Fermented Morinda citrifolia (Noni) Alleviates DNCB-Induced Atopic Dermatitis in NC/Nga Mice through Modulating Immune Balance and Skin Barrier Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of F.NONI
2.2. HPLC-UV Analysis of F.NONI
2.3. Animals
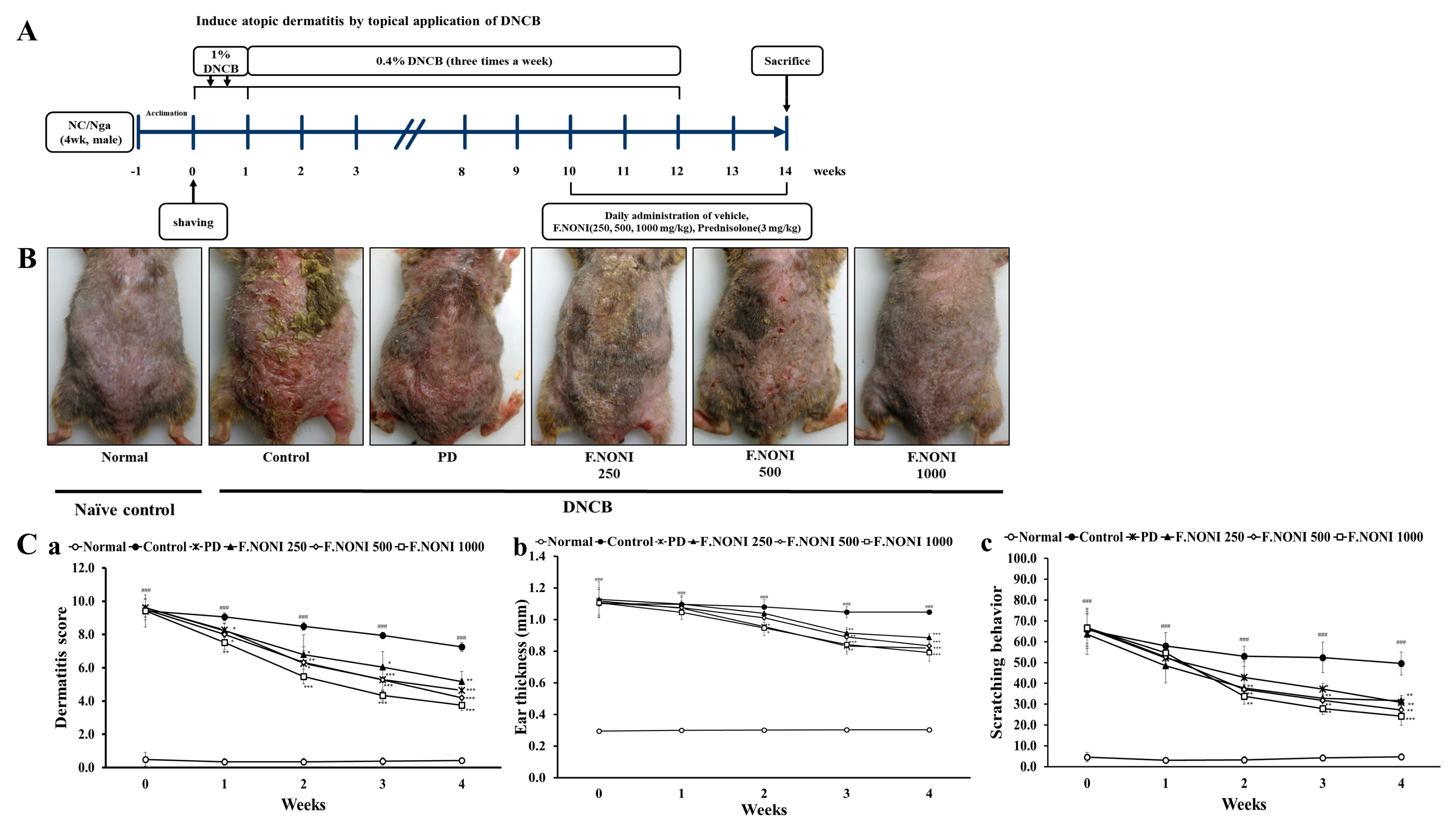
2.4. Induction of AD-Like Skin Lesions and F.NONI Treatment
2.5. Dermatitis Score and Ear Thickness
2.6. Scratching Behavior
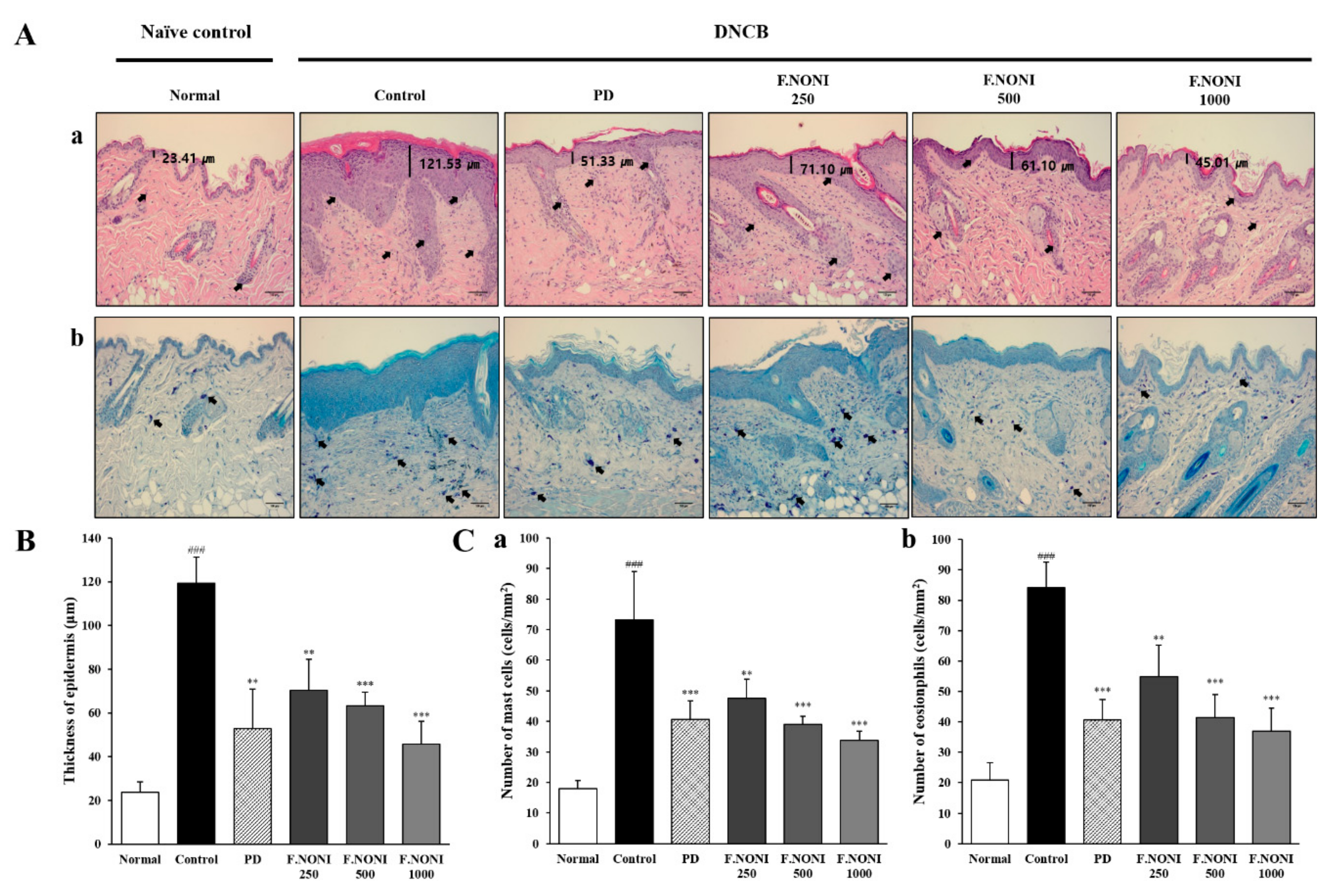
2.7. Histological Analysis
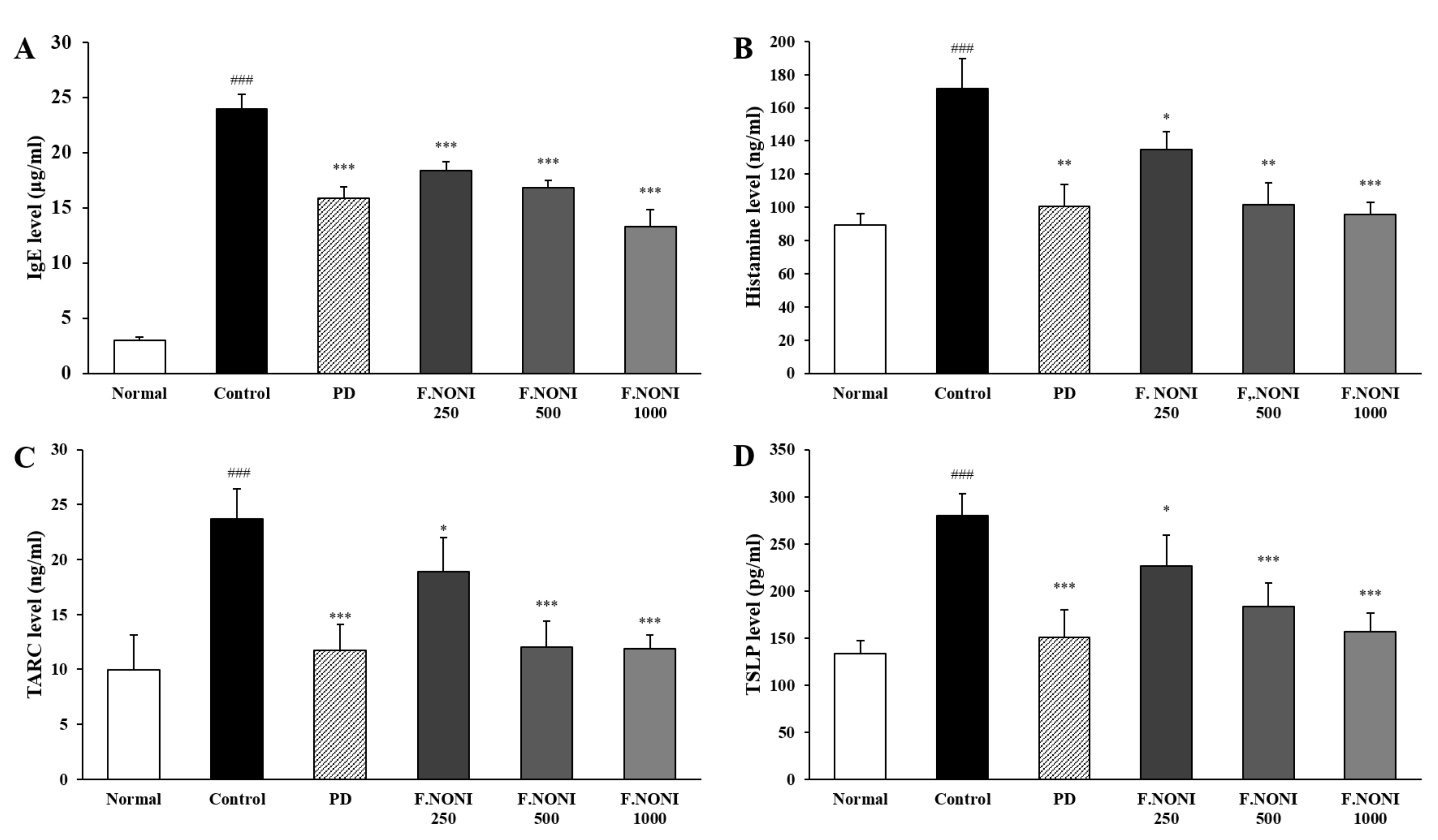
2.8. Serum Immunoglobulin and Cytokine Analysis
2.9. Splenocyte Isolation and Splenic Cytokine Analysis
2.10. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.11. Western Blot Analysis
2.12. Statistical Analysis
3. Results
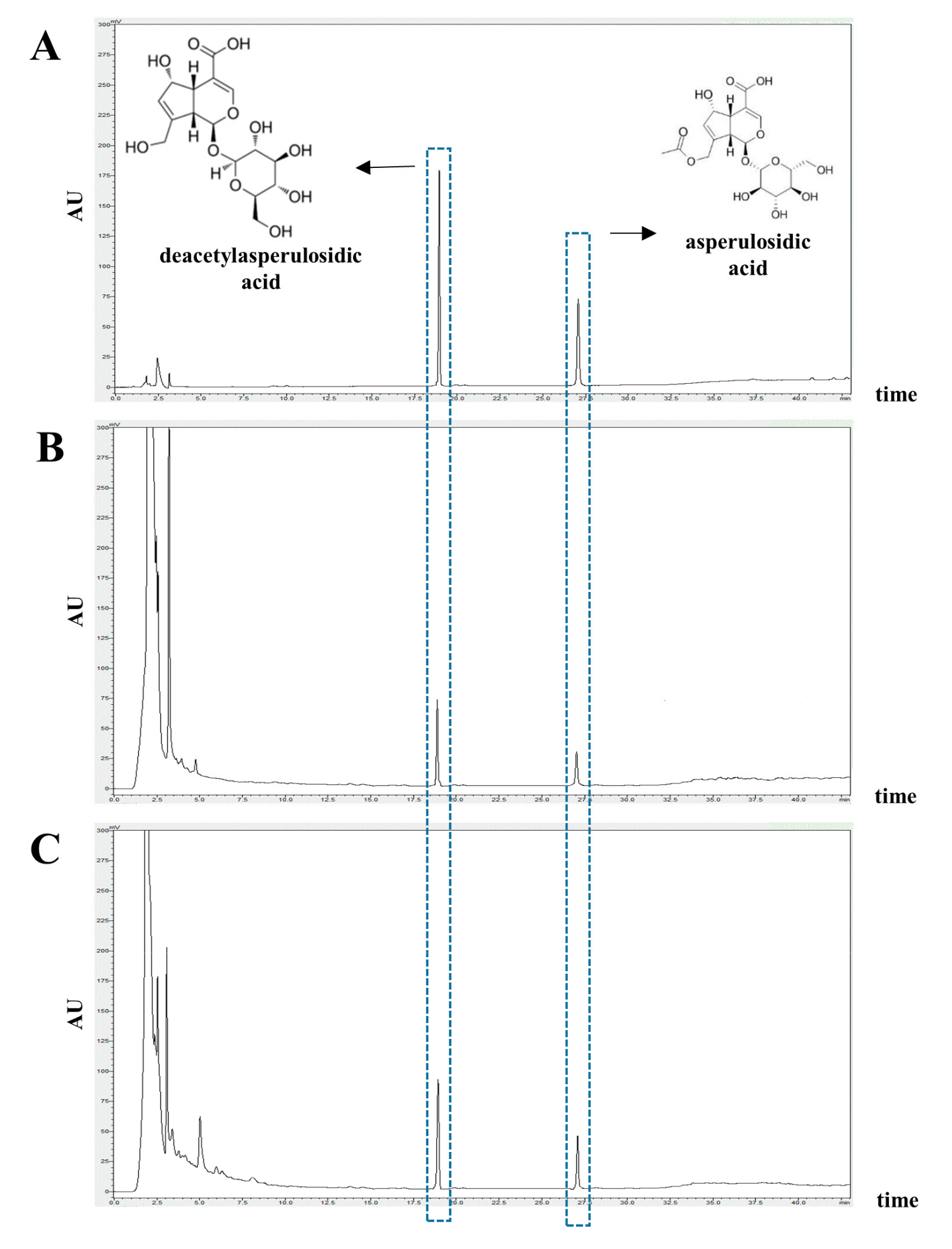
3.1. Identification and Quantification of Deacetylasperulosidic Acid (DAA) and Asperulosidic Acid (AA) in the F.NONI
3.2. F.NONI Attenuated DNCB-Induced AD-Like Skin Lesions and Scratching Behavior
3.3. F.NONI Inhibited Thickening of the Epidermis and Infiltration of Inflammatory Cells
3.4. F.NONI Regulated the Levels of Immunoglobulins and Cytokines in Serum
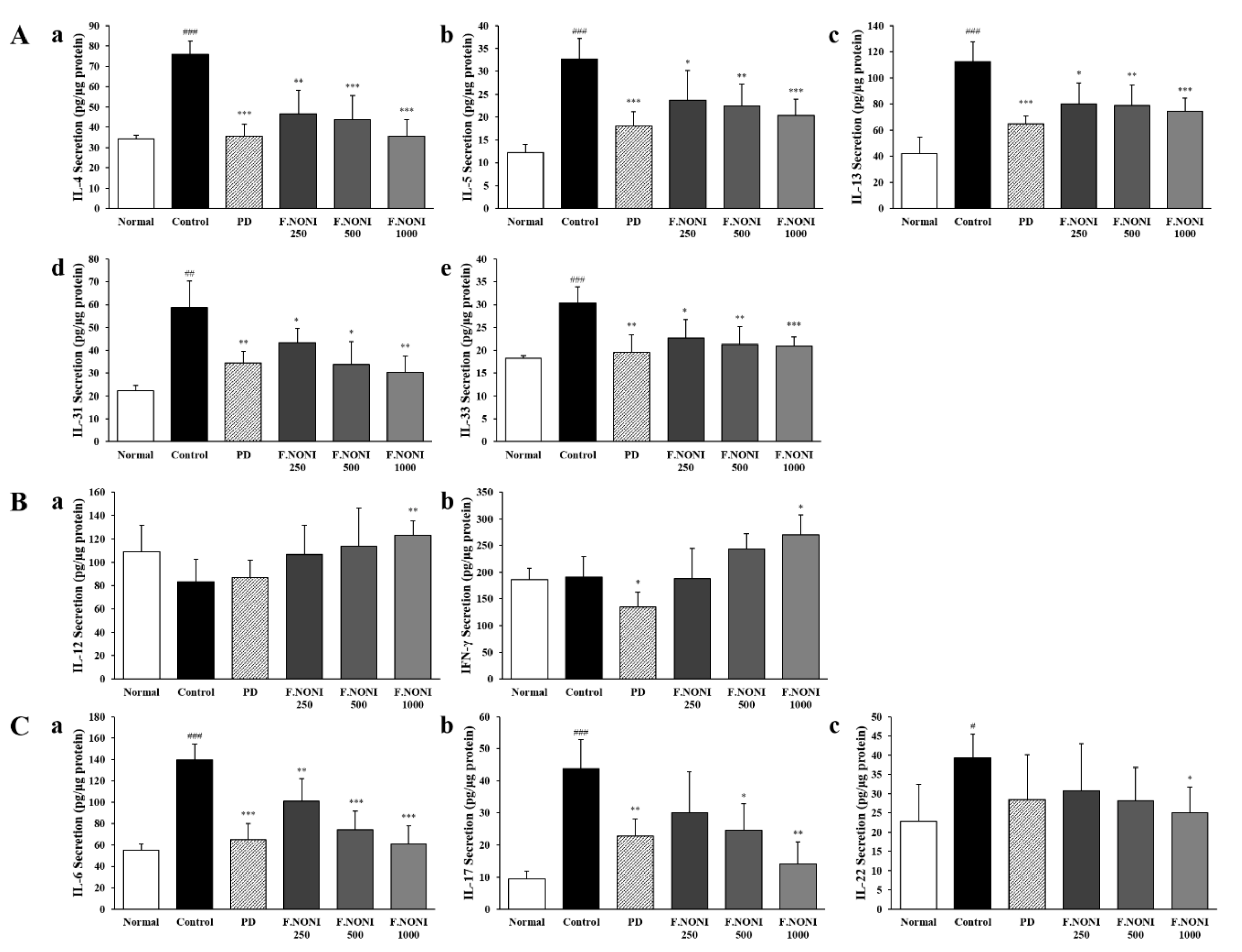
3.5. F.NONI Regulated the Balance of Cytokine Production in Splenocytes
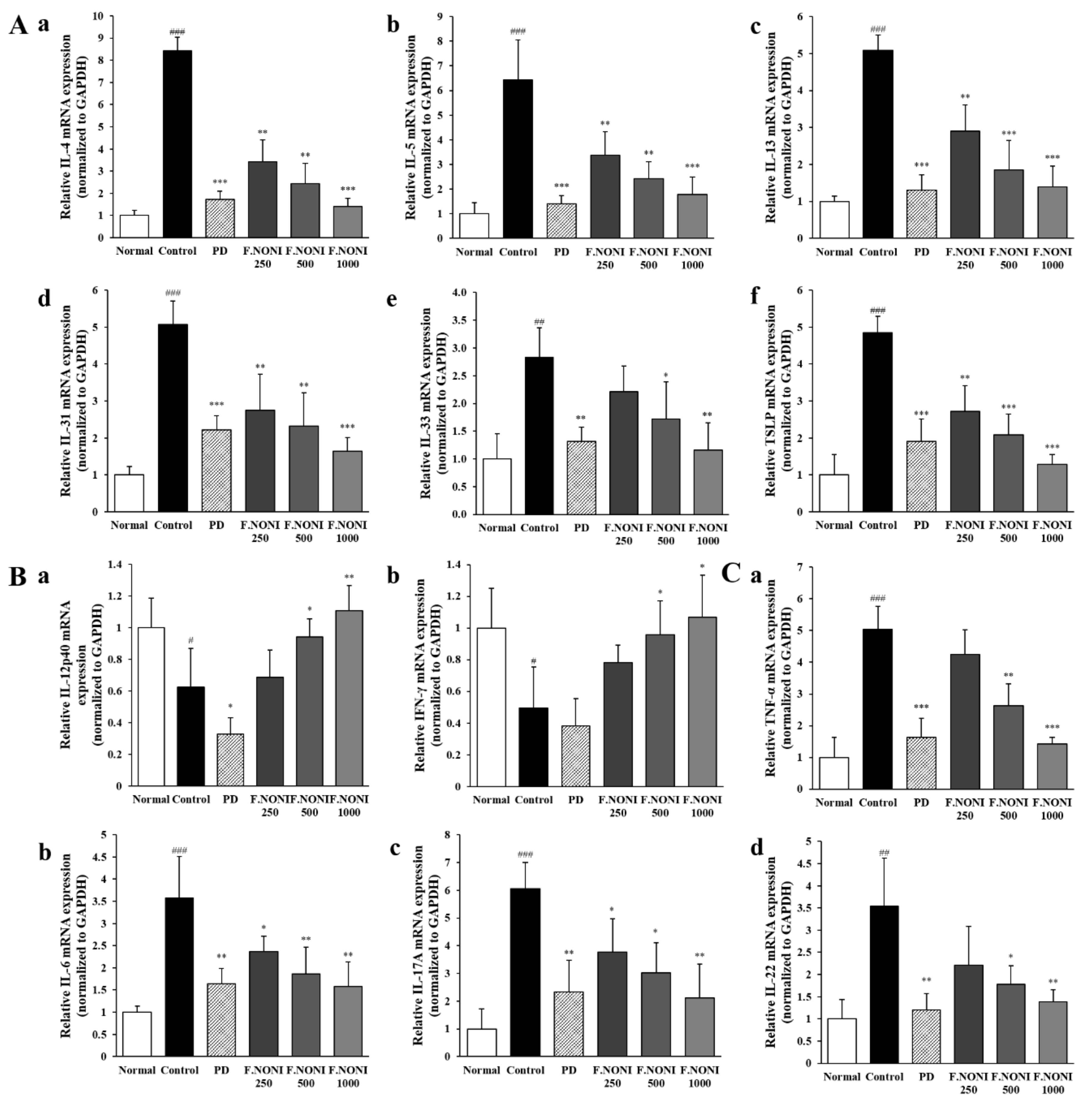
3.6. F.NONI Regulated DNCB-Induced Cytokine Gene Expression in Dorsal Skin
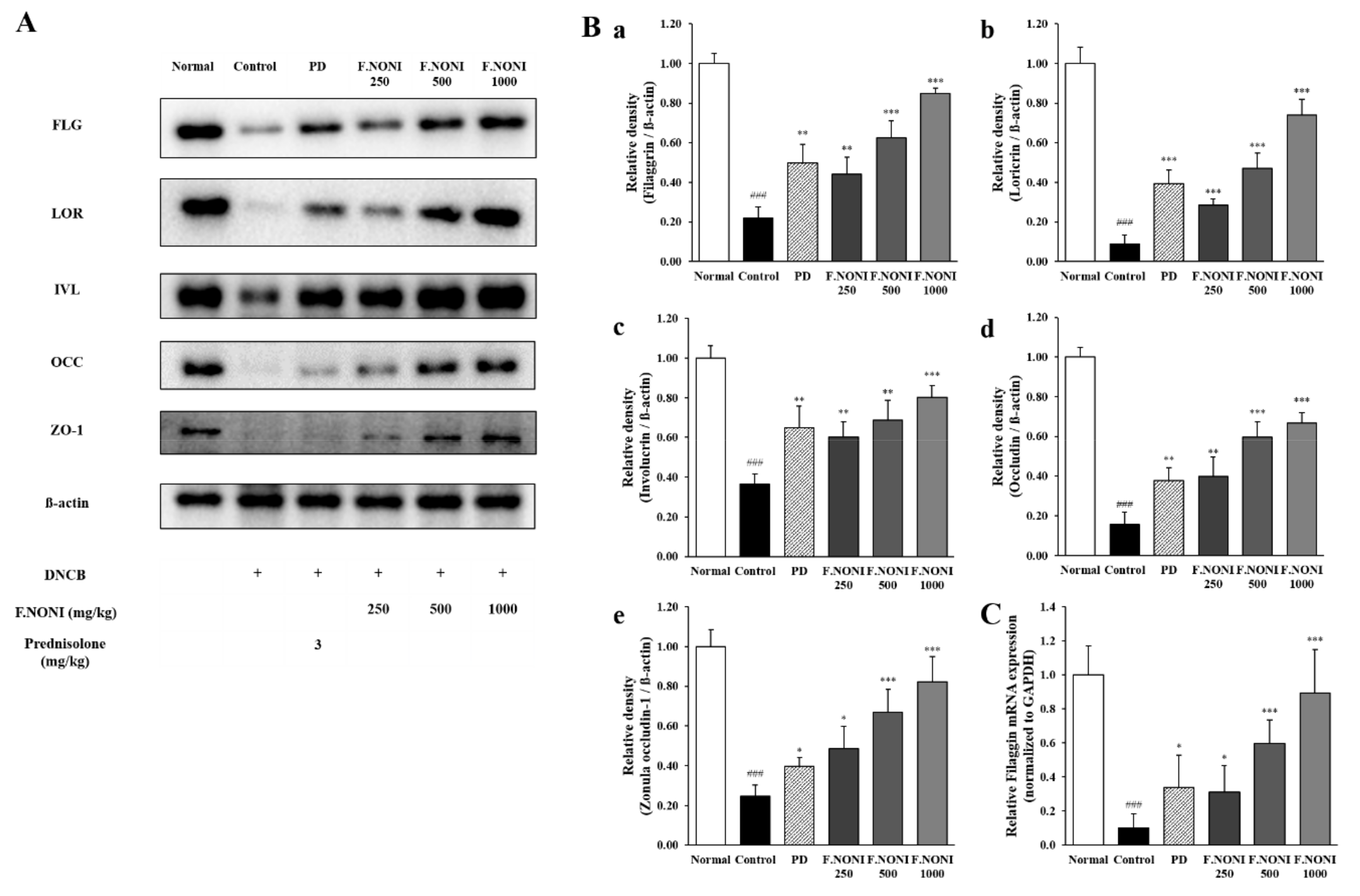
3.7. F.NONI Restored DNCB-Induced Defects in Skin Barrier Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rerknimitr, P.; Otsuka, A.; Nakashima, C.; Kabashima, K. The etiopathogenesis of atopic dermatitis: Barrier disruption, immunological derangement, and pruritus. Inflamm. Regen. 2017, 37, 14. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Thaçi, D.; Simpson, E.L.; Beck, L.A.; Bieber, T.; Blauvelt, A.; Papp, K.; Soong, W.; Worm, M.; Szepietowski, J.C.; Sofen, H. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: A randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet 2016, 387, 40–52. [Google Scholar] [CrossRef]
