Circulating Levels of the Soluble Receptor for AGE (sRAGE) during Escalating Oral Glucose Dosages and Corresponding Isoglycaemic i.v. Glucose Infusions in Individuals with and without Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Experimental Procedures
2.3. Assessment of Serum RAGE Concentrations
2.4. Statistical Methods
3. Results
3.1. Glucose Homeostasis during OGTT and IIGI
3.2. At baseline, sRAGE Concentrations were not Related to Anthropometric Characteristics or Measures of Glucose Homeostasis
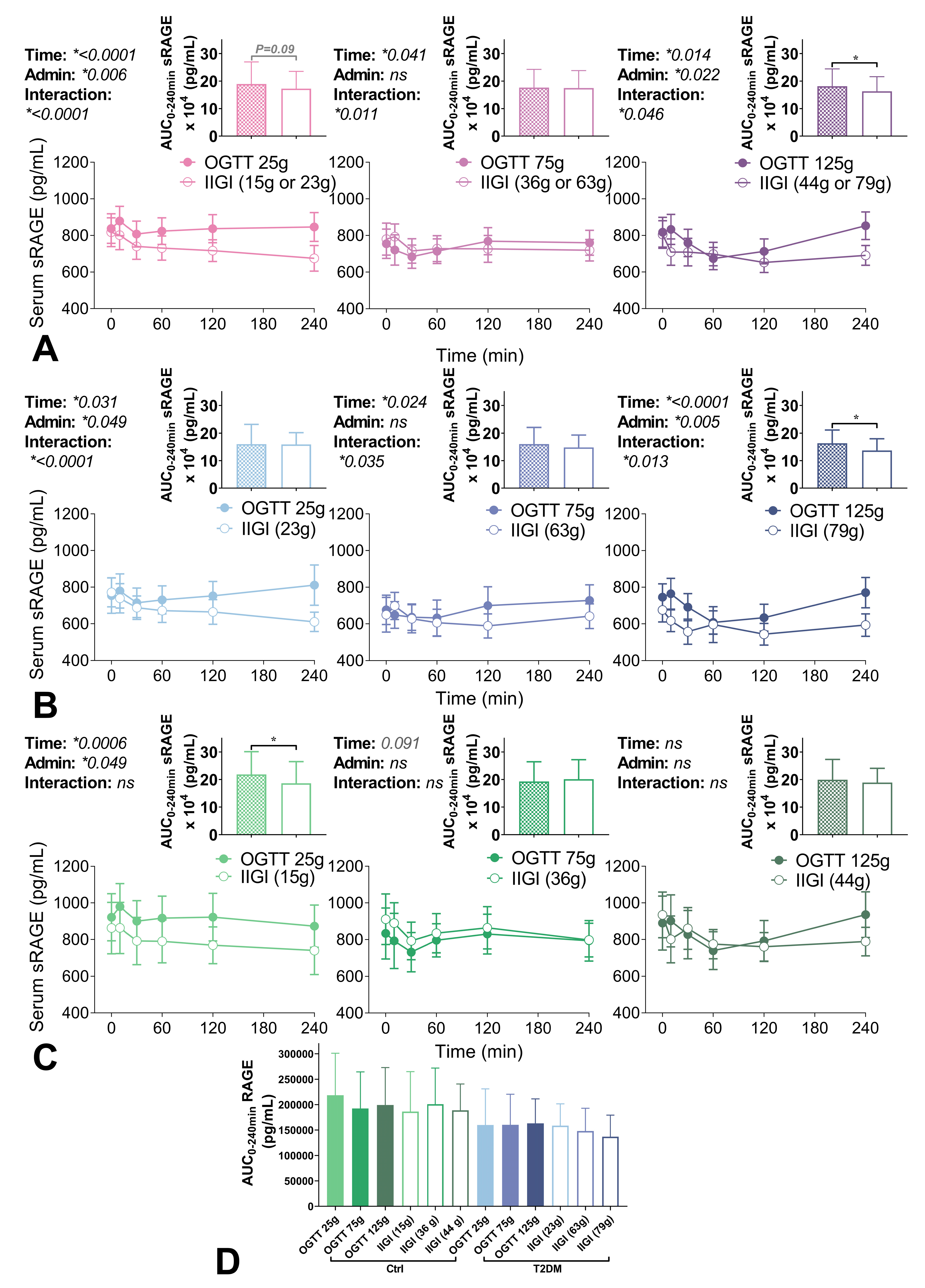
3.3. Circulating sRAGE Concentrations Following Glucose Administration
4. Discussion
4.1. Baseline Correlations
4.2. Temporal and Net Changes in sRAGE Differ Between Oral Ingestion and Infusion of Glucose
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deo, P.; Keogh, J.B.; Price, N.J.; Clifton, P.M. Effects of Weight Loss on Advanced Glycation End Products in Subjects with and without Diabetes: A Preliminary Report. Int. J. Environ. Res. Public Health 2017, 14, 1553. [Google Scholar] [CrossRef] [PubMed]
- Kilhovd, B.K.; Juutilainen, A.; Lehto, S.; Rönnemaa, T.; Torjesen, P.A.; Hanssen, K.F.; Laakso, M. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: A population-based 18 year follow-up study. Diabetologia 2007, 50, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.J.; Beulens, J.W.J.; van Dieren, S.; Scheijen, J.L.J.M.; van der A, D.L.; Spijkerman, A.M.W.; van der Schouw, Y.T.; Stehouwer, C.D.A.; Schalkwijk, G. Plasma Advanced Glycation End Products Are Associated With Incident Cardiovascular Events in Individuals With Type 2 Diabetes: A Case-Cohort Study With a Median Follow-up of 10 Years (EPIC-NL). Diabetes 2015, 64, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Nin, J.W.; Jorsal, A.; Ferreira, I.; Schalkwijk, C.G.; Prins, M.H.; Parving, H.-H.; Tarnow, L.; Rossing, P.; Stehouwer, C.D. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: A 12-year follow-up study. Diabetes Care 2011, 34, 442–447. [Google Scholar] [CrossRef]
- Gorst, C.; Kwok, C.S.; Aslam, S.; Buchan, I.; Kontopantelis, E.; Myint, P.K.; Heatlie, G.; Loke, Y.; Rutter, M.K.; Mamas, M.A. Long-term Glycemic Variability and Risk of Adverse Outcomes: A Systematic Review and Meta-analysis. Diabetes Care 2015, 38, 2354–2369. [Google Scholar] [CrossRef]
- Xie, J.; Méndez, J.D.; Méndez-Valenzuela, V.; Aguilar-Hernández, M.M. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell. Signal. 2013, 25, 2185–2197. [Google Scholar] [CrossRef]
