Short Communication: Oral Administration of Heat-killed Lactobacillus brevis KB290 in Combination with Retinoic Acid Provides Protection against Influenza Virus Infection in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Feeding Solution Preparation
2.3. Feeding Intervention
2.4. Influenza Virus
2.5. IAV Challenge
2.6. Bodyweight, Body Temperature, and General Health Score
2.7. Viral Titre
2.8. Statistical Analysis
2.8.1. Bodyweight Analysis
2.8.2. Viral Titre
3. Results
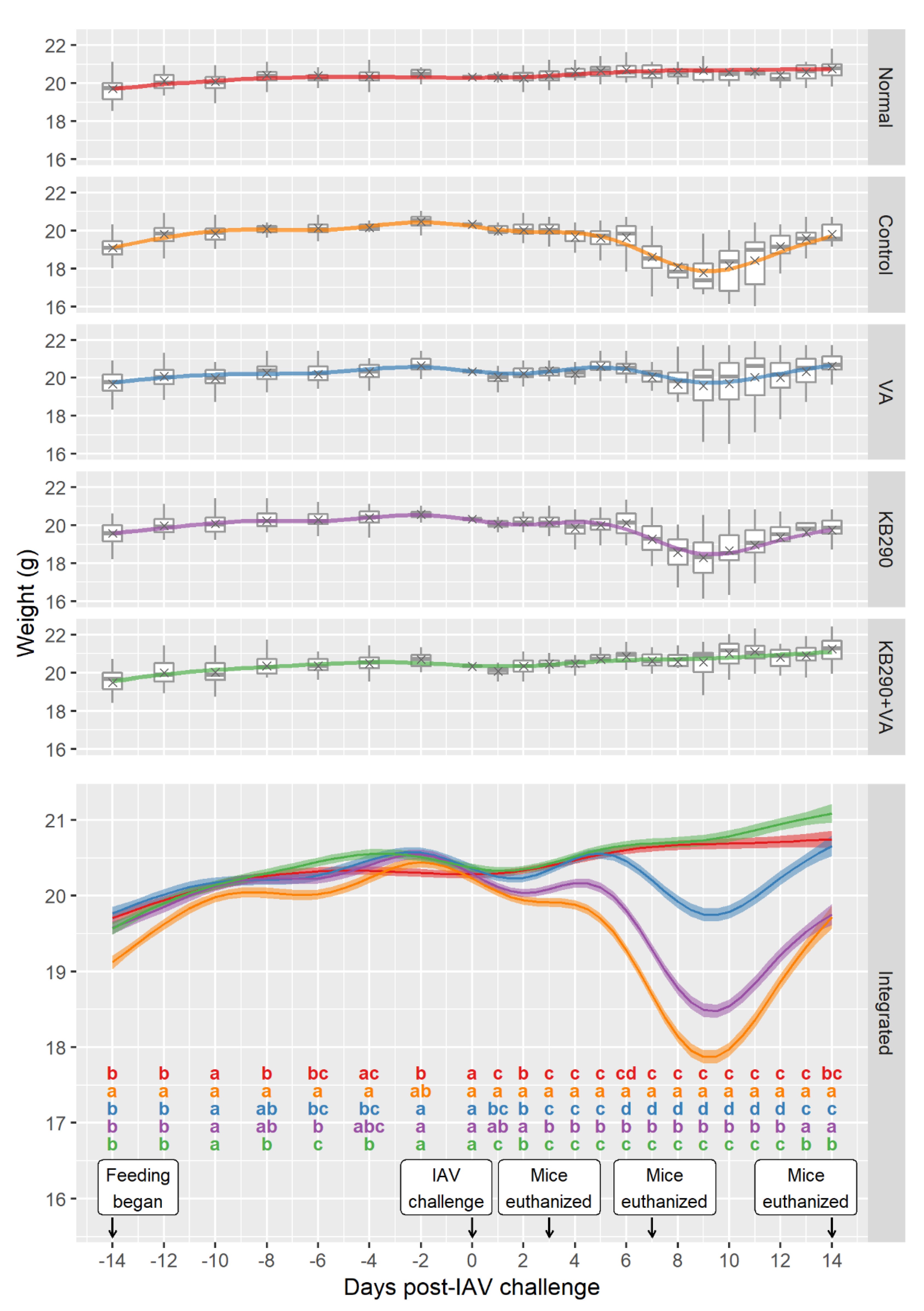
3.1. Bodyweight, General Health Score, and Body Temperature Changes
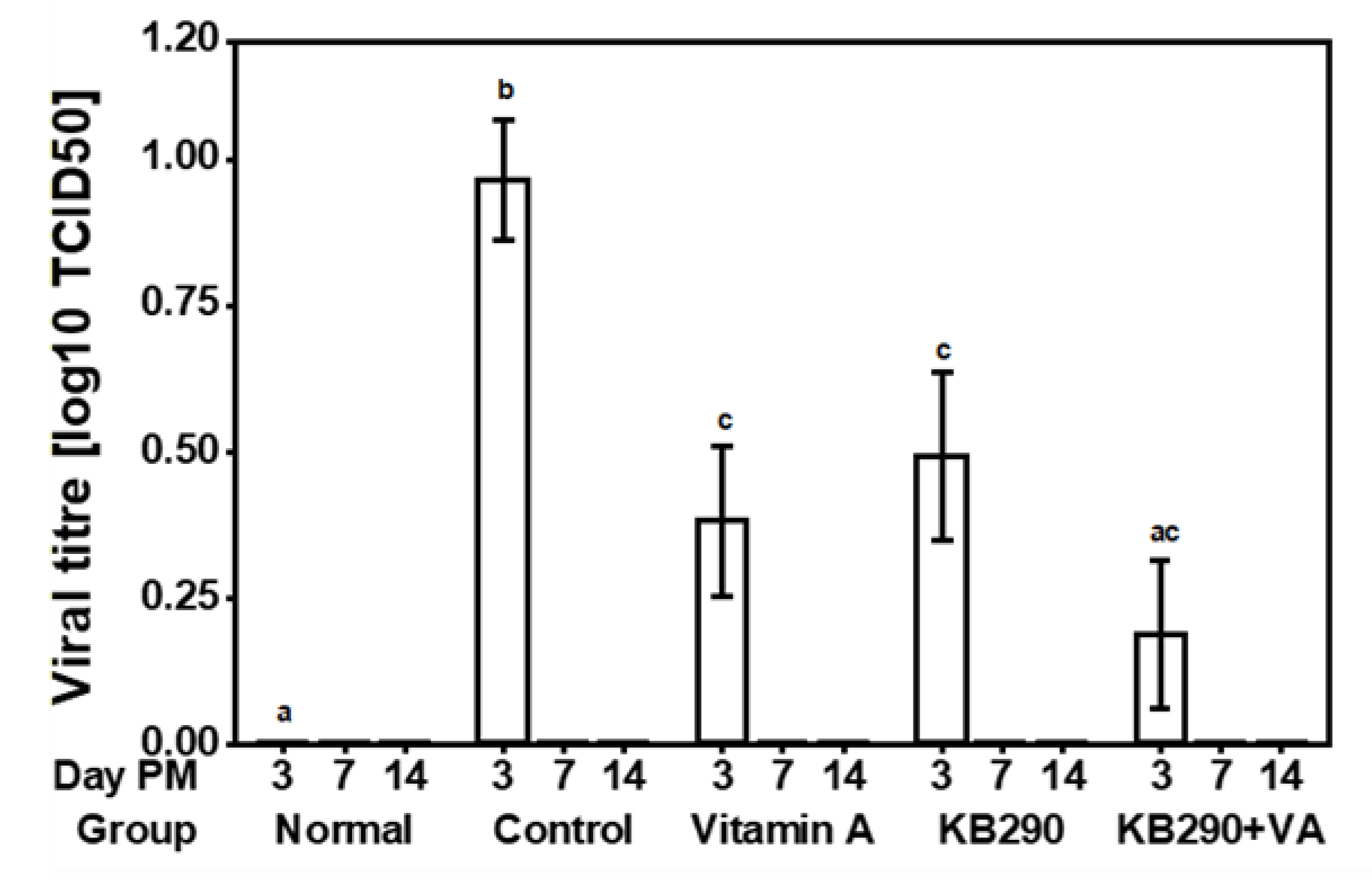
3.2. Viral Titre
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 18 March 2020).