- Williams, H.C. Atopic dermatitis. N. Engl. J. Med. 2005, 352, 2314–2324. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Paller, A.S. Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 2003, 112, S118–S127. [Google Scholar] [CrossRef]
- Nakajima, S.; Nomura, T.; Common, J.; Kabashima, K. Insights into atopic dermatitis gained from genetically defined mouse models. J. Allergy Clin. Immunol. 2019, 143, 13–25. [Google Scholar] [CrossRef]
- Egawa, G.; Kabashima, K. Barrier dysfunction in the skin allergy. Allergol. Int. 2018, 67, 3–11. [Google Scholar] [CrossRef]
- Egawa, G.; Kabashima, K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J. Allergy Clin. Immunol. 2016, 138, 350–358. [Google Scholar] [CrossRef]
- Novak, N.; Bieber, T. Allergic and nonallergic forms of atopic diseases. J. Allergy Clin. Immunol. 2003, 112, 252–262. [Google Scholar] [CrossRef]
- Stott, B.; Lavender, P.; Lehmann, S.; Pennino, D.; Durham, S.; Schmidt-Weber, C.B. Human IL-31 is induced by IL-4 and promotes TH2-driven inflammation. J. Allergy Clin. Immunol. 2013, 132, 446–454.e445. [Google Scholar] [CrossRef]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K. The pruritus-and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508.e524. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Choi, M.J.; Bak, D.-H.; Lee, B.C.; Ko, E.J.; Ahn, G.R.; Ahn, S.W.; Kim, M.J.; Na, J.; Kim, B.J. Topical administration of EGF suppresses immune response and protects skin barrier in DNCB-induced atopic dermatitis in NC/Nga mice. Sci. Rep. 2018, 8, 11895. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.Y.; Leung, D.M. Immune dysregulation in atopic dermatitis. Curr. Allergy Asthma Rep. 2006, 6, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Waljee, A.K.; Rogers, M.A.; Lin, P.; Singal, A.G.; Stein, J.D.; Marks, R.M.; Ayanian, J.Z.; Nallamothu, B.K. Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ 2017, 357, j1415. [Google Scholar] [CrossRef] [PubMed]
- Carr, W.W. Topical calcineurin inhibitors for atopic dermatitis: Review and treatment recommendations. Pediatr. Drugs 2013, 15, 303–310. [Google Scholar] [CrossRef]