- Colhoun, H.M.; Betteridge, D.J.; Durrington, P.; Hitaman, G.; Neil, A.; Livingstone, S.; Charlton-Menys, V.; Bao, W.; DeMicco, D.A.; Preston, G.M.; et al. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: An analysis from the CARDS trial. Diabetes 2011, 60, 2379–2385. [Google Scholar] [CrossRef]
- Fujisawa, K.; Katakami, N.; Kaneto, H.; Naka, T.; Takahara, M.; Sakamoto, F.; Irie, Y.; Miyashita, K.; Kubo, F.; Yasuda, T.; et al. Circulating soluble RAGE as a predictive biomarker of cardiovascular event risk in patients with type 2 diabetes. Atherosclerosis 2013, 227, 425–428. [Google Scholar] [CrossRef]
- Holst, J.J.; Gribble, F.; Horowitz, M.; Rayner, C.K. Roles of the Gut in Glucose Homeostasis. Diabetes Care 2016, 39, 884–892. [Google Scholar] [CrossRef]
- Nauck, M.A.; Homberger, E.; Siegel, E.G.; Allen, R.C.; Eaton, R.P.; Ebert, R.; Creutzfeldt, W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J. Clin. Endocrinol. Metab. 1986, 63, 492–498. [Google Scholar] [CrossRef]
- Bagger, J.I.; Knop, F.K.; Lund, A.; Vestergaard, H.; Holst, J.J.; Vilsbøll, T. Impaired regulation of the incretin effect in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2011, 96, 737–745. [Google Scholar] [PubMed]
- Nauck, M.; Stöckmann, F.; Ebert, R.; Creutzfeldt, W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986, 29, 46–52. [Google Scholar] [PubMed]
- Miranda, E.R.; Fuller, K.N.Z.; Perkins, R.K.; Beisswenger, P.J.; Farabi, S.S.; Quinn, L.; Haus, J.M. Divergent Changes in Plasma AGEs and sRAGE Isoforms Following an Overnight Fast in T1DM. Nutrients 2019, 11, 386. [Google Scholar] [CrossRef]
- Ojima, A.; Ishibashi, Y.; Matsui, T.; Maeda, S.; Nishino, Y.; Takeuchi, M.; Fukami, K.; Yamagishi, S. Glucagon-Like Peptide-1 Receptor Agonist Inhibits Asymmetric Dimethylarginine Generation in the Kidney of Streptozotocin-Induced Diabetic Rats by Blocking Advanced Glycation End Product–Induced Protein Arginine Methyltranferase-1 Expression. Am. J. Pathol. 2013, 182, 132–141. [Google Scholar] [PubMed]
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochem. Biophys. Res. Commun. 2010, 391, 1405–1408. [Google Scholar]
- Ishibashi, Y.; Nishino, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Glucagon-like peptide–1 suppresses advanced glycation end product–induced monocyte chemoattractant protein–1 expression in mesangial cells by reducing advanced glycation end product receptor level. Metabolism 2011, 60, 1271–1277. [Google Scholar] [PubMed]
- Bagger, J.I.; Knop, F.K.; Lund, A.; Holst, J.J.; Vilsbøll, T. Glucagon responses to increasing oral loads of glucose and corresponding isoglycaemic intravenous glucose infusions in patients with type 2 diabetes and healthy individuals. Diabetologia 2014, 57, 1720–1725. [Google Scholar]