- Guillemard, E.; Tondu, F.; Lacoin, F.; Schrezenmeir, J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br. J. Nutr. 2010, 103, 58–68. [Google Scholar] [CrossRef]
- Gasparini, R.; Bonanni, P.; Amicizia, D.; Bella, A.; Donatelli, I.; Cristina, M.L.; Panatto, D.; Luigi Lai, P. Influenza epidemiology in Italy two years after the 2009–2010 pandemic need to improve vaccination coverage. Hum. Vaccin Immunother. 2013, 9, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Takeshita, M.; Kikuchi, Y.; Dashnyam, B.; Kawahara, S.; Yoshida, H.; Watanabe, W.; Muguruma, M.; Kurokawa, M. Efficacy of oral administration of heat-killed probiotics from Mongolian dairy products against influenza infection in mice: Alleviation of influenza infection by its immunomodulatory activity through intestinal immunity. Int. Immunopharmacol. 2011, 11, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.-N.; Lee, D.-H.; Lee, Y.-N.; Park, J.-K.; Yuk, S.-S.; Yang, S.-Y.; Lee, H.-J.; Woo, S.-H.; Kim, H.-M.; Lee, J.-B.; et al. Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antivir. Res. 2012, 93, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; He, F.; Kubota, A.; Yoda, K.; Miyazawa, K.; Hiramatsu, M. Heat-killed Lactobacillus gasseri TMC0356 protects mice against influenza virus infection by stimulating gut and respiratory immune responses. FEMS Immunol. Med. Microbiol. 2012, 64, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Kechaou, N.; Chain, F.; Gratadoux, J.-J.; Blugeon, S.; Bertho, N.; Chevalier, C.; Le Goffic, R.; Courau, S.; Molimard, P.; Chatel, J.M.; et al. Identification of one novel candidate probiotic Lactobacillus plantarum strain active against influenza virus infection in mice by a large-scale screening. Appl. Environ. Microbiol. 2013, 79, 1491–1499. [Google Scholar] [CrossRef]
- Kiso, M.; Takano, R.; Sakabe, S.; Katsura, H.; Shinya, K.; Uraki, R.; Watanabe, S.; Saito, H.; Toba, M.; Kohda, N.; et al. Protective efficacy of orally administered, heat-killed Lactobacillus pentosus b240 against Influenza A virus. Sci. Rep. 2013, 3, 1563. [Google Scholar] [CrossRef]
- Lee, Y.-N.; Youn, H.-N.; Kwon, J.-H.; Lee, D.-H.; Park, J.-K.; Yuk, S.-S.; Erdene-Ochir, T.-O.; Kim, K.-T.; Lee, J.-B.; Park, S.-Y.; et al. Sublingual administration of Lactobacillus rhamnosus affects respiratory immune responses and facilitates protection against Influenza virus infection in mice. Antivir. Res. 2013, 98, 284–290. [Google Scholar] [CrossRef]
- Park, M.-K.; Ngo, V.; Kwon, Y.-M.; Lee, Y.-T.; Yoo, S.; Cho, Y.-H.; Hong, S.-M.; Hwang, H.S.; Ko, E.-J.; Jung, Y.-J.; et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS ONE 2013, 8, e75368. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.I.; Bae, J.-Y.; Yoo, K.; Kim, H.; Kim, I.-H.; Park, M.-S.; Lee, I. Effects of heat-killed Lactobacillus plantarum against Influenza viruses in mice. J. Microbiol. 2018, 56, 145–149. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Kunitoh-Asari, A.; Hayakawa, K.; Imai, S.; Kasuya, K.; Abe, K.; Adachi, Y.; Fukudome, S.-I.; Takahashi, Y.; Hachimura, S. Oral administration of Lactobacillus plantarum strain AYA enhances IgA secretion and provides survival protection against influenza virus infection in mice. PLoS ONE 2014, 9, e86416. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Moriya, T.; Sakai, F.; Ikeda, N.; Shiozaki, T.; Hosoya, T.; Nakagawa, H.; Miyazaki, T. Oral administration of Lactobacillus gasseri SBT2055 is effective for preventing influenza in mice. Sci. Rep. 2014, 4, 4638. [Google Scholar] [CrossRef] [PubMed]