- Matsuda, H.; Watanabe, N.; Geba, G.P.; Sperl, J.; Tsudzuki, M.; Hiroi, J.; Matsumoto, M.; Ushio, H.; Saito, S.; Askenase, P.W. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 1997, 9, 461–466. [Google Scholar] [CrossRef]
- Zhang, E.Y.; Chen, A.Y.; Zhu, B.T. Mechanism of dinitrochlorobenzene-induced dermatitis in mice: Role of specific antibodies in pathogenesis. PLoS ONE 2009, 4, e7703. [Google Scholar] [CrossRef]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal models of atopic dermatitis. J. Investig. Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef]
- Lee, J.W.; Wu, Q.; Jang, Y.P.; Choung, S.Y. Pinus densiflora bark extract ameliorates 2, 4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice by regulating Th1/Th2 balance and skin barrier function. Phytother. Res. 2018, 32, 1135–1143. [Google Scholar] [CrossRef]
- Kinghorn, A.D.; Chai, H.-B.; Sung, C.K.; Keller, W.J. The classical drug discovery approach to defining bioactive constituents of botanicals. Fitoterapia 2011, 82, 71–79. [Google Scholar] [CrossRef] [PubMed]
- McClatchey, W. From Polynesian healers to health food stores: Changing perspectives of Morinda citrifolia (Rubiaceae). Integr. Cancer Ther. 2002, 1, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Suto, H.; Matsuda, H.; Mitsuishi, K.; Hira, K.; Uchida, T.; Unno, T.; Ogawa, H.; Ra, C. NC/Nga mice: A mouse model for atopic dermatitis. Int. Arch. Allergy Immunol. 1999, 120, 70–75. [Google Scholar] [CrossRef]
- Takano, N.; Arai, I.; Kurachi, M. Analysis of the spontaneous scratching behavior by NC/Nga mice: A possible approach to evaluate antipruritics for subjects with atopic dermatitis. Eur. J. Pharmacol. 2003, 471, 223–228. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cook, M.E. Dietary Conjugated Linoleic Acid Decreased Cachexia, Macrophage Tumor Necrosis Factor-α Production, and Modifies Splenocyte Cytokines Production1. Exp. Biol. Med. 2003, 228, 51–58. [Google Scholar] [CrossRef]