- Basta, G.; Sironi, A.M.; Lazzerini, G.; Del Turco, S.; Buzzigoli, E.; Casolaro, A.; Natali, A.; Ferrannini, E.; Gastaldelli, A. Circulating soluble receptor for advanced glycation end products is inversely associated with glycemic control and S100A12 protein. J. Clin. Endocrinol. Metab. 2006, 91, 4628–4634. [Google Scholar]
- Tan, K.C.B.; Shiu, S.W.; Chow, W.S.; Leng, L.; Bucala, R.; Betteridge, D.J. Association between serum levels of soluble receptor for advanced glycation end products and circulating advanced glycation end products in type 2 diabetes. Diabetologia 2006, 49, 2756–2762. [Google Scholar]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating Glycotoxins and Dietary Advanced Glycation Endproducts: Two Links to Inflammatory Response, Oxidative Stress, and Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar]
- Sebeková, K.; Somoza, V.; Jarcusková, M.; Heidland, A.; Podracká, L. Plasma advanced glycation end products are decreased in obese children compared with lean controls. Int. J. Pediatr. Obes. 2009, 4, 112–118. [Google Scholar] [PubMed]
- Yamagishi, S.; Adachi, H.; Nakamura, K.; Matsui, T.; Jinnouchi, Y.; Takenaka, K.; Takeuchi, M.; Enomoto, M.; Furuki, K.; Hino, A.; et al. Positive association between serum levels of advanced glycation end products and the soluble form of receptor for advanced glycation end products in nondiabetic subjects. Metabolism 2006, 55, 1227–1231. [Google Scholar]
- Brinkley, T.E.; Leng, X.; Nicklas, B.J.; Kritchevsky, S.B.; Ding, J.; Kitzman, D.W.; Hundley, W.G. Racial differences in circulating levels of the soluble receptor for advanced glycation endproducts in middle-aged and older adults. Metabolism 2017, 70, 98–106. [Google Scholar]
- Semba, R.D.; Arab, L.; Sun, K.; Nicklett, E.J.; Ferrucci, L. Fat Mass Is Inversely Associated with Serum Carboxymethyl-Lysine, An Advanced Glycation End Product, in Adults. J. Nutr. 2011, 141, 1726–1730. [Google Scholar] [PubMed]
- Gaens, K.H.; Ferreira, I.; van de Waarenburg, M.P.; van Greevenbroek, M.M.; van der Kallen, C.J.; Dekker, J.M.; Nijpels, G.; Rensen, S.S.; Stehouwer, C.D.; Schalkwijk, C.G. Protein-Bound Plasma Nepsilon-(Carboxymethyl)lysine Is Inversely Associated With Central Obesity and Inflammation and Significantly Explain a Part of the Central Obesity-Related Increase in Inflammation: The Hoorn and CODAM Studies. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2707–2713. [Google Scholar] [PubMed]
