Effects of Lactobacillus plantarum Q180 on Postprandial Lipid Levels and Intestinal Environment: A Double-Blind, Randomized, Placebo-Controlled, Parallel Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Sample Collection
2.4. Blood Lipid Biomarker in Plasma
2.5. Intestinal Microbial Community Analysis
2.6. Intestinal Microbial Community Analysis
2.6.1. Fecal Microbial metaBolites: Biogenic Amines, SCFAs, Indoles and Phenols
2.6.2. Fecal Excretion Fat-Related Metabolites: Neutral Sterols and Bile Acids
2.7. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Changes in Fasting and Postprandial Blood Lipid Levels
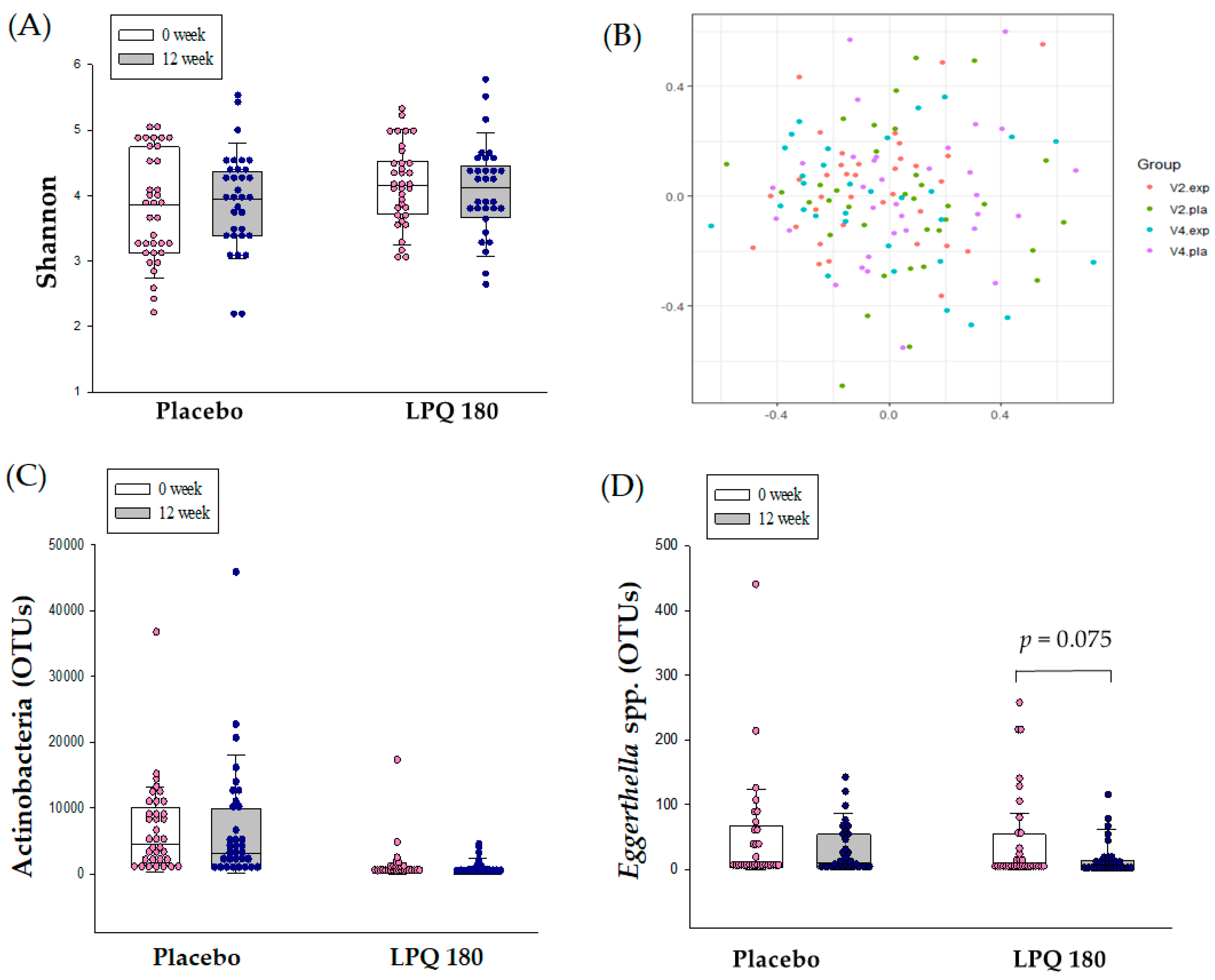
3.3. Intestinal Microbiome
3.4. Changes of Intestinal Microbial Metabolites in Feces
3.5. Correlation between the Baseline Intestinal Microbiome and Changes of Blood Lipid-Related Markers and Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; De Ferranti, S.; Després, J.P.; Fullerton, H.J. Heart disease and stroke statistics-2016 update. Am. Heart Assoc. Circ. 2016, 133, e38–e48. [Google Scholar] [CrossRef] [PubMed]
- Varbo, A.; Nordestgaard, B.G. Remnant cholesterol and triglyceride-rich lipoproteins in atherosclerosis progression and cardiovascular disease. Am. Heart Assoc. 2016, 35, 2133–2135. [Google Scholar] [CrossRef] [PubMed]
- Dron, J.S.; Hegele, R.A. Genetics of triglycerides and the risk of atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 31. [Google Scholar] [CrossRef]
- Caesar, R.; Fåk, F.; Bäckhed, F. Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J. Intern. Med. 2010, 268, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Moroti, C.; Magri, L.F.S.; de Rezende Costa, M.; Cavallini, D.C.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Ataie-Jafari, A.; Larijani, B.; Majd, H.A.; Tahbaz, F. Cholesterol-lowering effect of probiotic yogurt in comparison with ordinary yogurt in mildly to moderately hypercholesterolemic subjects. Ann. Nutr. Metab. 2009, 54, 22–27. [Google Scholar] [CrossRef]
- Andrade, S.; Borges, N. Effect of fermented milk containing Lactobacillus acidophilus and Bifidobacterium longum on plasma lipids of women with normal or moderately elevated cholesterol. J. Dairy Res. 2009, 76, 469–474. [Google Scholar] [CrossRef]
- Asan-Ozusaglam, M.; Gunyakti, A. Lactobacillus fermentum strains from human breast milk with probiotic properties and cholesterol-lowering effects. Food Sci. Biotechnol. 2019, 28, 501–509. [Google Scholar] [CrossRef]