- Mimura, T.; Shinozaki, Y.; Kawasaki, H.; Iwamura, H. JTP-27536 [(+)-1, 3-dihydroxy-2-hydroxymethylpropyl-2-ammonium 2-[(R)-3-cyclo-hexyl-1-phenylpropyl]-1, 3-dioxo-2, 3-dihydro-1H-isoindole-5-carboxylate monohydrate], a novel inhibitor of immunoglobulins and interleukin-5 with anti-inflammatory properties in mouse allergic dermatitis model. J. Pharmacol. Exp. Ther. 2005, 314, 293–301. [Google Scholar]
- Sugiura, K.; Shamoto, M.; Sakamoto, N.; Shinzato, M.; Osada, A.; Sugiura, M.; Hayakawa, R.; Kato, Y. It is true that, when Langerhans cells migrate from the skin to the lymph node, they are transported via lymph vessels. Dermatology 2003, 206, 222–224. [Google Scholar] [CrossRef]
- Novak, N.; Bieber, T. The role of dendritic cell subtypes in the pathophysiology of atopic dermatitis. J. Am. Acad. Dermatol. 2005, 53, S171–S176. [Google Scholar] [CrossRef]
- Boguniewicz, M.; Fonacier, L.; Leung, D.Y. Atopic and Contact Dermatitis. In Clinical Immunology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 611–624. [Google Scholar]
- Sohn, E.; Kang, S.C.; Jang, S.-A.; Kwon, J.E.; Pyo, S.; Koo, H.J. Fermented Korean red ginseng ameliorates 1-chloro-2, 4-dinitrobenzene-induced atopic dermatitis via MAPKs/NF-κB pathway in mice. FASEB J. 2016, 30. [Google Scholar] [CrossRef]
- Yin, J.; Yoon, S.; Ahn, H.; Lee, M. Inhibitory activity of allergic contact dermatitis and atopic dermatitis-like skin in BALB/c mouse through oral administration of fermented barks of Alnus sibirica. Molecules 2018, 23, 450. [Google Scholar] [CrossRef] [PubMed]
- West, B.J.; Deng, S.; Isami, F.; Uwaya, A.; Jensen, C.J. The potential health benefits of noni juice: A review of human intervention studies. Foods 2018, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Palu, A.K.; Kim, A.H.; West, B.J.; Deng, S.; Jensen, J.; White, L. The effects of Morinda citrifolia L.(noni) on the immune system: Its molecular mechanisms of action. J. Ethnopharmacol. 2008, 115, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Coutinho de Sousa, B.; Reis Machado, J.; da Silva, M.V.; da Costa, T.A.; Lazo-Chica, J.E.; Degasperi, T.D.P.; Rodrigues Junior, V.; Sales-Campos, H.; Uber Bucek, E.; Freire Oliveira, C.J. Morinda citrifolia (noni) fruit juice reduces inflammatory cytokines expression and contributes to the maintenance of intestinal mucosal integrity in DSS experimental colitis. Mediat. Inflamm. