Vitamin and Mineral Supplementation Practices in Preterm Infants: A Survey of Australian and New Zealand Neonatal Intensive and Special Care Units
Abstract
1. Introduction
2. Materials and Methods
3. Results
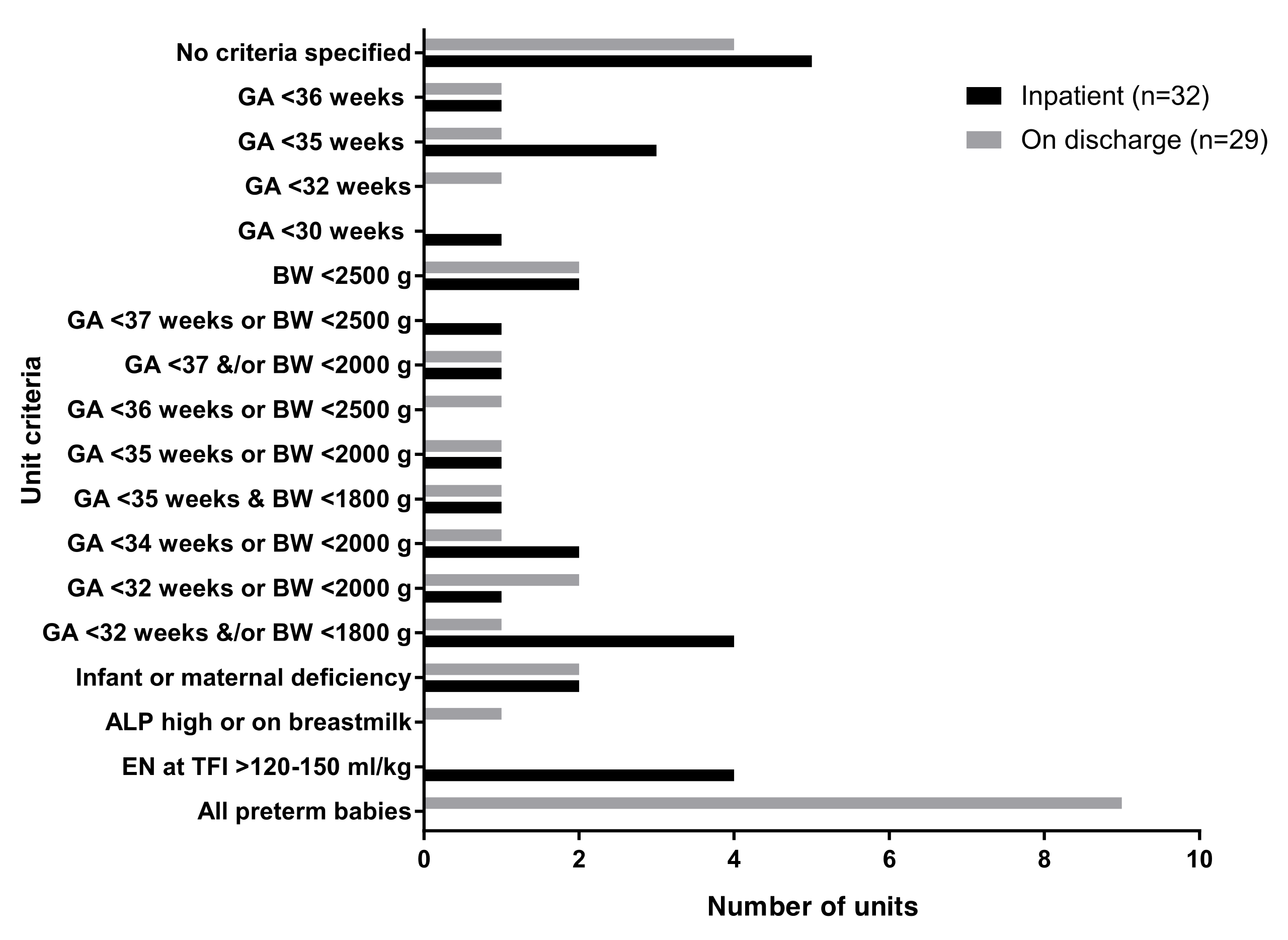
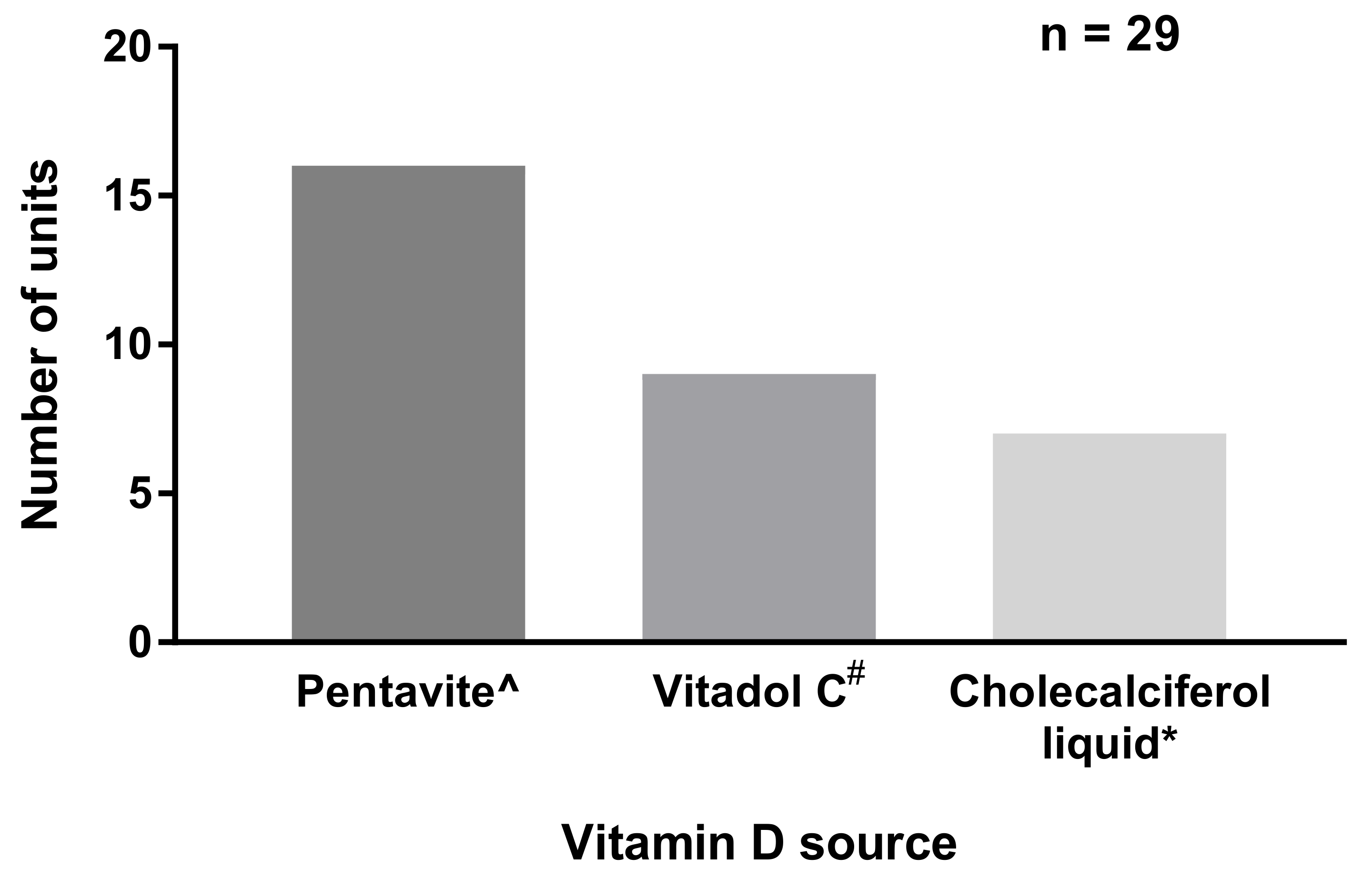
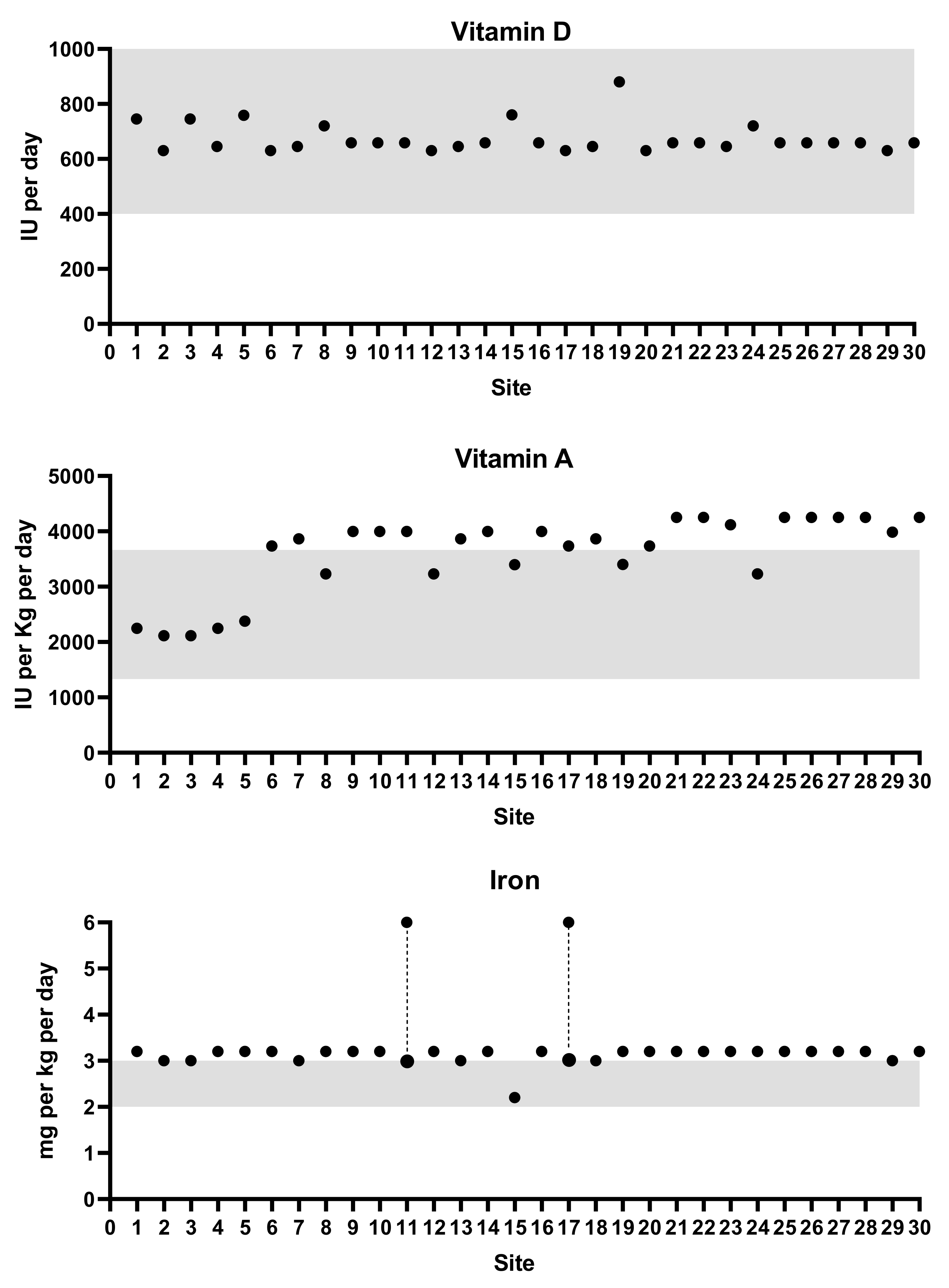
3.1. Vitamin D Supplementation
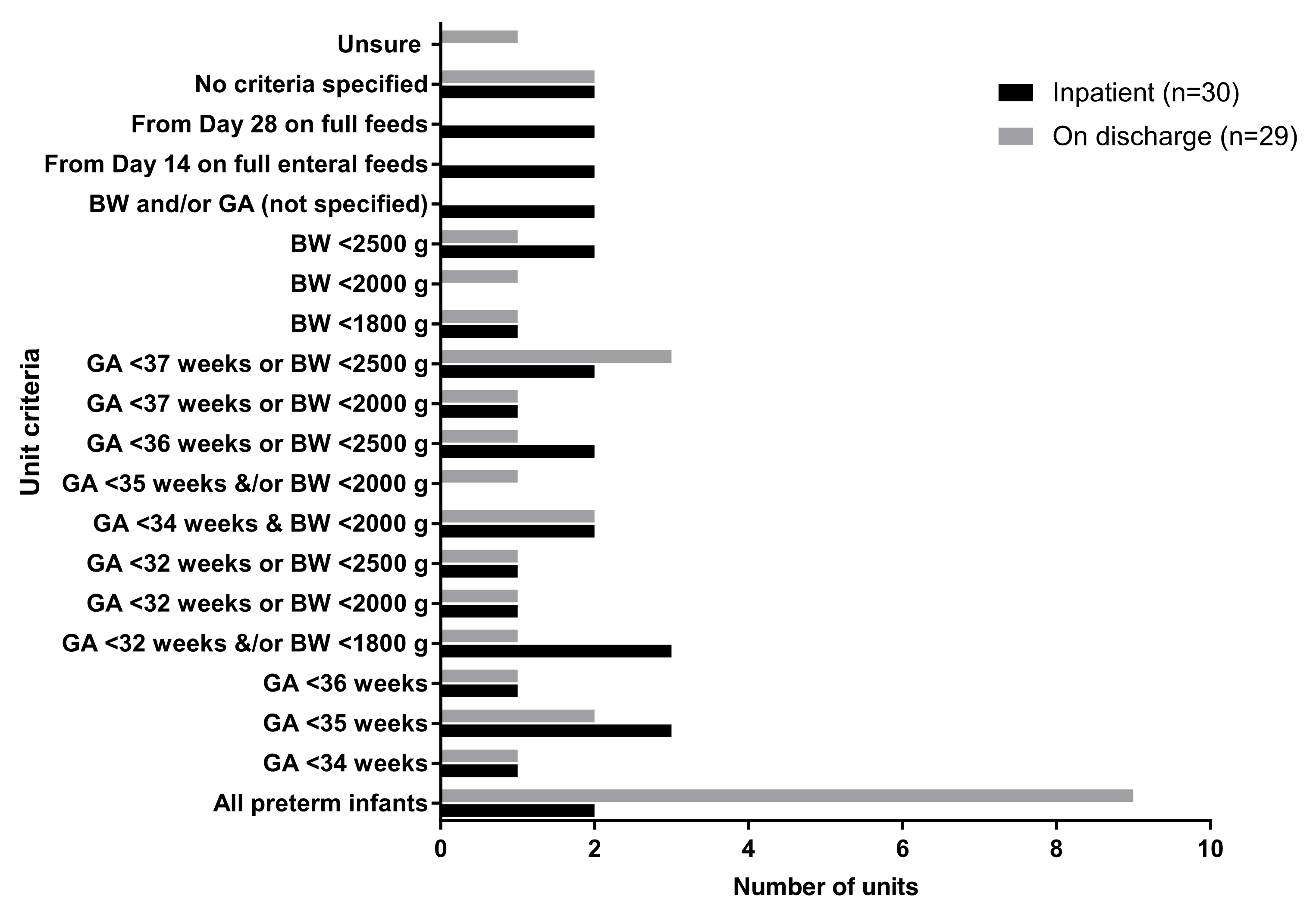
3.2. Iron Supplementation
3.3. Other Vitamins and Minerals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koletzko, B.; Poindexter, B.; Uauy, R. (Eds.) Recommended Nutrient Intake Levels for Stable, Fully Enterally Fed Very Low Birth Weight Infants. In Nutritional Care of Preterm Infants Scientific Basis and Practical Guidelines; Karger: Basel, Switzerland, 2014; pp. 297–299. [Google Scholar]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; de Curtis, M.; Darmaun, D.; Decsi, T.; Domellof, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.C.; Uauy, R.; Koletzko, B.; Zlotkin, S.H. Nutrition of the Preterm Infant: Scientific Basis and Practical Guidelines, 2nd ed.; Digital Education Publishing, Inc.: Cincinnati, OH, USA, 2005. [Google Scholar]
- Onwuneme, C.; Molloy, E.J. Question 2: Vitamin D intake for preterm infants: How much do they really need? Arch. Dis. Child. 2018, 103, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Onwuneme, C.; Martin, F.; McCarthy, R.; Carroll, A.; Segurado, R.; Murphy, J.; Twomey, A.; Murphy, N.; Kilbane, M.; McKenna, M.; et al. The association of Vitamin D status with acute respiratory morbidity in preterm infants. J. Pediatr. 