- Gaens, K.H.; Goossens, G.H.; Niessen, P.M.; van Greevenbroek, M.M.; van der Kallen, C.J.; Niessen, H.W.; Rensen, S.S.; Buurman, W.A.; Greve, J.W.M.; Blaak, E.E.; et al. Nepsilon-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1199–1208. [Google Scholar] [PubMed]
- Cohen, M.J.; Carles, M.; Brohi, K.; Calfee, C.S.; Rahn, P.; Call, M.S.; Chesebro, B.B.; West, M.A.; Pittet, J.F. Early Release of soluble RAGE After Severe Trauma in Humans. J. Trauma 2010, 68, 1273–1278. [Google Scholar]
- Jensen, L.J.N.; Lindberg, S.; Hoffmann, S.; Iversen, A.Z.; Pedersen, S.H.; Mogelvang, R.; Galatius, S.; Flyvbjerg, A.; Jensen, J.S.; Bjerre, M. Dynamic changes in sRAGE levels and relationship with cardiac function in STEMI patients. Clin. Biochem. 2015, 48, 297–301. [Google Scholar]
- Joly, P.; Masse, C.; Dwivedi, D.; Liaw, P.; Marshall, J.; Berthiaume, Y.; Charbonney, E. DNA and sRAGE circulation in the early phase after polytrauma. Crit. Care 2015, 19, 314. [Google Scholar]
- Uhle, F.; Lichtenstern, C.; Brenner, T.; Fleming, T.; Koch, C.; Hecker, A.; Heiss, C.; Nawroth, P.P.; Hofer, S.; Weigand, M.A.; et al. Role of the RAGE Axis during the Immune Response after Severe Trauma: A Prospective Pilot Study. Mediat. Inflamm. 2015, 2015, 691491. [Google Scholar] [CrossRef]
- Meertens, J.H.; Nienhuis, H.L.; Lefrandt, J.D.; Schalkwijk, C.G.; Nyyssonen, K.; Ligtenberg, J.J.M.; Smit, A.J.; Zijlstra, J.G.; Mulder, D.J. The course of skin and serum biomarkers of advanced glycation endproducts and its association with oxidative stress, inflammation, disease severity, and mortality during ICU admission in critically Ill patients: Results from a prospective pilot study. PLoS ONE 2016, 11, e0160893. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sato, E.; Fujiwara, N.; Kawagoe, Y.; Suzuki, T.; Ueda, Y.; Yamada, S.; Shoji, H.; Takeuchi, M.; Ueda, S.; et al. Circulating levels of advanced glycation end products (AGE) and interleukin-6 (IL-6) are independent determinants of serum asymmetric dimethylarginine (ADMA) levels in patients with septic shock. Pharmacol. Res. 2009, 60, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Schulman, C.I.; Uribarri, J.; Cai, W.; Manning, R.; Landy, D.C.; Gallardo, M.; Castillo, Á.; Namias, N.; Striker, G.E.; Livingstone, A.; et al. Increased circulating advanced glycation endproducts (AGEs) in acute trauma patients. Clin. Chem. Lab. Med. 2014, 52, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.K.; Miranda, E.R.; Karstoft, K.; Beisswenger, P.J.; Solomon, T.P.J.; Haus, J.M. Experimental Hyperglycemia Alters Circulating Concentrations and Renal Clearance of Oxidative and Advanced Glycation End Products in Healthy Obese Humans. Nutrients 2019, 11, 532. [Google Scholar] [CrossRef]