- Waki, N.; Yajima, N.; Suganuma, H.; Buddle, B.M.; Luo, D.; Heiser, A.; Zheng, T. Oral administration of Lactobacillus brevis KB290 to mice alleviates clinical symptoms following influenza virus infection. Lett. Appl. Microbiol. 2014, 58, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Belkacem, N.; Serafini, N.; Wheeler, R.; Derrien, M.; Boucinha, L.; Couesnon, A.; Cerf-Bensussan, N.; Gomperts Boneca, I.; Di Santo, J.P.; Taha, M.-K.; et al. Lactobacillus paracasei feeding improves immune control of influenza infection in mice. PLoS ONE 2017, 12, e0184976. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Kim, J.I.; Park, S.; Yoo, K.; Kim, I.-H.; Joo, W.; Ryu, B.H.; Park, M.S.; Lee, I.; Park, M.-S. Effects of Lactobacillus plantarum and Leuconostoc mesenteroides probiotics on Human Seasonal and Avian Influenza Viruses. J. Microbiol Biotechnol. 2018, 28, 893–901. [Google Scholar] [CrossRef]
- Takahashi, E.; Sawabuchi, T.; Kimoto, T.; Sakai, S.; Kido, H. Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 feeding enhances humoral immune responses, which are suppressed by the antiviral neuraminidase inhibitor oseltamivir in influenza A virus-infected mice. J. Dairy Sci. 2019, 102, 9559–9569. [Google Scholar] [CrossRef]
- Ishibashi, N.; Yamazaki, S. Probiotics and safety. Am. J. Clin. Nutr. 2001, 73, 465s–470s. [Google Scholar] [CrossRef]
- Liong, M.-T. Safety of probiotics: Translocation and infection. Nutr. Rev. 2008, 66, 192–202. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Forssten, S.; Hibberd, A.A.; Lyra, A.; Stahl, B. Probiotic approach to prevent antibiotic resistance. Ann. Med. 2016, 48, 246–255. [Google Scholar] [CrossRef]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The Genus Enterococcus: Between probiotic potential and safety concerns-an update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health benefits of Heat-Killed (Tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hirose, Y.; Yamamoto, Y.; Yoshikai, Y.; Murosaki, S. Daily intake of heat-killed Lactobacillus plantarum L-137 improves inflammation and lipid metabolism in overweight healthy adults: A randomized-controlled trial. Eur. J. Nutr. 2019. [Google Scholar] [CrossRef]
- Warda, A.K.; Rea, K.; Fitzgerald, P.; Hueston, C.; Gonzalez-Tortuero, E.; Dinan, T.G.; Hill, C. Heat-killed lactobacilli alter both microbiota composition and behaviour. Behav. Brain Res. 2019, 362, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Russell, W.M.; Douglas-Escobar, M.; Hauser, N.; Lopez, M.; Neu, J. Live and Heat-Killed Lactobacillus rhamnosus GG: Effects on proinflammatory and anti-inflammatory Cytokines/Chemokines in gastrostomy-fed infant rats. Pediatr. Res. 2009, 66, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.-X.; Chang, B.; Wang, B.-Y.; Liu, W.-X.; Jiang, M. Live and heat-killed probiotic: Effects on chronic experimental colitis induced by dextran sulfate sodium (DSS) in rats. Int. J. Clin. Exp. Med. 2015, 8, 20072–20078. [Google Scholar]
- Ciandrini, E.; Campana, R.; Baffone, W. Live and heat-killed Lactobacillus spp. interfere with Streptococcus mutans and Streptococcus oralis during biofilm development on titanium surface. Arch. Oral Biol. 2017, 78, 48–57. [Google Scholar] [CrossRef]
- Penkert, R.R.; Jones, B.G.; Häcker, H.; Partridge, J.F.; Hurwitz, J.L. Vitamin A differentially regulates cytokine expression in respiratory epithelial and macrophage cell lines. Cytokine 2017, 91, 1–5. [Google Scholar] [CrossRef]
- Nobuta, Y.; Inoue, T.; Suzuki, S.; Arakawa, C.; Yakabe, T.; Ogawa, M.; Yajima, N. The efficacy and the safety of Lactobacillus brevis KB290 as a human probiotic. Int. J. Probio. Prebio. 2009, 4, 263–270. [Google Scholar]