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Peng, L.; Jensen, C.J.; Deng, S.; West, B.J. Noni juice reduces lipid peroxidation–derived DNA adducts in heavy smokers. Food Sci. Nutr. 2013, 1, 141–149. [Google Scholar] [CrossRef]
- Cimanga, K.; Hermans, N.; Apers, S.; Van Miert, S.; Van den Heuvel, H.; Claeys, M.; Pieters, L.; Vlietinck, A. Complement-Inhibiting Iridoids from Morinda morindoides. J. Nat. Prod. 2003, 66, 97–102. [Google Scholar] [CrossRef]
- Li, B.; Zhang, D.-M.; Luo, Y.-M.; Chen, X.-G. Three New and Antitumor Anthraquinone Glycosides from Lasianthus acuminatissimus MERR. Chem. Pharm. Bull. 2006, 54, 297–300. [Google Scholar] [CrossRef]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef]
- Werfel, T.; Allam, J.-P.; Biedermann, T.; Eyerich, K.; Gilles, S.; Guttman-Yassky, E.; Hoetzenecker, W.; Knol, E.; Simon, H.-U.; Wollenberg, A. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 336–349. [Google Scholar] [CrossRef]
- Asahina, R.; Maeda, S. A review of the roles of keratinocyte-derived cytokines and chemokines in the pathogenesis of atopic dermatitis in humans and dogs. Adv. Vet. Dermatol. 2017, 8, 15–25. [Google Scholar]
- Agrawal, R.; Woodfolk, J.A. Skin barrier defects in atopic dermatitis. Curr. Allergy Asthma Rep. 2014, 14, 433. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, A.; Kubo, A.; Beck, L.A. Skin barrier disruption: A requirement for allergen sensitization? J. Investig. Dermatol. 2012, 132, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, J.S.; Cho, D.H.; Park, H.J. Molecular mechanisms of cutaneous inflammatory disorder: Atopic dermatitis. Int. J. Mol. Sci. 2016, 17, 1234. [Google Scholar] [CrossRef]
- Brandt, E.B.; Sivaprasad, U. Th2 cytokines and atopic dermatitis. J. Clin. Cell. Immunol. 2011, 2, 110. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Braathen, L.; Simon, H.U. Eosinophils and atopic dermatitis. Allergy 2004, 59, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.-C.; Meller, S.; Rieker, J. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Furue, M. T helper type 2 signatures in atopic dermatitis. J. Cutan. Immunol. Allergy 2018, 1, 93–99. [Google Scholar] [CrossRef]
