Effect of Organic Food Intake on Nitrogen Stable Isotopes
Abstract
1. Introduction
2. Materials and Methods
2.1. 15N Natural Isotopic Abundance of Organic Plants
2.2. Effect of Organic Plant Protein Intake on Body 15N Natural Isotopic Abundance
3. Results
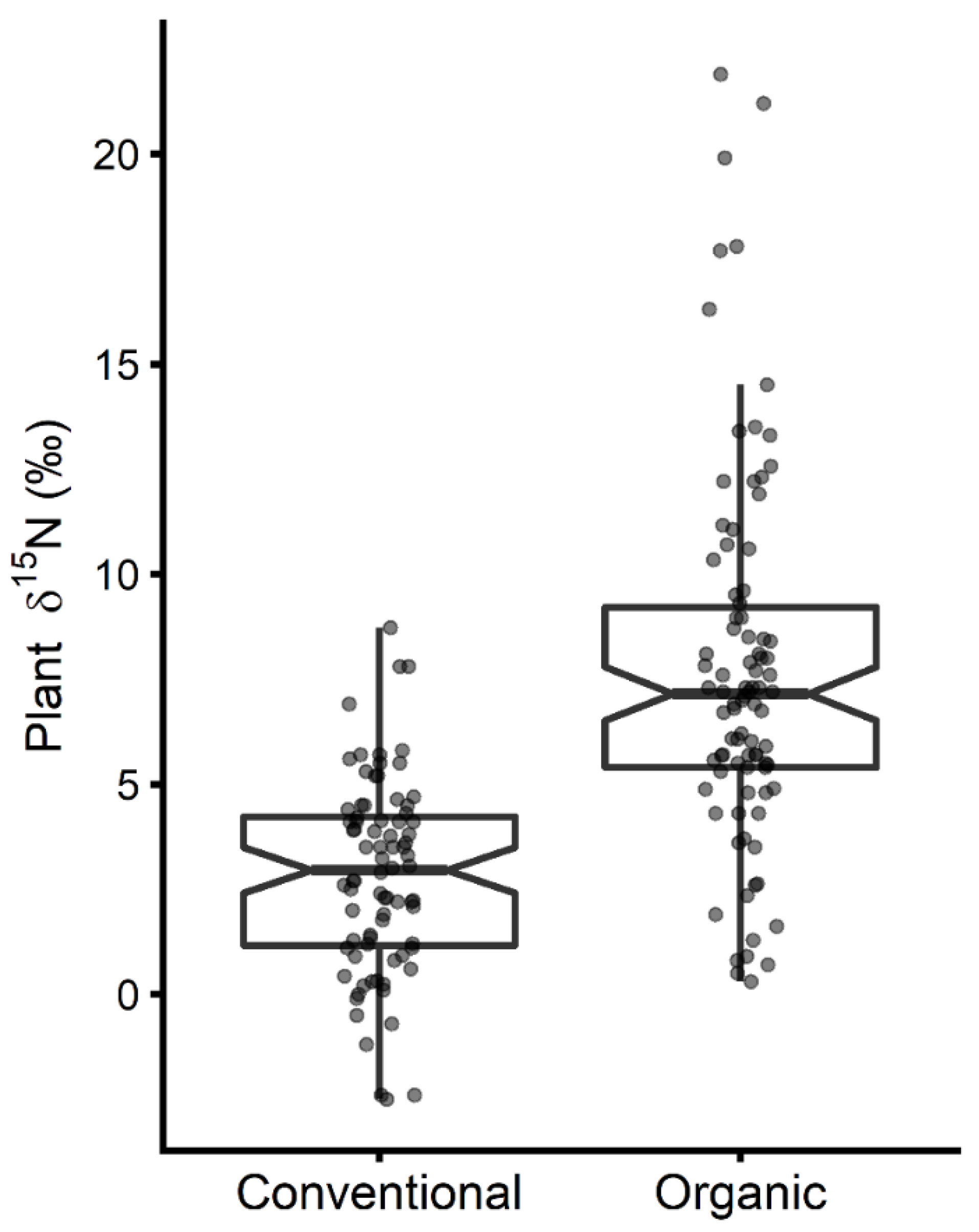
3.1. 15N Natural Isotopic Abundance of Organic Plants
3.2. Effect of Organic Plant Protein Intake on Body 15N Isotopic Abundance
4. Discussion
4.1. Organic Farming Impacts Plant Nitrogen Isotopic Composition
4.2. Organic Plant Intake Affects the Performance of δ15N as a Biomarker
4.3. Animal Protein Sources Affect the Performance of δ15N as a Biomarker
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Votruba, S.B.; Shaw, P.A.; Oh, E.J.; Venti, C.A.; Bonfiglio, S.; Krakoff, J.; O’Brien, D.M. Associations of plasma, RBCs, and hair carbon and nitrogen isotope ratios with fish, meat, and sugar-sweetened beverage intake in a 12-wk inpatient feeding study. Am. J. Clin. Nutr. 2019, 110, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.Y.; Lampe, J.W.; Tinker, L.F.; Neuhouser, M.L.; Beresford, S.A.; Niles, K.R.; Mossavar-Rahmani, Y.; Snetselaar, L.G.; Van Horn, L.; Prentice, R.L. Serum nitrogen and carbon stable isotope ratios meet biomarker criteria for fish and animal protein intake in a controlled feeding study of a Women’s Health Initiative Cohort. J. Nutr. 2018, 148, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Petzke, K.J.; Boeing, H.; Klaus, S.; Metges, C.C. Carbon and nitrogen stable isotopic composition of hair protein and amino acids can be used as biomarkers for animal-derived dietary protein intake in humans. J. Nutr. 2005, 135, 1515–1520. [Google Scholar] [CrossRef]
- Kuhnle, G.G.; Joosen, A.M.; Kneale, C.J.; O’Connell, T.C. Carbon and nitrogen isotopic ratios of urine and faeces as novel nutritional biomarkers of meat and fish intake. Eur. J. Nutr. 2013, 52, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.S.; Cooper, A.J.; O’Connell, T.C.; Kuhnle, G.G.; Kneale, C.K.; Mulligan, A.M.; Luben, R.N.; Brage, S.; Khaw, K.-T.; Wareham, N.J. Serum carbon and nitrogen stable isotopes as potential biomarkers of dietary intake and their relation with incident type 2 diabetes: The EPIC-Norfolk study. Am. J. Clin. Nutr. 2014, 100, 708–718. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.M. Stable isotope ratios as biomarkers of diet for health research. Annu. Rev. Nutr. 2015, 35, 565–594. [Google Scholar] [CrossRef]
- Macko, S.A.; Estep, M.L.F.; Engel, M.H.; Hare, P. Kinetic fractionation of stable nitrogen isotopes during amino acid transamination. Geochim. Cosmochim. Acta 1986, 50, 2143–2146. [Google Scholar] [CrossRef]
- Mantha, O.L.; Polakof, S.; Huneau, J.F.; Mariotti, F.; Poupin, N.; Zalko, D.; Fouillet, H. Early changes in tissue amino acid metabolism and nutrient routing in rats fed a high-fat diet: Evidence from natural isotope abundances of nitrogen and carbon in tissue proteins. Br. J. Nutr. 2018, 119, 981–991. [Google Scholar] [CrossRef]