- Park, S.-Y.; Cho, S.-A.; Kim, S.-H.; Lim, S.-D. Physiological characteristics and anti-obesity effect of Lactobacillus plantarum Q180 isolated from feces. Korean J. Food Sci. Anim. Resour. 2014, 34, 647. [Google Scholar] [CrossRef]
- Park, S.-Y.; Cho, S.-A.; Lim, S.-D. Application of response surface methodology (RSM) for optimization of anti-obesity effect in fermented milk by lactobacillus plantarum Q180. Korean J. Food Sci. Anim. Resour. 2014, 34, 836. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, S.; Lim, S.-D. The Inhibitory Effect of L. plantarum Q180 on Adipocyte Differentiation in 3T3-L1 and Reduction of Adipocyte Size in Mice Fed High-fat Diet. Korean J. Food Sci. Anim. Resour. 2018, 38, 99. [Google Scholar] [PubMed]
- Park, S.-Y.; Seong, K.-S.; Lim, S.-D. Anti-obesity effect of yogurt fermented by Lactobacillus plantarum Q180 in diet-induced obese rats. Korean J. Food Sci. Anim. Resour. 2016, 36, 77. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, K.J.; Park, S.-Y.; Lim, Y.; Kwon, O.; Lee, J.H.; Kim, J.Y. Differential responses of endothelial integrity upon the intake of microencapsulated garlic, tomato extract or a mixture: A single-intake, randomized, double-blind, placebo-controlled crossover trial. Food Funct. 2018, 9, 5426–5435. [Google Scholar] [CrossRef]
- Kim, J.; Yang, Y.; Yang, Y.; Oh, S.; Hong, Y.-C.; Lee, E.; Kwon, O. Diet quality scores and oxidative stress in Korean adults. Eur. J. Clin. Nutr. 2011, 65, 1271. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ahn, Y.; Lim, Y.; Kim, J.; Kim, J.; Kwon, O. Daily nutritional dose supplementation with antioxidant nutrients and phytochemicals improves DNA and LDL stability: A double-blind, randomized, and placebo-controlled trial. Nutrients 2013, 5, 5218–5232. [Google Scholar] [CrossRef] [PubMed]
- Scanu, A.; Page, I.H. Separation and characterization of human serum chylomicrons. J. Exp. Med. 1959, 109, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, M. Determination of biogenic amines as dansyl derivatives in intestinal digesta and feces by reversed phase HPLC. Chromatographia 2002, 55, 297–300. [Google Scholar] [CrossRef]
- Costabile, A.; Klinder, A.; Fava, F.; Napolitano, A.; Fogliano, V.; Leonard, C.; Gibson, G.R.; Tuohy, K.M. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: A double-blind, placebo-controlled, crossover study. Br. J. Nutr. 2008, 99, 110–120. [Google Scholar] [CrossRef]