- Saeki, H.; Tamaki, K. Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J. Dermatol. Sci. 2006, 43, 75–84. [Google Scholar] [CrossRef]
- Biedermann, T.; Skabytska, Y.; Kaesler, S.; Volz, T. Regulation of T cell immunity in atopic dermatitis by microbes: The yin and yang of cutaneous inflammation. Front. Immunol. 2015, 6, 353. [Google Scholar] [CrossRef]
- Sun, L.; He, C.; Nair, L.; Yeung, J.; Egwuagu, C.E. Interleukin 12 (IL-12) family cytokines: Role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine 2015, 75, 249–255. [Google Scholar] [CrossRef]
- Boguniewicz, M.; Jaffe, H.S.; Izu, A.; Sullivan, M.J.; York, D.; Geha, R.S.; Leung, D.Y. Recombinant gamma interferon in treatment of patients with atopic dermatitis and elevated IgE levels. Am. J. Med. 1990, 88, 365–370. [Google Scholar] [CrossRef]
- Shershakova, N.; Baraboshkina, E.; Andreev, S.; Purgina, D.; Struchkova, I.; Kamyshnikov, O.; Nikonova, A.; Khaitov, M. Anti-inflammatory effect of fullerene C 60 in a mice model of atopic dermatitis. J. Nanobiotechnol. 2016, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; Krueger, J.G.; Guttman-Yassky, E. Skin barrier and immune dysregulation in atopic dermatitis: An evolving story with important clinical implications. J. Allergy Clin. Immunol. Pract. 2014, 2, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Krueger, J.G.; Guttman-Yassky, E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J. Allergy Clin. Immunol. 2015, 135, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.-H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133. [Google Scholar] [CrossRef] [PubMed]
- Michalak-Stoma, A.; Pietrzak, A.; Szepietowski, J.C.; Zalewska-Janowska, A.; Paszkowski, T.; Chodorowska, G. Cytokine network in psoriasis revisited. Eur. Cytokine Netw. 2011, 22, 160–168. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y. Epidermal barrier in atopic dermatitis. Allergy Asthma Immunol. Res. 2012, 4, 12–16. [Google Scholar] [CrossRef]