- Huneau, J.-F.; Mantha, O.L.; Hermier, D.; Mathé, V.; Galmiche, G.; Mariotti, F.; Fouillet, H. Natural Isotope Abundances of Carbon and Nitrogen in Tissue Proteins and Amino Acids as Biomarkers of the Decreased Carbohydrate Oxidation and Increased Amino Acid Oxidation Induced by Caloric Restriction under a Maintained Protein Intake in Obese Rats. Nutrients 2019, 11, 1087. [Google Scholar] [CrossRef]
- Martinelli, L.A.; Nardoto, G.B.; Perez, M.A.; Junior, G.A.; Fracassi, F.C.; Oliveira, J.G.; Ottani, I.S.; Lima, S.H.; Mazzi, E.A.; Gomes, T.F. Carbon and Nitrogen Isotope Ratios of Food and Beverage in Brazil. Molecules 2020, 25, 1457. [Google Scholar] [CrossRef]
- Huelsemann, F.; Koehler, K.; Braun, H.; Schaenzer, W.; Flenker, U. Human dietary δ15N intake: Representative data for principle food items. Am. J. Phys. Anthropol. 2013, 152, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-J.; Kwak, J.-H.; Lim, S.-S.; Park, H.-J.; Chang, S.X.; Lee, S.-M.; Arshad, M.A.; Yun, S.-I.; Kim, H.-Y. Synthetic fertilizer and livestock manure differently affect δ15N in the agricultural landscape: A review. Agric. Ecosyst. Environ. 2017, 237, 1–15. [Google Scholar] [CrossRef]
- Bateman, A.S.; Kelly, S.D. Fertilizer nitrogen isotope signatures. Isot. Environ. Health Stud. 2007, 43, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Inácio, C.T.; Chalk, P.M.; Magalhães, A.M. Principles and limitations of stable isotopes in differentiating organic and conventional foodstuffs: 1. Plant products. Crit. Rev. Food Sci. Nutr. 2015, 55, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.S.; Kelly, S.D.; Jickells, T.D. Nitrogen isotope relationships between crops and fertilizer: Implications for using nitrogen isotope analysis as an indicator of agricultural regime. J. Agric. Food Chem. 2005, 53, 5760–5765. [Google Scholar] [CrossRef]
- Willer, H.; Lernoud, J. The World of Organic Agriculture. Statistics and Emerging Trends 2019; Research Institute of Organic Agriculture (FiBL): Frick, Switzerland; IFOAM–Organics International: Bonn, Germany, 2019; ISBN 3-03736-119-0. [Google Scholar]
- Poupin, N.; Bos, C.; Mariotti, F.; Huneau, J.-F.; Tomé, D.; Fouillet, H. The nature of the dietary protein impacts the tissue-to-diet 15N discrimination factors in laboratory rats. PLoS ONE 2011, 6, e28046. [Google Scholar] [CrossRef][Green Version]
- Poupin, N.; Mariotti, F.; Huneau, J.-F.; Hermier, D.; Fouillet, H. Natural isotopic signatures of variations in body nitrogen fluxes: A compartmental model analysis. PLoS Comput. Biol. 2014, 10, e1003865. [Google Scholar] [CrossRef]
- Del Amor, F.M.; Navarro, J.; Aparicio, P.M. Isotopic discrimination as a tool for organic farming certification in sweet pepper. J. Environ. Qual. 2008, 37, 182–185. [Google Scholar] [CrossRef]
- Rogers, K.M. Nitrogen isotopes as a screening tool to determine the growing regimen of some organic and nonorganic supermarket produce from New Zealand. J. Agric. Food Chem. 2008, 56, 4078–4083. [Google Scholar] [CrossRef]