2015, 166, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Bhayat, S.; Gowda, H. Impact on chronic lung disease of early versus late vitamin D supplementation in very low birth weight infants. J. Perinat. Med. 2015, 43. [Google Scholar] [CrossRef]
- Cetinkaya, M.; Cekmez, F.; Buyukkale, G.; Erener-Ercan, T.; Demir, F.; Tunc, T.; Aydin, F.N.; Aydemir, G. Lower vitamin D levels are associated with increased risk of early-onset neonatal sepsis in term infants. J. Perinatol. 2015, 35, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Qasem, W.A.; Friel, J.K. An overview of iron in term breast-fed infants. Clin. Med. Insights Pediatr. 2015, 9, 79–84. [Google Scholar] [CrossRef]
- Lozoff, B.; Smith, J.B.; Kaciroti, N.; Clark, K.M.; Guevara, S.; Jimenez, E. Functional significance of early-life iron deficiency: Outcomes at 25 years. J. Pediatr. 2013, 163, 1260–1266. [Google Scholar] [CrossRef]
- Mills, R.J.; Davies, M.W. Enteral iron supplementation in preterm and low birth weight infants. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Long, H.; Yi, J.M.; Hu, P.L.; Li, Z.B.; Qiu, W.Y.; Wang, F.; Zhu, S. Benefits of iron supplementation for low birth weight infants: A systematic review. BMC Pediatr. 2012, 12, 99. [Google Scholar] [CrossRef]