- Roggerio, A.; Strunz, C.M.C.; Pacanaro, A.P.; Leal, D.P.; Takada, J.Y.; Avakian, S.D.; Mansur, A.D.P. Gene Expression of Sirtuin-1 and Endogenous Secretory Receptor for Advanced Glycation End Products in Healthy and Slightly Overweight Subjects after Caloric Restriction and Resveratrol Administration. Nutrients 2018, 10, 937. [Google Scholar] [CrossRef]
- Brett, J.; Schmidt, A.M.; Du Yan, S.; Zou, Y.S.; Weidman, E.; Pinsky, D.; Nowygrod, R.; Neeper, M.; Przysiecki, C.; Shaw, A.; et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am. J. Pathol. 1993, 143, 1699–1712. [Google Scholar]
- Puddu, A.; Mach, F.; Nencioni, A.; Viviani, G.L.; Montecucco, F. An Emerging Role of Glucagon-Like Peptide-1 in Preventing Advanced-Glycation-End-Product-Mediated Damages in Diabetes. Mediat. Inflamm. 2013, 2013, 591056. [Google Scholar] [CrossRef]
- Yamagishi, S.-I.; Fukami, K.; Matsui, T. Crosstalk between advanced glycation end products (AGEs)-receptor RAGE axis and dipeptidyl peptidase-4-incretin system in diabetic vascular complications. Cardiovasc. Diabetol. 2015, 14, 1–12. [Google Scholar] [CrossRef]
- Nguyen, D.-V.; Linderholm, A.; Haczku, A.; Kenyon, N. Obesity-related, metabolic asthma: A new role for glucagon-like peptide 1 agonists. Lancet Respir. Med. 2017, 5, 162–164. [Google Scholar] [CrossRef]
- Kajikawa, M.; Nakashima, A.; Fujimura, N.; Maruhashi, T.; Iwamoto, Y.; Iwamoto, A.; Matsumoto, T.; Oda, N.; Hidaka, T.; Kihara, Y.; et al. Ratio of Serum Levels of AGEs to Soluble Form of RAGE Is a Predictor of Endothelial Function. Diabetes Care 2015, 38, 119–125. [Google Scholar] [CrossRef]
- Yu, Y.; Hanssen, K.F.; Kalyanaraman, V.; Chirindel, A.; Jenkins, A.J.; Nankervis, A.J.; Torjesen, P.A.; Scholz, H.; Henriksen, T.; Lorentzen, B.; et al. Reduced soluble receptor for advanced glycation end-products (sRAGE) scavenger capacity precedes pre-eclampsia in Type 1 diabetes. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- De Courten, B.; De Courten, M.P.; Soldatos, G.; Dougherty, S.L.; Straznicky, N.; Schlaich, M.; Sourris, K.C.; Chand, V.; Scheijen, J.L.; Kingwell, B.A.; et al. Diet low in advanced glycation end products increases insulin sensitivity in healthy overweight individuals: A double-blind, randomized, crossover trial. Am. J. Clin. Nutr. 2016, 103, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Woodward, M.; Neal, B.; Li, Q.; Pickering, R.; Marre, M.; Williams, B.; Perkovic, V.; Cooper, M.E.; Zoungas, S.; et al. Relationship Between Levels of Advanced Glycation End Products and Their Soluble Receptor and Adverse Outcomes in Adults With Type 2 Diabetes. Diabetes Care 2015, 38, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