- Kim, B.S.; Siracusa, M.C.; Saenz, S.A.; Noti, M.; Monticelli, L.A.; Sonnenberg, G.F.; Hepworth, M.R.; Van Voorhees, A.S.; Comeau, M.R.; Artis, D. TSLP elicits IL-33–independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 2013, 5, ra116–ra170. [Google Scholar] [CrossRef]
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786. [Google Scholar] [CrossRef]
- Nakai, K.; Yoneda, K.; Hosokawa, Y.; Moriue, T.; Presland, R.B.; Fallon, P.G.; Kabashima, K.; Kosaka, H.; Kubota, Y. Reduced expression of epidermal growth factor receptor, E-cadherin, and occludin in the skin of flaky tail mice is due to filaggrin and loricrin deficiencies. Am. J. Pathol. 2012, 181, 969–977. [Google Scholar] [CrossRef]
- Kubo, A.; Nagao, K.; Yokouchi, M.; Sasaki, H.; Amagai, M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 2009, 206, 2937–2946. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.; Elias, P.M.; Crumrine, D.; Lin, T.-K.; Brandner, J.M.; Hachem, J.-P.; Presland, R.B.; Fleckman, P.; Janecke, A.R.; Sandilands, A. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am. J. Pathol. 2011, 178, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Batista, D.; Perez, L.; Orfali, R.; Zaniboni, M.; Samorano, L.; Pereira, N.; Sotto, M.; Ishizaki, A.; Oliveira, L.; Sato, M. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sequence (Forward) | Sequence (Reverse) |
|---|---|---|
| IFN-γ | ATG AAC GCT ACA CAC TGC ATC | CCA TCC TTT TGC CAG TTC CTC |
| IL-4 | ACG GGA GAA GGG ACG CCA T | GAA GCC GTA CAG ACG AGC TCA |
| IL-5 | CAA AAA GAG AAG TGT GGC GAG G | TAG ATA GGA GCA GGA AGC CC |
| IL-6 | TAG TCC TTC CTA CCC CAA TTT CC | TTG GTC CTT AGC CAC TCC TTC |
| IL-12 | GCA GAA AGG TGC GTT CCT CG | ATG TGC AGG TGT GGT TGG GC |
| IL-13 | CCT GGC TCT TGC TTG CCT T | GGT CTT GTG TGA TGT TGC TCA |
| IL-17A | AAG GCA GCA GCG ATC ATC C | GGA ACG GTT GAG GTA GTC TGA G |
| IL-22 | CGA TCT CTG ATG GCT GTC CT | ACG CAA GCA TTT CTC AGA GA |
| IL-31 | TCA GCA GAC GAA TCA ATA CAG C | TCG CTC AAC ACT TTG ACT TTC T |