- Leaf, A.; Subramanian, S.; Cherian, S. Vitamins for preterm infants. Curr. Paediatr. 2004, 14, 298–305. [Google Scholar] [CrossRef]
- Backstrom, M.C.; Maki, R.; Kuusela, A.L.; Sievanen, H.; Koivisto, A.M.; Ikonen, R.S.; Kouri, T.; Maki, M. Randomised controlled trial of vitamin D supplementation on bone density and biochemical indices in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 1999, 80, F161–F166. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.W.; Krug-Wispe, S.; Neylan, M.; Succop, P.; Oestreich, A.E.; Tsang, R.C. Effect of three levels of vitamin D intake in preterm infants receiving high mineral-containing milk. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Pittard, W.B.; Geddes, K.M.; Hulsey, T.C.; Hollis, B.W. How Much Vitamin D for Neonates. Am. J. Dis. Child. 1991, 145, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Pinto, K.; Collins, C.T.; Gibson, R.A.; Andersen, C.C. Vitamin D in preterm infants: A prospective observational study. J. Paediatr. Child Health 2015, 51, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, C.K.; Sankar, M.J.; Agarwal, R.; Pratap, O.T.; Jain, V.; Gupta, N.; Gupta, A.K.; Deorari, A.K.; Paul, V.K.; Sreenivas, V. Trial of daily vitamin D supplementation in preterm infants. Pediatrics 2014, 133, e628–e634. [Google Scholar] [CrossRef]
- Anderson-Berry, A.; Thoene, M.; Wagner, J.; Lyden, E.; Jones, G.; Kaufmann, M.; Van Ormer, M.; Hanson, C. Randomized trial of two doses of vitamin D3 in preterm infants <32 weeks: Dose impact on achieving desired serum 25(OH)D3 in a NICU population. PLoS ONE 2017, 12, e0185950. [Google Scholar] [CrossRef]