| No Diabetes (Control) n = 8 | Type 2 Diabetes n = 8 | p Value | Baseline Associations to sRAGE, r, p | |||
|---|---|---|---|---|---|---|
| Control a | Type 2 Diabetes a | All b | ||||
| Sex (M:F) | 3:5 | 3:5 | ns | - | - | - |
| Age (years) | 56.5 ± 11.4 | 57.0 ± 11.9 | ns | 0.124 ns | −0.050 ns | 0.025 ns |
| BMI (kg/m2) | 28.9 ± 2.1 | 29.5 ± 3.2 | ns | −0.212 ns | −0.024 ns | −0.150 ns |
| Waist circumference (cm) | 101.8 ± 12.40 | 105.0 ± 10.18 | ns | −0.425 ns | −0.3631 ns | −0.451 0.08 |
| Systolic Blood Pressure (mmHg) | 125.9 ± 14.08 | 131.4 ± 14.73 | ns | 0.078 ns | 0.240 ns | 0.089 ns |
| HbA1C (%) | 5.4 ± 0.3 | 7.0 ± 0.7 | <0.001 | −0.488 ns | 0.266 ns | −0.293 ns |
| Duration of diabetes (months) | - | 8.4 ± 11.8 | - | - | 0.609 ns | - |
| Plasma glucose t = 120 mins (mmol/L) | 6.0 ± 0.6 | 16.0 ± 2.7 | <0.001 | 0.455 ns | −0.496 ns | −0.147 ns |
| Fasting plasma insulin (mmol/L) | 56.42 ± 20.93 | 86.34 ± 39.51 | ns | −0.072 ns | −0.207 ns | −0.260 ns |
| Peak C-peptide (75 g OGTT) | 3.839 ± 0.90 | 2.798 ± 0.93 | 0.039 | −0.391 ns | −0.188 ns | −0.100 ns |
| Fasting serum sRAGE (pg/mL) | 892.2 ± 371.1 | 712.0 ± 222.1 | ns | - | - | - |
| AUC0–240 sRAGE (ng/mL) | ||||
|---|---|---|---|---|
| Controls | Type 2 Diabetes | p Value | All | |
| 25 g OGTT | 218.44 ± 82.79 | 159.92 ± 71.18 | ns | 181.89 ± 80.47 |
| Matched IIGI | 186.29 ± 78.61 | 158.73 ± 42.90 | ns | 172.81 ± 62.81 |
| 75 g OGTT | 192.50 ± 71.90 | 160.26 ± 60.27 | ns | 176.38 ± 66.22 |
| Matched IIGI | 200.99 ± 71.07 | 148.03 ± 44.94 | 0.09 | 174.51 ± 63.62 |
| 125 g OGTT | 199.34 ± 73.68 | 163.10 ± 48.29 | ns | 181.22 ± 63.02 |
| Matched IIGI | 188.85 ± 51.84 | 136.99 ± 42.45 | * 0.046 | 162.92 ± 53.03 |
| Intra-group variation OGTT (p value) | ns | ns | ns | |
| Intra-group variation IIGI (p value) | ns | * 0.0033 p(trend) < 0.001 r2 = 0.045 | ns | |
| Controls | Type 2 Diabetes | All | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Insulin | Glucagon | GLP1 | GIP | Glucose | Insulin | Glucagon | GLP1 | GIP | Glucose | Insulin | Glucagon | GLP1 | GIP | |
| 25 g OGTT | 0.095 ns b | 0.143 ns b | 0.095 ns b | −0.071 ns b | 0.524 ns b | −0.338 ns a | 0.088 ns a | −0.085 ns a | −0.604 ns a | 0.334 ns a | −0.359 ns b | 0.15 ns b | −0.233 ns b | −0.444 0.087 b | 0.365 ns b |
| Matched IIGI | 0.503 ns b | 0.214 ns b | −0.119 ns b | −0.047 ns b | 0.539 ns b | −0.388 ns a | −0.530 ns a | −0.224 ns a | −0.705 0.051 a | −0.0126 ns a | −0.003 ns b | −0.227 ns b | −0.191 ns b | −0.327 ns b | 0.225 ns b |