| IL-33 | TCC AAC TCC AAG ATT TCC CCG | CAT GCA GTA GAC ATG GCA GAA |
| Pro-FLG | GAA TCC ATA TTT ACA GCA AAG CAC CTT G | GGT ATG TCC AAT GTG ATT GCA CGA TTG |
| TNF-α | CAC AAG ATG CTG GGA CAG TGA | TCC TTG ATG GTG GTG CAT GA |
| TSLP | TGC AAG TAC TAG TAC GGA TGG GGC | GGA CTT CTT GTG CCA TTT CCT GAG |
| GAPDH | CGG CCG CAT CTT CTT GTG | CCG ACC TTC ACC ATT TTG TCT AC |
| Sample | DAA | AA |
|---|---|---|
| Noni | 9.303 ± 0.146 | 1.470 ± 0.065 |
| F.NONI | 13.219 ± 0.146 | 2.266 ± 0.065 |
| Groups | Concentration (mg/kg Body Weight) | Serum Level (μg/mL) | Normalized to Normal IgG1/IgG2a | |
|---|---|---|---|---|
| IgG1 | IgG2a | |||
| Normal | - | 722.6 ± 107.9 | 597.8 ± 279.5 | 1.0 |
| Control | - | 2414.9 ± 215.4 ### | 1687.8 ± 268.4 ## | 1.8 ## |
| PD | 3 | 1299.7 ± 317.7 *** | 1038.9 ± 152.4 *** | 1.2 * |
| F.NONI | 250 | 1992.1 ± 251.9 * | 1368.5 ± 140.3 | 1.7 |
| 500 | 1688.7 ± 258.5 ** | 1389.5 ± 242.8 | 1.4 * | |
| 1000 | 1273.6 ± 231.8 *** | 1485.8 ± 116.5 | 1.1 ** | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Seong, G.S.; Choung, S.Y. Fermented Morinda citrifolia (Noni) Alleviates DNCB-Induced Atopic Dermatitis in NC/Nga Mice through Modulating Immune Balance and Skin Barrier Function. Nutrients 2020, 12, 249. https://doi.org/10.3390/nu12010249
Kim SH, Seong GS, Choung SY. Fermented Morinda citrifolia (Noni) Alleviates DNCB-Induced Atopic Dermatitis in NC/Nga Mice through Modulating Immune Balance and Skin Barrier Function. Nutrients. 2020; 12(1):249. https://doi.org/10.3390/nu12010249
Chicago/Turabian StyleKim, Sung Ho, Geum Su Seong, and Se Young Choung. 2020. "Fermented Morinda citrifolia (Noni) Alleviates DNCB-Induced Atopic Dermatitis in NC/Nga Mice through Modulating Immune Balance and Skin Barrier Function" Nutrients 12, no. 1: 249. https://doi.org/10.3390/nu12010249
APA StyleKim, S. H., Seong, G. S., & Choung, S. Y. (2020). Fermented Morinda citrifolia (Noni) Alleviates DNCB-Induced Atopic Dermatitis in NC/Nga Mice through Modulating Immune Balance and Skin Barrier Function. Nutrients, 12(1), 249. https://doi.org/10.3390/nu12010249