- Fort, P.; Salas, A.A.; Nicola, T.; Craig, C.M.; Carlo, W.A.; Ambalavanan, N. A comparison of 3 Vitamin D dosing regimens in extremely preterm infants: A randomized controlled trial. J. Pediatr. 2016, 174, 132–138. [Google Scholar] [CrossRef]
- Jin, H.X.; Wang, R.S.; Chen, S.J.; Wang, A.P.; Liu, X.Y. Early and late Iron supplementation for low birth weight infants: A meta-analysis. Ital. J. Pediatr. 2015, 41, 16. [Google Scholar] [CrossRef]
- Steinmacher, J.; Pohlandt, F.; Bode, H.; Sander, S.; Kron, M.; Franz, A.R. Randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1301 grams: Neurocognitive development at 5.3 years’ corrected age. Pediatrics 2007, 120, 538–546. [Google Scholar] [CrossRef]
- Aggarwal, D.; Sachdev, H.P.S.; Nagpal, J.; Singh, T.; Mallika, V. Haematological effect of iron supplementation in breast fed term low birth weight infants. Arch. Dis. Child. 2005, 90, 26–29. [Google Scholar] [CrossRef]
- Berglund, S.; Westrup, B.; Domellof, M. Iron Supplements Reduce the Risk of Iron Deficiency Anemia in Marginally Low Birth Weight Infants. Pediatrics 2010, 126, E874–E883. [Google Scholar] [CrossRef] [PubMed]