| 75 g OGTT | 0.65 0.08 a | 0.376 ns a | 0.17 ns a | 0.119 ns a | 0.205 ns a | −0.143 ns b | −0.262 ns b | −0.238 ns b | −0.881 * 0.007 b | −0.095 ns b | −0.112 ns b | 0.026 ns b | −0.212 ns b | −0.194 ns b | 0.076 ns b |
| Matched IIGI | −0.024 ns b | 0.286 ns b | −0.071 ns b | −0.405 ns b | 0.619 ns b | 0.043 ns a | −0.096 ns a | 0.038 ns a | −0.795 * 0.018 a | 0.205 ns a | −0.353 ns b | −0.065 ns b | −0.171 ns b | −0.615 * 0.013 b | 0.285 ns b |
| 125 g OGTT | 0.674 ns a | 0.403 ns a | 0.322 ns a | −0.390 ns a | 0.204 ns a | −0.238 ns b | −0.381 ns b | −0.524 ns b | −0.310 ns b | 0.167 ns b | −0.241 ns b | 0.082 ns b | −0.365 ns b | −0.335 ns b | 0.165 ns b |
| Matched IIGI | 0.238 ns b | −0.024 ns b | 0.00 ns b | −0.286 ns b | 0.238 ns b | 0.061 ns a | −0.178 ns a | −0.204 ns a | −0.538 ns a | 0.249 ns a | −0.356 ns b | −0.185 ns b | −0.268 ns b | −0.371 ns b | 0.131 ns b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotheringham, A.K.; Bagger, J.I.; Borg, D.J.; McCarthy, D.A.; Holst, J.J.; Vilsbøll, T.; Knop, F.K.; Forbes, J.M. Circulating Levels of the Soluble Receptor for AGE (sRAGE) during Escalating Oral Glucose Dosages and Corresponding Isoglycaemic i.v. Glucose Infusions in Individuals with and without Type 2 Diabetes. Nutrients 2020, 12, 2928. https://doi.org/10.3390/nu12102928
Fotheringham AK, Bagger JI, Borg DJ, McCarthy DA, Holst JJ, Vilsbøll T, Knop FK, Forbes JM. Circulating Levels of the Soluble Receptor for AGE (sRAGE) during Escalating Oral Glucose Dosages and Corresponding Isoglycaemic i.v. Glucose Infusions in Individuals with and without Type 2 Diabetes. Nutrients. 2020; 12(10):2928. https://doi.org/10.3390/nu12102928
Chicago/Turabian StyleFotheringham, Amelia K., Jonatan I. Bagger, Danielle J. Borg, Domenica A. McCarthy, Jens J. Holst, Tina Vilsbøll, Filip K. Knop, and Josephine M. Forbes. 2020. "Circulating Levels of the Soluble Receptor for AGE (sRAGE) during Escalating Oral Glucose Dosages and Corresponding Isoglycaemic i.v. Glucose Infusions in Individuals with and without Type 2 Diabetes" Nutrients 12, no. 10: 2928. https://doi.org/10.3390/nu12102928
APA StyleFotheringham, A. K., Bagger, J. I., Borg, D. J., McCarthy, D. A., Holst, J. J., Vilsbøll, T., Knop, F. K., & Forbes, J. M. (2020). Circulating Levels of the Soluble Receptor for AGE (sRAGE) during Escalating Oral Glucose Dosages and Corresponding Isoglycaemic i.v. Glucose Infusions in Individuals with and without Type 2 Diabetes. Nutrients, 12(10), 2928. https://doi.org/10.3390/nu12102928