- Flickinger, E.; Schreijen, E.; Patil, A.; Hussein, H.; Grieshop, C.; Merchen, N.; Fahey, G., Jr. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J. Anim. Sci. 2003, 81, 2008–2018. [Google Scholar] [CrossRef]
- Park, K.; Kim, M.; Woo, K.; Lee, N.; Paik, H. Rapid Analysis of Major Putrefactive Metabolites by GC and GC/MSD. Korean J. Biotechnol. Bioeng. 2003. [Google Scholar] [CrossRef]
- Fechner, A.; Fenske, K.; Jahreis, G. Effects of legume kernel fibres and citrus fibre on putative risk factors for colorectal cancer: A randomised, double-blind, crossover human intervention trial. Nutr. J. 2013, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Jahreis, G. Determination of underivatised sterols and bile acid trimethyl silyl ether methyl esters by gas chromatography–mass spectrometry–single ion monitoring in faeces. J. Chromatogr. B 2004, 813, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kim, M.; Ahn, Y.-T.; Sim, J.-H.; Choi, I.-D.; Lee, S.-H.; Lee, J.H. The triglyceride-lowering effect of supplementation with dual probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032: Reduction of fasting plasma lysophosphatidylcholines in nondiabetic and hypertriglyceridemic subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, K. Effect of globin digest on blood lipids. Jpn. J. Nutr. Food 1999, 52, 71–77. [Google Scholar]
- Takase, H.; Shoji, K.; Hase, T.; Tokimitsu, I. Effect of diacylglycerol on postprandial lipid metabolism in non-diabetic subjects with and without insulin resistance. Atherosclerosis 2005, 180, 197–204. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Kim, M.; Chae, J.S.; Ahn, Y.-T.; Sim, J.-H.; Choi, I.-D.; Lee, S.-H.; Lee, J.H. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduces fasting triglycerides and enhances apolipoprotein AV levels in non-diabetic subjects with hypertriglyceridemia. Atherosclerosis 2015, 241, 649–656. [Google Scholar] [CrossRef]
- Xiao, J.; Kondo, S.; Takahashi, N.; Miyaji, K.; Oshida, K.; Hiramatsu, A.; Iwatsuki, K.; Kokubo, S.; Hosono, A. Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers. J. Dairy Sci. 2003, 86, 2452–2461. [Google Scholar] [CrossRef]
- Fuentes, M.C.; Lajo, T.; Carrión, J.M.; Cuñé, J. A randomized clinical trial evaluating a proprietary mixture of Lactobacillus plantarum strains for lowering cholesterol 1. Mediterr. J. Nutr. Metab. 2016, 9, 125–135. [Google Scholar] [CrossRef]