- Berglund, S.K.; Chmielewska, A.; Starnberg, J.; Westrup, B.; Hagglof, B.; Norman, M.; Domellof, M. Effects of iron supplementation of low-birth-weight infants on cognition and behavior at 7 years: A randomized controlled trial. Pediatr. Res. 2018, 83, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Brion, L.P.; Bell, E.F.; Raghuveer, T.S. Vitamin E supplementation in preterm infants: Systematic review. J. Investig. Med. 2003, 51, S420–S421. [Google Scholar]
- Cormack, B.; Sinn, J.; Lui, K.; Tudehope, D. Australasian neonatal intensive care enteral nutrition survey: Implications for practice. J. Paediatr. Child Health 2013, 49, E340–E347. [Google Scholar] [CrossRef] [PubMed]
- Darlow, B.A.; Graham, P.J.; Rojas-Reyes, M.X. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Rakshasbhuvankar, A.; Patole, S.; Simmer, K.; Pillow, J.J. Enteral vitamin A for reducing severity of bronchopulmonary dysplasia in extremely preterm infants: A randomised controlled trial. BMC Pediatr. 2017, 17. [Google Scholar] [CrossRef]
| Nutrient | Koletzko 2014 [1] (Per kg/Day) | ESPGHAN 2010 [2] (Per kg/Day) | Tsang 2005 [3] (Per kg/Day) |
|---|---|---|---|
| Vitamin A (IU) | 1332–3663 | 1320–3300 | 700–1500 |
| Vitamin E (mg α-TE) | 2.2–11 | 2.2–11 | 4–8 |
| Vitamin D (IU) | 400–1000 per day from milk and supplement | 800–1000 per day | 150–400 |
| Folic acid (µg) | 35–100 | 35–100 | 25–50 |
| Iron (mg) | 2–3 | 2–3 | 2–4 |
| Zinc (mg) | 1.4–2.5 | 1.1–2.0 | 1–3 |
| Calcium (mg) | 120–200 | 120–140 | 100–220 |
| Phosphorus (mg) | 60–140 | 60–90 | 60–140 |
| Dose (mg/kg/day) | Inpatients N = 29 | At Discharge N = 29 |
|---|---|---|
| 1.8 | 4 (14%) | 5 (17%) |