- Murakami, K.; Habukawa, C.; Nobuta, Y.; Moriguchi, N.; Takemura, T. The effect of Lactobacillus brevis KB290 against irritable bowel syndrome: A placebo-controlled double-blind crossover trial. Biopsychosoc. Med. 2012, 6, 16. [Google Scholar] [CrossRef]
- Kishi, A.; Uno, K.; Matsubara, Y.; Okuda, C.; Kishida, T. Effect of the oral administration of Lactobacillus brevis subsp. coagulans on interferon-alpha producing capacity in humans. J. Am. Coll. Nutr. 1996, 15, 408–412. [Google Scholar] [CrossRef]
- Fukui, Y.; Sasaki, E.; Fuke, N.; Nakai, Y.; Ishijima, T.; Abe, K.; Yajima, N. Effect of Lactobacillus brevis KB290 on the cell-mediated cytotoxic activity of mouse splenocytes: A DNA microarray analysis. Br. J. Nutr. 2013, 2, 1–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Waki, N.; Matsumoto, M.; Fukui, Y.; Suganuma, H. Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among schoolchildren: An open-label pilot study. Lett. Appl. Microbiol. 2014, 59, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Long, K.Z.; García, C.; Santos, J.I.; Rosado, J.L.; Hertzmark, E.; DuPont, H.L.; Ko, G. Vitamin A supplementation has divergent effects on norovirus infections and clinical symptoms among Mexican children. J. Infect. Dis. 2007, 196, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Long, K.Z.; Garcıa, C.; Ko, G.; Santos, J.I.; Al Mamun, A.; Rosado, J.L.; DuPont, H.L.; Nathakumar, N. Vitamin A modifies the intestinal chemokine and cytokine responses to norovirus infection in Mexican children. J. Nutr. 2011, 141, 957–963. [Google Scholar] [CrossRef]
- Hall, J.A.; Cannons, J.L.; Grainger, J.R.; Dos Santos, L.M.; Hand, T.W.; Naik, S.; Wohlfert, E.A.; Chou, D.B.; Oldenhove, G.; Robinson, M.; et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity 2011, 34, 435–447. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Teixeira, F.; Sato, M.N. Impact of retinoic acid on immune cells and inflammatory diseases. Mediat. Inflamm. 2018, 2018, 3067126. [Google Scholar] [CrossRef]
- Svensson, M.; Johansson-Lindbom, B.; Zapata, F.; Jaensson, E.; Austenaa, L.M.; Blomhoff, R.; Agace, W.W. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 2008, 1, 38–48. [Google Scholar] [CrossRef]
- Zeng, R.; Oderup, C.; Yuan, R.; Lee, M.; Habtezion, A.; Hadeiba, H.; Butcher, E.C. Retinoic acid regulates the development of a gut-homing precursor for intestinal dendritic cells. Mucosal Immunol. 2013, 6, 847–856. [Google Scholar] [CrossRef]
- Bakdash, G.; Vogelpoel, L.T.; van Capel, T.M.; Kapsenberg, M.L.; de Jong, E.C. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015, 8, 265–278. [Google Scholar] [CrossRef]
- Matsumiya, T.; Stafforini, D.M. Function and regulation of retinoic acid-inducible gene-I. Crit. Rev. Immunol. 2010, 30, 489–513. [Google Scholar] [CrossRef]
- Lee, H.; Ko, G. Antiviral effect of vitamin A on norovirus infection via modulation of the gut microbiome. Sci. Rep. 2016, 6, 25835. [Google Scholar] [CrossRef] [PubMed]
- Surman, S.L.; Jones, B.G.; Sealy, R.E.; Rudraraju, R.; Hurwitz, J.L. Oral retinyl palmitate or retinoic acid corrects mucosal IgA responses toward an intranasal influenza virus vaccine in vitamin A deficient mice. Vaccine 2014, 32, 2521–2524. [Google Scholar] [CrossRef] [PubMed]
- Cottey, R.; Rowe, C.A.; Bender, B.S. Influenza Virus. Curr. Protoc. Immunol. 2001, 42, 19.11.1–19.11.32. [Google Scholar] [CrossRef] [PubMed]
- Eisfeld, A.J.; Neumann, G.; Kawaoka, Y. Influenza a virus isolation, culture and identification. Nat. Protoc. 2014, 9, 2663–2681. [Google Scholar] [CrossRef]
- Mei, J.; Riedel, N.; Grittner, U.; Endres, M.; Banneke, S.; Emmrich, J.V. Body temperature measurement in mice during acute illness: Implantable temperature transponder versus surface infrared thermometry. Sci. Rep. 2018, 8, 3526. [Google Scholar] [CrossRef]