- Simons, L.A.; Amansec, S.G.; Conway, P. Effect of Lactobacillus fermentum on serum lipids in subjects with elevated serum cholesterol. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 531–535. [Google Scholar] [CrossRef]
- Jones, M.; Martoni, C.; Prakash, S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: A randomized controlled trial. Eur. J. Clin. Nutr. 2012, 66, 1234. [Google Scholar] [CrossRef]
- Potts, J.L.; Fisher, R.M.; Humphreys, S.M.; Coppack, S.W.; Gibbons, G.F.; Frayn, K.N. Peripheral triacylglycerol extraction in the fasting and post-prandial states. Clin. Sci. 1991, 81, 621–626. [Google Scholar] [CrossRef]
- Devaraj, S.; Hemarajata, P.; Versalovic, J. The human gut microbiome and body metabolism: Implications for obesity and diabetes. Clin. Chem. 2013, 59, 617–628. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, D.H.; Kim, H.S.; Kim, Y.-K. An exploratory study on the effect of daily fruits and vegetable juice on human gut microbiota. Food Sci. Biotechnol. 2018, 27, 1377–1386. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Steven, R.G.; Nelson, K.E.; David, A.R. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635. [Google Scholar] [CrossRef]
- Artis, M.C.A.A.D. The intestinal microbiota in health and disease: The influence of microbial products on immune cell homeostasis. Curr. Opin. Gastroenterol. 2009, 25, 496–502. [Google Scholar]
- Lahti, L.; Salonen, A.; Kekkonen, R.A.; Salojärvi, J.; Jalanka-Tuovinen, J.; Palva, A.; Orešič, M.; De Vos, W.M. Associations between the human intestinal microbiota, Lactobacillus rhamnosus GG and serum lipids indicated by integrated analysis of high-throughput profiling data. PeerJ 2013, 1, e32. [Google Scholar] [CrossRef]
- Fu, J.; Bonder, M.J.; Cenit, M.C.; Tigchelaar, E.; Maatman, A.; Dekens, J.A.; Brandsma, E.; Marczynska, J.; Imhann, F.; Weersma, R.K.; et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ. Res. 2015, 117, 817–824. [Google Scholar] [CrossRef]
- Fuller, R.; Glenn, R. Probiotics and prebiotics: Microflora management for improved gut health. Clin. Microbiol. Infect. 1998, 4, 477–480. [Google Scholar] [CrossRef][Green Version]
