p-value | p-value | |
| Weight (kg) | 98.6 (15.5) | 96.3 (14.4) | 97.6 (12.4) | 94.6 (12.0) | <0.001 | 0.42 |
| Waist circumference (cm) | 112.4 (12.4) | 108.3 (11.4) | 111.9 (9.0) | 108.5 (9.6) | <0.001 | 0.54 |
| BMI (kg/m2) | 32.2 (4.3) | 31.4 (3.8) | 31.8 (2.5) | 30.8 (2.6) | <0.001 | 0.47 |
| Systolic blood pressure (mm Hg) | 144 (19) | 143 (17) | 141 (16) | 140 (14) | 0.84 | 0.95 |
| Diastolic blood pressure (mm Hg) | 85 (9) | 80 (6) | 86 (9) | 80 (9) | 0.004 | 0.62 |
| Fasting plasma glucose (mmol/L) | 8.3 (1.5) | 7.3 (1.3) | 8.4 (1.9) | 7.8 (1.9) | 0.035 | 0.57 |
| 2hr plasma glucose (mmol/L) | 15.2 (3.5) | 14.2 (3.4) | 17.0 (3.5) | 14.8 (3.2) | 0.001 | 0.23 |
| HbA1c (mmol/mol) | 49.79 (6.62) | 46.86 (5.84) | 53.63 (10.66) | 46.68 (6.09) | . | 0.02 + |
| Fasting plasma insulin (pmol/L) | 85.1 (39.1) | 75.3 (29.8) | 84.0 (30.1) | 81.1 (24.3) | 0.72 | 0.20 |
| Total cholesterol (mmol/L) | 3.9 (0.9) | 3.8 (0.9) | 3.8 (0.7) | 3.8 (0.7) | 0.59 | 0.38 |
| HDL cholesterol (mmol/L) | 1.17 (0.31) | 1.18 (0.25) | 1.07 (0.23) | 1.14 (0.17) | 0.048 | 0.60 |
| LDL cholesterol (mmol/L) | 2.0 (0.7) | 1.9 (0.7) | 2.1 (0.6) | 2.0 (0.7) | 0.29 | 0.83 |
| Triglycerides (mmol/L) | 1.6 (0.6) | 1.4 (0.7) | 1.4 (0.7) | 1.3 (0.5) | 0.50 | 0.30 |
| Calcidiol (nmol/L) | 67.4 (29.3) | 89.3 (17.6) | 59.8 (17.3) | 55.5 (18.3) | <0.01 ^ | |
| Leucine | 0.19 (0.02) | 0.19 (0.03) | 0.19 (0.03) | 0.19 (0.03) | 0.42 | 0.70 |
| Fat mass (kg) | 32.5 (9.9) | 29.7 (9.0) | 30.3 (6.3) | 27.9 (6.4) | <0.001 | 0.51 |
| VAT (cm2) | 181 (73) | 156 (64) | 181 (49) | 158 (50) | <0.001 | 0.36 |
| ASMM (kg) | 26.8 (5.0) | 27.6 (5.3) | 27.9 (4.4) | 27.9 (4.4) | 0.66 | 0.29 |
| Matsuda index | 2.68 (1.23) | 2.93 (1.48) | 2.62 (0.89) | 2.70 (0.66) | 0.66 | 0.35 |
| Disposition index | 0.88 (0.55) | 0.84 (0.66) | 0.71 (0.67) | 0.83 (0.48) | . | 0.045 # |
| Hepatic insulin resistance index | 2012.9 (1216.5) | 1541.5 (752.4) | 1912.0 (829.6) | 1632.5 (558.3) | 0.17 | 0.26 |
| Muscle insulin sensitivity index | −3.25 (2.72) | −2.99 (3.07) | −3.25 (1.72) | −3.83 (2.64) | 0.91 | 0.37 |
| Adipose tissue insulin resistance index | 62.71 (46.29) | 44.52 (21.56) | 53.09 (20.65) | 48.66 (21.61) | 0.21 | 0.58 |
| 10-RM leg press (kg) | 124 (36) | 178 (66) | 148 (67) | 220 (79) | 0.03 * | |
| Knee extension power (Watt) | 184 (48) | 200 (47) | 203 (72) | 229 (74) | 0.01 | 0.98 |
| VO2peak (L/min) | 1.68 (0.50) | 1.76 (0.63) | 2.03 (0.30) | 2.08 (0.45) | 0.60 | 0.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasman, W.J.; Memelink, R.G.; de Vogel-Van den Bosch, J.; Begieneman, M.P.V.; van den Brink, W.J.; Weijs, P.J.M.; Wopereis, S. Obese Older Type 2 Diabetes Mellitus Patients with Muscle Insulin Resistance Benefit from an Enriched Protein Drink during Combined Lifestyle Intervention: The PROBE Study. Nutrients 2020, 12, 2979. https://doi.org/10.3390/nu12102979
Pasman WJ, Memelink RG, de Vogel-Van den Bosch J, Begieneman MPV, van den Brink WJ, Weijs PJM, Wopereis S. Obese Older Type 2 Diabetes Mellitus Patients with Muscle Insulin Resistance Benefit from an Enriched Protein Drink during Combined Lifestyle Intervention: The PROBE Study. Nutrients. 2020; 12(10):2979. https://doi.org/10.3390/nu12102979
Chicago/Turabian StylePasman, Wilrike J., Robert G. Memelink, Johan de Vogel-Van den Bosch, Mark P. V. Begieneman, Willem J. van den Brink, Peter J. M. Weijs, and Suzan Wopereis. 2020. "Obese Older Type 2 Diabetes Mellitus Patients with Muscle Insulin Resistance Benefit from an Enriched Protein Drink during Combined Lifestyle Intervention: The PROBE Study" Nutrients 12, no. 10: 2979. https://doi.org/10.3390/nu12102979
APA StylePasman, W. J., Memelink, R. G., de Vogel-Van den Bosch, J., Begieneman, M. P. V., van den Brink, W. J., Weijs, P. J. M., & Wopereis, S. (2020). Obese Older Type 2 Diabetes Mellitus Patients with Muscle Insulin Resistance Benefit from an Enriched Protein Drink during Combined Lifestyle Intervention: The PROBE Study. Nutrients, 12(10), 2979. https://doi.org/10.3390/nu12102979