| 2.4 | 3 (10%) | 3 (10%) |
| 3 | 17 (59%) | 17 (59%) |
| 6 | 2 (7%) | 3 (10%) |
| Not specified | 3 (10%) | 1 (3%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliver, C.; Watson, C.; Crowley, E.; Gilroy, M.; Page, D.; Weber, K.; Messina, D.; Cormack, B. Vitamin and Mineral Supplementation Practices in Preterm Infants: A Survey of Australian and New Zealand Neonatal Intensive and Special Care Units. Nutrients 2020, 12, 51. https://doi.org/10.3390/nu12010051
Oliver C, Watson C, Crowley E, Gilroy M, Page D, Weber K, Messina D, Cormack B. Vitamin and Mineral Supplementation Practices in Preterm Infants: A Survey of Australian and New Zealand Neonatal Intensive and Special Care Units. Nutrients. 2020; 12(1):51. https://doi.org/10.3390/nu12010051
Chicago/Turabian StyleOliver, Colleen, Caitlin Watson, Elesa Crowley, Melissa Gilroy, Denise Page, Katrina Weber, Deanna Messina, and Barbara Cormack. 2020. "Vitamin and Mineral Supplementation Practices in Preterm Infants: A Survey of Australian and New Zealand Neonatal Intensive and Special Care Units" Nutrients 12, no. 1: 51. https://doi.org/10.3390/nu12010051
APA StyleOliver, C., Watson, C., Crowley, E., Gilroy, M., Page, D., Weber, K., Messina, D., & Cormack, B. (2020). Vitamin and Mineral Supplementation Practices in Preterm Infants: A Survey of Australian and New Zealand Neonatal Intensive and Special Care Units. Nutrients, 12(1), 51. https://doi.org/10.3390/nu12010051