- Gibson, G.R. Effects of Consumption of Probiotics and Prebiotics on Serum Lipid Levels in Humans. Crit. Rev. Biochem. Mol. Biol. 2002, 37, 259–281. [Google Scholar]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Verbeke, K.A.; Boobis, A.R.; Chiodini, A.; Edwards, C.A.; Franck, A.; Kleerebezem, M.; Nauta, A.; Raes, J.; Van Tol, E.A.; Tuohy, K.M. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr. Res. Rev. 2015, 28, 42–66. [Google Scholar] [CrossRef]
- Fan, P.; Song, P.; Li, L.; Huang, C.; Chen, J.; Yang, W.; Qiao, S.; Wu, G.; Zhang, G.; Ma, X. Roles of biogenic amines in intestinal signaling. Curr. Protein Pept. Sci. 2017, 18, 532–540. [Google Scholar] [CrossRef]
- Granado-Serrano, A.B.; Martín-Garí, M.; Sánchez, V.; Solans, M.R.; Berdún, R.; Ludwig, I.A.; Rubió, L.; Vilaprinyó, E.; Portero-Otín, M.; Serrano, J. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 2019, 9, 1772. [Google Scholar] [CrossRef]
- Ze, X.; David, Y.B.; Laverde-Gomez, J.A.; Dassa, B.; Sheridan, P.O.; Duncan, S.H.; Louis, P.; Henrissat, B.; Juge, N.; Koropatkin, N.M. Unique organization of extracellular amylases into amylosomes in the resistant starch-utilizing human colonic Firmicutes bacterium Ruminococcus bromii. MBio 2015, 6, e01058-15. [Google Scholar] [CrossRef]
- Haas, K.N.; Blanchard, J.L. Kineothrix alysoides, gen. nov., sp. nov., a saccharolytic butyrate-producer within the family Lachnospiraceae. Int. J. Syst. Evol. Microbiol. 2017, 67, 402–410. [Google Scholar] [CrossRef]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.; Flament, C.; Lepage, P.; Roberti, M.P. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef]
- Ulbrich, K.; Reichardt, N.; Braune, A.; Kroh, L.W.; Blaut, M.; Rohn, S. The microbial degradation of onion flavonol glucosides and their roasting products by the human gut bacteria Eubacterium ramulus and Flavonifractor plautii. Food Res. Int. 2015, 67, 349–355. [Google Scholar] [CrossRef]
- Kaci, G.; Goudercourt, D.; Dennin, V.; Pot, B.; Doré, J.; Ehrlich, S.D.; Renault, P.; Blottière, H.M.; Daniel, C.; Delorme, C. Anti-inflammatory properties of Streptococcus salivarius, a commensal bacterium of the oral cavity and digestive tract. Appl. Environ. Microbiol. 2014, 80, 928–934. [Google Scholar] [CrossRef]
- Ramanan, P.; Barreto, J.N.; Osmon, D.R.; Tosh, P.K. Rothia bacteremia: A 10-year experience at Mayo Clinic, Rochester, Minnesota. J. Clin. Microbiol. 2014, 52, 3184–3189. [Google Scholar] [CrossRef]
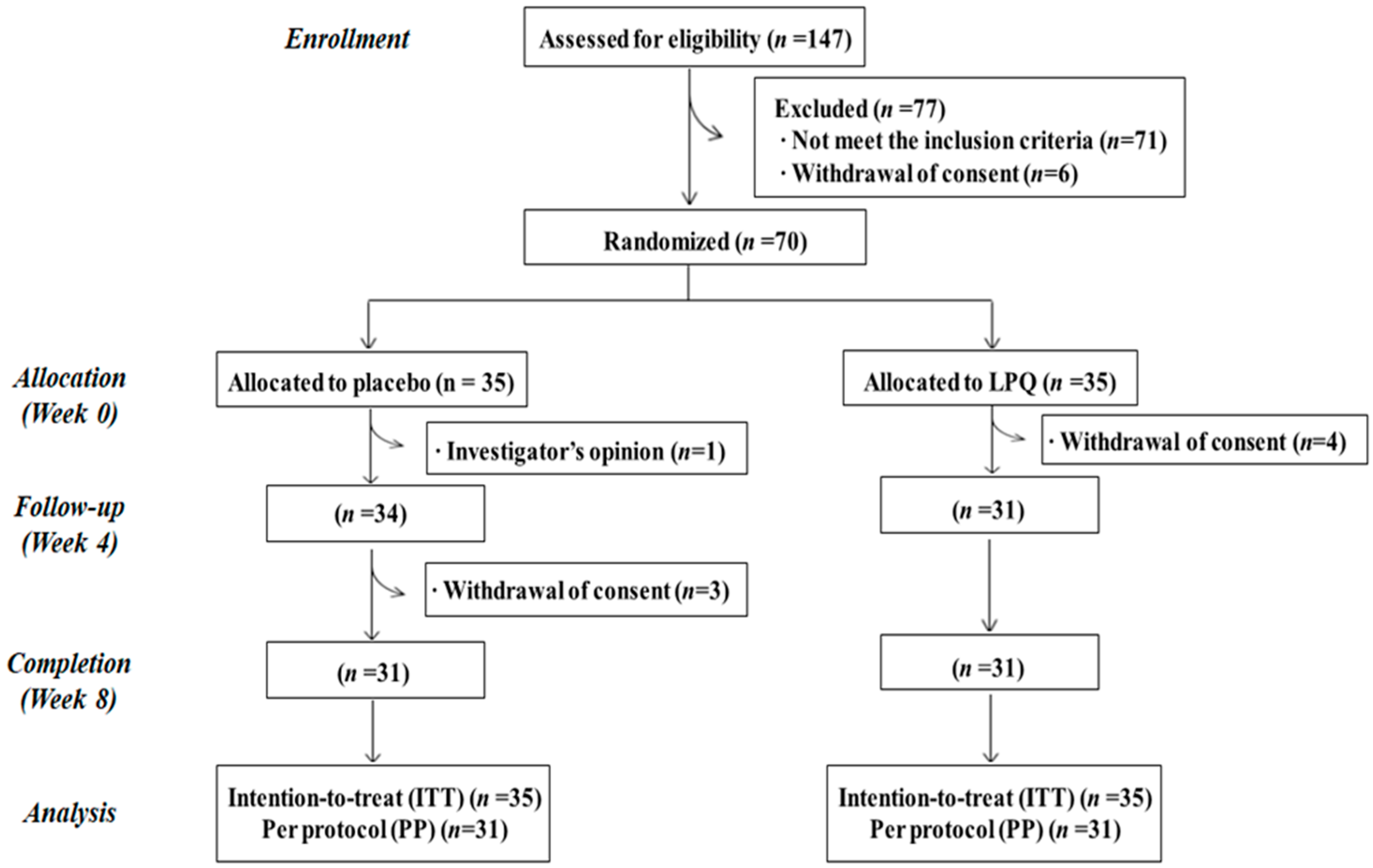
| Variables | Placebo (n = 35) | LPQ 180 (n = 35) | p-Value 2 |