- Verenitch, S.; Mazumder, A. Isotopic characterization as a screening tool in authentication of organic produce commercially available in western North America. Isot. Environ. Health Stud. 2015, 51, 332–343. [Google Scholar] [CrossRef]
- Camilleri, G.M.; Verger, E.O.; Huneau, J.-F.; Carpentier, F.; Dubuisson, C.; Mariotti, F. Plant and animal protein intakes are differently associated with nutrient adequacy of the diet of French adults. J. Nutr. 2013, 143, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.M.; Egnell, M.; Huneau, J.-F.; Mariotti, F. Plant protein intake and dietary diversity are independently associated with nutrient adequacy in French adults. J. Nutr. 2016, 146, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Agarwal, S.; Lieberman, H.R.; Fulgoni, V.L. Sources and amounts of animal, dairy, and plant protein intake of US adults in 2007–2010. Nutrients 2015, 7, 7058–7069. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-I.; Lim, S.-S.; Lee, G.-S.; Lee, S.-M.; Kim, H.-Y.; Ro, H.-M.; Choi, W.-J. Natural 15 N abundance of paddy rice (Oryza sativa L.) grown with synthetic fertilizer, livestock manure compost, and hairy vetch. Biol. Fertil. Soils 2011, 47, 607–617. [Google Scholar] [CrossRef]
- Laursen, K.H.; Mihailova, A.; Kelly, S.; Epov, V.; Bérail, S.; Schjørring, J.K.; Donard, O.; Larsen, E.H.; Pedentchouk, N.; Marca-Bell, A. Is it really organic?—Multi-isotopic analysis as a tool to discriminate between organic and conventional plants. Food Chem. 2013, 141, 2812–2820. [Google Scholar] [CrossRef]
- Schmidt, H.; Roßmann, A.; Voerkelius, S.; Schnitzler, W.H.; Georgi, M.; Graßmann, J.; Zimmermann, G.; Winkler, R. Isotope characteristics of vegetables and wheat from conventional and organic production. Isot. Environ. Health Stud. 2005, 41, 223–228. [Google Scholar] [CrossRef]
- Inácio, C.T.; Chalk, P.M. Principles and limitations of stable isotopes in differentiating organic and conventional foodstuffs: 2. Animal products. Crit. Rev. Food Sci. Nutr. 2017, 57, 181–196. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
L. Mantha, O.; Laxmi Patel, M.; Hankard, R.; De Luca, A. Effect of Organic Food Intake on Nitrogen Stable Isotopes. Nutrients 2020, 12, 2965. https://doi.org/10.3390/nu12102965
L. Mantha O, Laxmi Patel M, Hankard R, De Luca A. Effect of Organic Food Intake on Nitrogen Stable Isotopes. Nutrients. 2020; 12(10):2965. https://doi.org/10.3390/nu12102965
Chicago/Turabian StyleL. Mantha, Olivier, Maya Laxmi Patel, Régis Hankard, and Arnaud De Luca. 2020. "Effect of Organic Food Intake on Nitrogen Stable Isotopes" Nutrients 12, no. 10: 2965. https://doi.org/10.3390/nu12102965
APA StyleL. Mantha, O., Laxmi Patel, M., Hankard, R., & De Luca, A. (2020). Effect of Organic Food Intake on Nitrogen Stable Isotopes. Nutrients, 12(10), 2965. https://doi.org/10.3390/nu12102965