- Kawase, M.; He, F.; Kubota, A.; Harata, G.; Hiramatsu, M. Oral administration of lactobacilli from human intestinal tract protects mice against influenza virus infection. Lett. Appl. Microbiol. 2010, 51, 6–10. [Google Scholar] [CrossRef]
- Barfod, K.K.; Roggenbuck, M.; Hansen, L.H.; Schjørring, S.; Larsen, S.T.; Sørensen, S.J.; Krogfelt, K.A. The murine lung microbiome in relation to the intestinal and vaginal bacterial communities. BMC Microbiol. 2013, 13, 303. [Google Scholar] [CrossRef]
- Pritzl, C.J.; Seo, Y.-J.; Xia, C.; Vijayan, M.; Stokes, Z.D.; Hahm, B. A ceramide analogue stimulates dendritic cells to promote T cell responses upon virus infections. J. Immunol. 2015, 194, 4339–4349. [Google Scholar] [CrossRef]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Lenth, R.; Buerkner, P.; Herve, M.; Love, J.; Riebl, H.; Singmann, H. Emmeans: Estimated Marginal Means, aka Least-Squares Means, R Package Version 1.4.8. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 21 September 2020).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Drummond, G.B.; Vowler, S.L. Different tests for a difference: How do we do research? J. Physiol. 2012, 590, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Ludbrook, J.; Dudley, H. Why permutation tests are superior to T and F tests in biomedical research. Am. Stat. 1998, 52, 127–132. [Google Scholar] [CrossRef]
- Maxime, H. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R Package Version 0.9-77. 2020. Available online: https://CRAN.R-project.org/package=RVAideMemoire (accessed on 21 September 2020).
- Sun, K.; Torres, L.; Metzger, D.W. A detrimental effect of Interleukin-10 on protective pulmonary humoral immunity during primary Influenza A virus infection. J. Virol. 2010, 84, 5007–5014. [Google Scholar] [CrossRef] [PubMed]
- Langlois, R.A.; Legge, K.L. Plasmacytoid dendritic cells enhance mortality during lethal influenza infections by eliminating virus specific CD8 T Cells. J. Immunol. 2010, 184, 4440–4446. [Google Scholar] [CrossRef]
- Pang, I.K.; Pillai, P.S.; Iwasaki, A. Efficient influenza A virus replication in the respiratory tract requires signals from TLR7 and RIG-I. Proc. Natl. Acad. Sci. USA 2013, 110, 13910–13915. [Google Scholar] [CrossRef]
- Keef, E.; Zhang, L.A.; Swigon, D.; Urbano, A.; Ermentrout, G.B.; Matuszewski, M.; Toapanta, F.R.; Ross, T.M.; Parker, R.S.; Clermont, G. Discrete dynamical modeling of influenza virus infection suggests age-dependent differences in immunity. J. Virol. 2017, 91, e00395–e00417. [Google Scholar] [CrossRef]
- Brandes, M.; Klauschen, F.; Kuchen, S.; Germain, R.N. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell 2013, 154, 197–212. [Google Scholar] [CrossRef]
- Kumova, O.K.; Fike, A.J.; Thayer, J.L.; Nguyen, L.T.; Mell, J.C.; Pascasio, J.; Stairiker, C.; Leon, L.G.; Katsikis, P.D.; Carey, A.J. Lung transcriptional unresponsiveness and loss of early influenza virus control in infected neonates is prevented by intranasal Lactobacillus rhamnosus GG. PLoS Pathog. 2019, 15, e1008072. [Google Scholar] [CrossRef]
- Goto, H.; Sagitani, A.; Ashida, N.; Kato, S.; Hirota, T.; Shinoda, T.; Yamamoto, N. Anti-influenza virus effects of both live and non-live Lactobacillus acidophilus L-92 accompanied by the activation of Innate immunity. Br. J. Nutr. 2013, 110, 1810–1818. [Google Scholar] [CrossRef]
- Jung, Y.-J.; Lee, Y.-T.; Ngo, V.L.; Cho, Y.-H.; Ko, E.-J.; Hong, S.-M.; Kim, K.-H.; Jang, J.-H.; Oh, J.-S.; Park, M.-K.; et al. Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci. Rep. 2017, 7, 17360. [Google Scholar] [CrossRef]