|---|---|---|---|
| Gender (male/female) | 12/23 | 12/23 | 1.000 |
| Age (year) | 48.3 ± 13.2 | 48.3 ± 11.6 | 1.000 |
| Menopause (Y/N/NA) | 14/9/12 | 13/10/12 | 0.956 |
| Postmenopausal period (month) | 118.1 ± 101.2 | 114.8 ± 91.2 | 0.930 |
| Body weight (kg) | 68.9 ± 11.1 | 69.8 ± 11.2 | 0.745 |
| BMI (kg/m2) | 25.7 ± 2.5 | 26.4 ± 2.5 | 0.209 |
| Waist circumference (cm) | 85.6 ± 6.7 | 86.7 ± 7.5 | 0.493 |
| Hip circumference (cm) | 96.7 ± 4.8 | 97.1 ± 6.4 | 0.737 |
| Alcohol drinker (Y/N) | 20/15 | 15/20 | 0.232 |
| Alcohol amount (g/week, per drinker) | 44.9 ± 61.2 | 27.1 ± 26.3 | 0.254 |
| Smoker (Y/N) | 5/30 | 4/31 | 1.000 |
| Smoking amount (cigarettes/day, per smoker) | 9.0 ± 2.2 | 7.0 ± 4.2 | 0.390 |
| Physical Activity (MET-min/week) | 1335.8 ± 1150 | 1757.1 ± 2375 | 0.350 |
| Blood lipid profiles | |||
| TG (mg/dL) | 134.2 ± 66.7 | 133.4 ± 58.9 | 0.958 |
| TC (mg/dL) | 206.3 ± 37.1 | 196.8 ± 41.6 | 0.316 |
| LDL-C (mg/dL) | 120.5 ± 31.3 | 114.4 ± 36.4 | 0.458 |
| HDL-C (mg/dL) | 50.1 ± 11.8 | 46.9 ± 11.4 | 0.253 |
| VLDL-C (mg/dL) | 26.8 ± 13.3 | 26.7 ± 11.8 | 0.958 |
| Blood pressure | |||
| SBP (mmHg) | 120.0 ± 13.4 | 122.9 ± 15.1 | 0.410 |
| DBP (mmHg) | 71.7 ± 8.1 | 73.0 ± 10.2 | 0.543 |
| RFS | 23.5 ± 6.9 | 23.6 ± 6.8 | 0.944 |
| MEDIFITCTS score | 36.4 ± 22.2 | 38.6 ± 24.0 | 0.695 |
| Dietary intake | |||
| Energy (kcal/day) | 1402.4 ± 429.5 | 1549.0 ± 377.5 | 0.156 |
| Carbohydrate (g/day) | 195.2 ± 74.9 | 226.2 ± 63.0 | 0.081 |
| Protein (g/day) | 59.9 ± 20.1 | 58.3 ± 17.2 | 0.729 |
| Fat (g/day) | 42.4 ± 17.7 | 45.6 ± 20.5 | 0.506 |
| Sodium (mg/day) | 3187.3 ± 1542 | 2995.6 ± 1041.4 | 0.564 |
| Variables | Placebo (n = 35) | LPQ 180 (n = 35) | p-Value 2 |
|---|---|---|---|
| Fasting Lipid | |||
| TG (mg/dL) | 20.6 ± 9.9 | −2.0 ± 9.9 | 0.243 |
| TC (mg/dL) | 1.6 ± 3.6 | −8.1 ± 3.6 | 0.165 |
| LDL-C (mg/dL) | 13.8 ± 2.8 | 2.3 ± 3.0 | 0.042 |
| HDL-C (mg/dL) | 4.0 ± 1.1 | 3.1 ± 1.1 | 0.682 |
| VLDL-C (mg/dL) | 4.1 ± 2.0 | −0.4 ± 2.0 | 0.243 |
| Chylomicron TG (mg/dL) | 38.9 ± 8.9 | 25.0 ± 9.5 | 0.442 |
| ApoB-48 (ng/mL) | 1.0 ± 4.7 | −11.2 ± 5.0 | 0.202 |
| ApoB-100 (ng/mL) | 47.8 ± 13.8 | −38.2 ± 14.6 | 0.003 |
| Postprandial lipid | |||
| TG | |||
| AUC (mg min / dL) | |||
| 0–2 h | 4112 ± 1458 | −183 ± 1550 | 0.160 |
| 0–4 h | 7821 ± 2729 | −1655 ± 2901 | 0.099 |
| 0–6 h | 11,714 ± 3808 | −4171 ± 4049 | 0.049 |
| 2–4 h | 3708 ± 1336 | −1473 ± 1421 | 0.067 |
| 4–6 h | 3893 ± 1326 | −2516 ± 1410 | 0.023 |
| Excursion (mg/dL) | 19 ± 12 | −26 ± 13 | 0.075 |
| Cmax (mg/dL) | 53 ± 15 | −26 ± 16 | 0.016 |
| Tmax (min) | −14 ± 11 | 16 ± 12 | 0.208 |
| Chylomicron TG | |||
| AUC (mg min / dL) | |||
| 0–2 h | 5234 ± 1178 | 2756 ± 1253 | 0.314 |
| 0–4 h | 9921 ± 2168 | 4781 ± 2305 | 0.257 |
| 0–6 h | 14,744 ± 3014 | 5413 ± 3204 | 0.140 |
| 2–4 h | 4687 ± 1035 | 2025 ± 1101 | 0.219 |
| 4–6 h | 4823 ± 1045 | 632 ± 1111 | 0.058 |
| Excursion (mg/dL) | 7 ± 10 | −33 ± 11 | 0.067 |
| Cmax (mg/dL) | 51 ± 12 | −7 ± 13 | 0.020 |
| Tmax (min) | −14 ± 12 | −5 ± 13 | 0.735 |
| ApoB-48 | |||
| AUC (ng min / mL) | |||
| 0–2 h | 640 ± 509 | −1017 ± 541 | 0.122 |
| 0–4 h | 1515 ± 1164 | −2320 ± 1238 | 0.117 |
| 0–6 h | 2240 ± 1323 | −2818 ± 1407 | 0.070 |
| 2–4 h | 875 ± 656 | −1304 ± 698 | 0.115 |
| 4–6 h | 725 ± 274 | −498 ± 292 | 0.036 |
| Excursion (ng/mL) | 12 ± 7 | 6 ± 7 | 0.672 |
| Cmax (ng/mL) | 14 ± 5 | −6 ± 5 | 0.063 |
| Tmax (min) | −51 ± 20 | −10 ± 22 | 0.341 |
| ApoB-100 | |||
| AUC (ng min / mL) | |||
| 0–2 h | 5779 ± 1535 | −3597 ± 1632 | 0.005 |
| 0–4 h | 9047 ± 3046 | −7560 ± 3239 | 0.011 |
| 0–6 h | 10,212 ± 4492 | −11,442 ± 4776 | 0.024 |
| 2–4 h | 3269 ± 1637 | −3963 ± 1740 | 0.038 |
| 4–6 h | 1165 ± 1595 | −3882 ± 1696 | 0.132 |
| Excursion (ng/mL) | −14 ± 9 | −3 ± 9 | 0.560 |
| Cmax (ng/mL) | 45 ± 12 | −35 ± 13 | 0.003 |
| Tmax (min) | −65 ± 29 | −26 ± 31 | 0.519 |
| Variables | Placebo (n = 35) | LPQ 180 (n = 35) | p-Value 2 |
|---|---|---|---|
| Total Biogenic amines(mg/g) | 3.62 ± 2.91 | −0.62 ± 3.01 | 0.459 |
| Short chain fatty acid (µg/g) | |||
| Unbranched SCFA | 40.83 ± 28.95 | −4.22 ± 30.57 | 0.433 |
| Branched SCFA | 4.09 ± 7.62 | −3.39 ± 8.05 | 0.620 |
| Total SCFA | 44.93 ± 35.69 | −7.61 ± 37.69 | 0.458 |
| Total Indoles and phenols (µg/g) | 44.27 ± 78.54 | −334.49 ± 86.33 | 0.019 |
| Total Neutral sterol (µg/g) | 69.84 ± 39.65 | 65.20 ± 39.65 | 0.952 |
| Bile acids (µg/g) | |||
| Primary | −0.54 ± 0.50 | 0.70 ± 0.50 | 0.205 |
| Secondary | 36.23 ± 27.21 | 36.01 ± 27.21 | 0.997 |
| Total bile acid | 35.69 ± 27.20 | 36.70 ± 27.20 | 0.985 |
| Variables | Placebo (n = 31) | LPQ 180 (n = 31) | |||
|---|---|---|---|---|---|
| r 2 | p | r 2 | p | ||
| Ruminococcus bromii | Δ TG | 0.118 | 0.689 | −0.325 | 0.075 |
| Δ TC | 0.119 | 0.523 | −0.327 | 0.073 | |
| Δ Apo B | 0.032 | 0.864 | −0.331 | 0.069 | |