- Nagai, T.; Makino, S.; Ikegami, S.; Itoh, H.; Yamada, H. Effects of oral administration of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 and its exopolysaccharides against influenza virus infection in mice. Int. Immunopharmacol. 2011, 11, 2246–2250. [Google Scholar] [CrossRef] [PubMed]
- Zelaya, H.; Tada, A.; Vizoso-Pinto, M.G.; Salva, S.; Kanmani, P.; Aguero, G.; Alvarez, S.; Kitazawa, H.; Villena, J. Nasal priming with immunobiotic Lactobacillus rhamnosus modulates inflammation-coagulation interactions and reduces influenza virus-associated pulmonary damage. Inflamm. Res. 2015, 64, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, E.; Suzuki, S.; Fukui, Y.; Yajima, N. Cell-bound exopolysaccharides of Lactobacillus brevis KB290 enhance cytotoxic activity of mouse splenocytes. J. Appl. Microbiol. 2015, 118, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Pino-Lagos, K.; Guo, Y.; Noelle, R.J. Retinoic acid: A key player in immunity. Biofactors 2010, 36, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Hathcock, J.N.; Hattan, D.G.; Jenkins, M.Y.; McDonald, J.T.; Sundaresan, P.R.; Wilkening, V.L. Evaluation of Vitamin A toxicity. Am. J. Clin. Nutr. 1990, 52, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Gerald, Y.M.; James, K.K.; Wei-Sek, H. Vitamin A hepatotoxicity in multiple family members. Hepatology 1988, 8, 272–275. [Google Scholar] [CrossRef]
- Anroop, B.N.; Shery, J. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- National Institute of Health (NIH). Vitamin A. Available online: https://ods.od.nih.gov/factsheets/VitaminA-Consumer/ (accessed on 14 September 2020).
- Lobo, G.P.; Hessel, S.; Eichinger, A.; Noy, N.; Moise, A.R.; Wyss, A.; Palczewski, K.; Lintig, J.V. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta, beta-carotene absorption and vitamin A production. Fed. Am. Soc. Exp. Biol. J. 2010, 24, 1656–1666. [Google Scholar] [CrossRef]
- Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Meyskens, F.L., Jr.; Omenn, G.S.; Valanis, B.; Williams, J.H., Jr. The Beta-Carotene and Retinol Efficacy Trial: Incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping Beta-Carotene and Retinol Supplements. J. Natl. Cancer Inst. 2004, 96, 1743–1750. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satomi, S.; Khanum, S.; Miller, P.; Suzuki, S.; Suganuma, H.; Heiser, A.; Gupta, S.K. Short Communication: Oral Administration of Heat-killed Lactobacillus brevis KB290 in Combination with Retinoic Acid Provides Protection against Influenza Virus Infection in Mice. Nutrients 2020, 12, 2925. https://doi.org/10.3390/nu12102925
Satomi S, Khanum S, Miller P, Suzuki S, Suganuma H, Heiser A, Gupta SK. Short Communication: Oral Administration of Heat-killed Lactobacillus brevis KB290 in Combination with Retinoic Acid Provides Protection against Influenza Virus Infection in Mice. Nutrients. 2020; 12(10):2925. https://doi.org/10.3390/nu12102925
Chicago/Turabian StyleSatomi, Shohei, Sofia Khanum, Poppy Miller, Shigenori Suzuki, Hiroyuki Suganuma, Axel Heiser, and Sandeep K Gupta. 2020. "Short Communication: Oral Administration of Heat-killed Lactobacillus brevis KB290 in Combination with Retinoic Acid Provides Protection against Influenza Virus Infection in Mice" Nutrients 12, no. 10: 2925. https://doi.org/10.3390/nu12102925
APA StyleSatomi, S., Khanum, S., Miller, P., Suzuki, S., Suganuma, H., Heiser, A., & Gupta, S. K. (2020). Short Communication: Oral Administration of Heat-killed Lactobacillus brevis KB290 in Combination with Retinoic Acid Provides Protection against Influenza Virus Infection in Mice. Nutrients, 12(10), 2925. https://doi.org/10.3390/nu12102925