| Δ Indole and phenols | 0.017 | 0.929 | −0.447 | 0.012 | |
| Kineothrix alysoides | Δ TC | −0.012 | 0.950 | −0.345 | 0.058 |
| Δ LDL-C | −0.106 | 0.571 | −0.420 | 0.019 | |
| Barnesiella intestinihominis | Δ TG | 0.040 | 0.892 | −0.407 | 0.023 |
| Δ Biogenic amines | −0.056 | 0.766 | −0.318 | 0.082 | |
| Flavonifractor plautii | Δ HDL-C | 0.174 | 0.351 | 0.414 | 0.021 |
| Streptococcus salivarius | Δ SCFA | 0.163 | 0.382 | 0.508 | 0.004 |
| Δ primary bile acids | −0.228 | 0.218 | −0.520 | 0.003 | |
| Rothia mucilaginosa | Δ SCFA | 0.105 | 0.574 | 0.518 | 0.003 |
| Δ primary bile acids | −0.227 | 0.219 | −0.556 | 0.001 | |
| Variables | Placebo (n = 31) | LPQ 180 (n = 31) | |||
|---|---|---|---|---|---|
| r 2 | p | r 2 | p | ||
| Biogenic amines | Δ TG AUC | −0.243 | 0.204 | −0.348 | 0.104 |
| Δ CM TG Cmax | −0.326 | 0.104 | −0.373 | 0.080 | |
| Δ Apo B-48 Cmax | −0.156 | 0.428 | −0.437 | 0.033 | |
| Indole and phenols | Δ HDL-C | 0.219 | 0.237 | 0.407 | 0.023 |
| Neutral sterol | Δ ApoB-100 | −0.111 | 0.575 | −0.308 | 0.092 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.E.; Kim, M.S.; Shim, K.W.; Kim, Y.-I.; Chu, J.; Kim, B.-K.; Choi, I.S.; Kim, J.Y. Effects of Lactobacillus plantarum Q180 on Postprandial Lipid Levels and Intestinal Environment: A Double-Blind, Randomized, Placebo-Controlled, Parallel Trial. Nutrients 2020, 12, 255. https://doi.org/10.3390/nu12010255
Park YE, Kim MS, Shim KW, Kim Y-I, Chu J, Kim B-K, Choi IS, Kim JY. Effects of Lactobacillus plantarum Q180 on Postprandial Lipid Levels and Intestinal Environment: A Double-Blind, Randomized, Placebo-Controlled, Parallel Trial. Nutrients. 2020; 12(1):255. https://doi.org/10.3390/nu12010255
Chicago/Turabian StylePark, Ye Eun, Min Seo Kim, Kyung Won Shim, You-Il Kim, Jaeryang Chu, Byoung-Kook Kim, In Suk Choi, and Ji Yeon Kim. 2020. "Effects of Lactobacillus plantarum Q180 on Postprandial Lipid Levels and Intestinal Environment: A Double-Blind, Randomized, Placebo-Controlled, Parallel Trial" Nutrients 12, no. 1: 255. https://doi.org/10.3390/nu12010255
APA StylePark, Y. E., Kim, M. S., Shim, K. W., Kim, Y.-I., Chu, J., Kim, B.-K., Choi, I. S., & Kim, J. Y. (2020). Effects of Lactobacillus plantarum Q180 on Postprandial Lipid Levels and Intestinal Environment: A Double-Blind, Randomized, Placebo-Controlled, Parallel Trial. Nutrients, 12(1), 255. https://doi.org/10.3390/nu12010255





