Researching New Therapeutic Approaches for Abdominal Visceral Pain Treatment: Preclinical Effects of an Assembled System of Molecules of Vegetal Origin
Abstract
1. Introduction
2. Material and Methods
2.1. System of Molecules Composition
2.2. Animals
2.3. Induction of Colitis
2.4. Treatments
2.5. Assessment of Visceral Sensitivity
2.6. Macroscopic and Microscopic Analysis of Tissue Damage
2.7. Statistical Analysis
3. Results
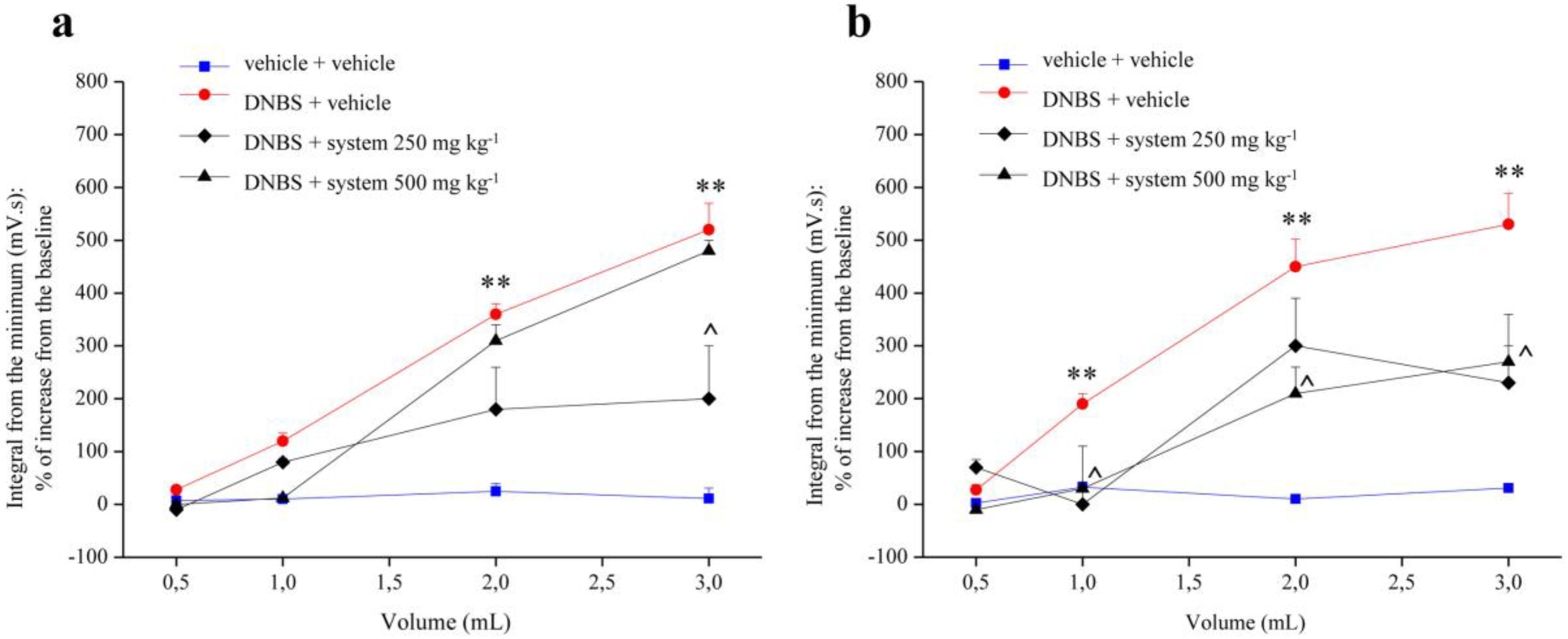
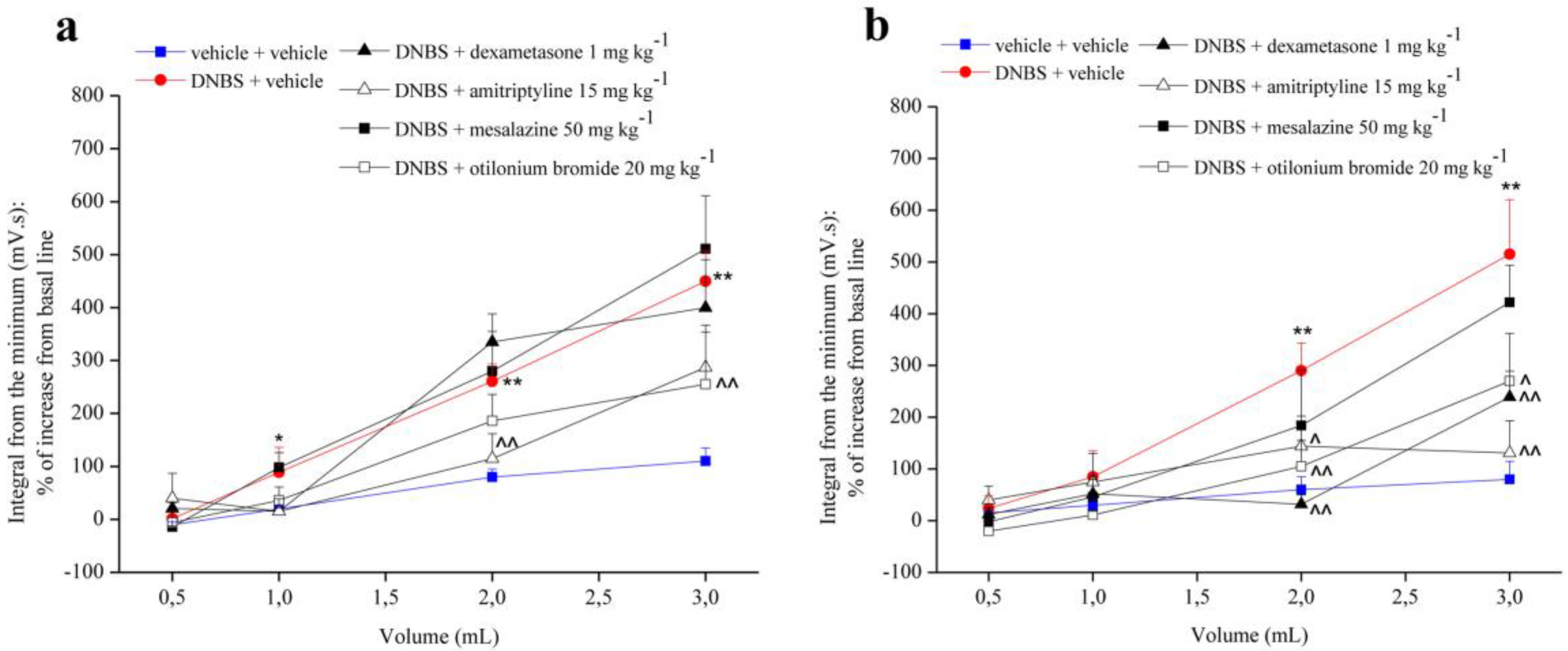
3.1. Effect of Two Different doses of System and Reference Drugs on Visceral Hypersensitivity
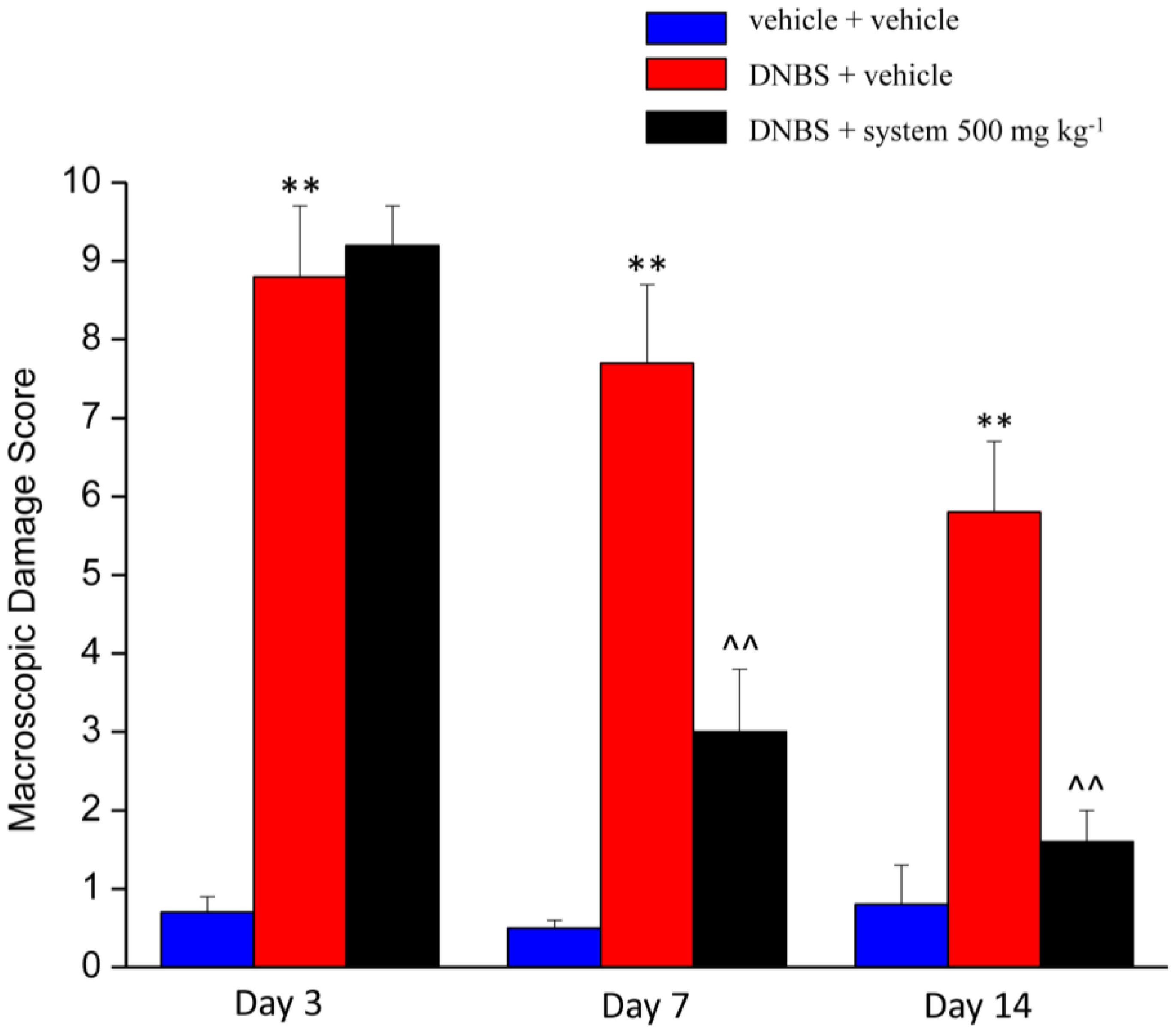
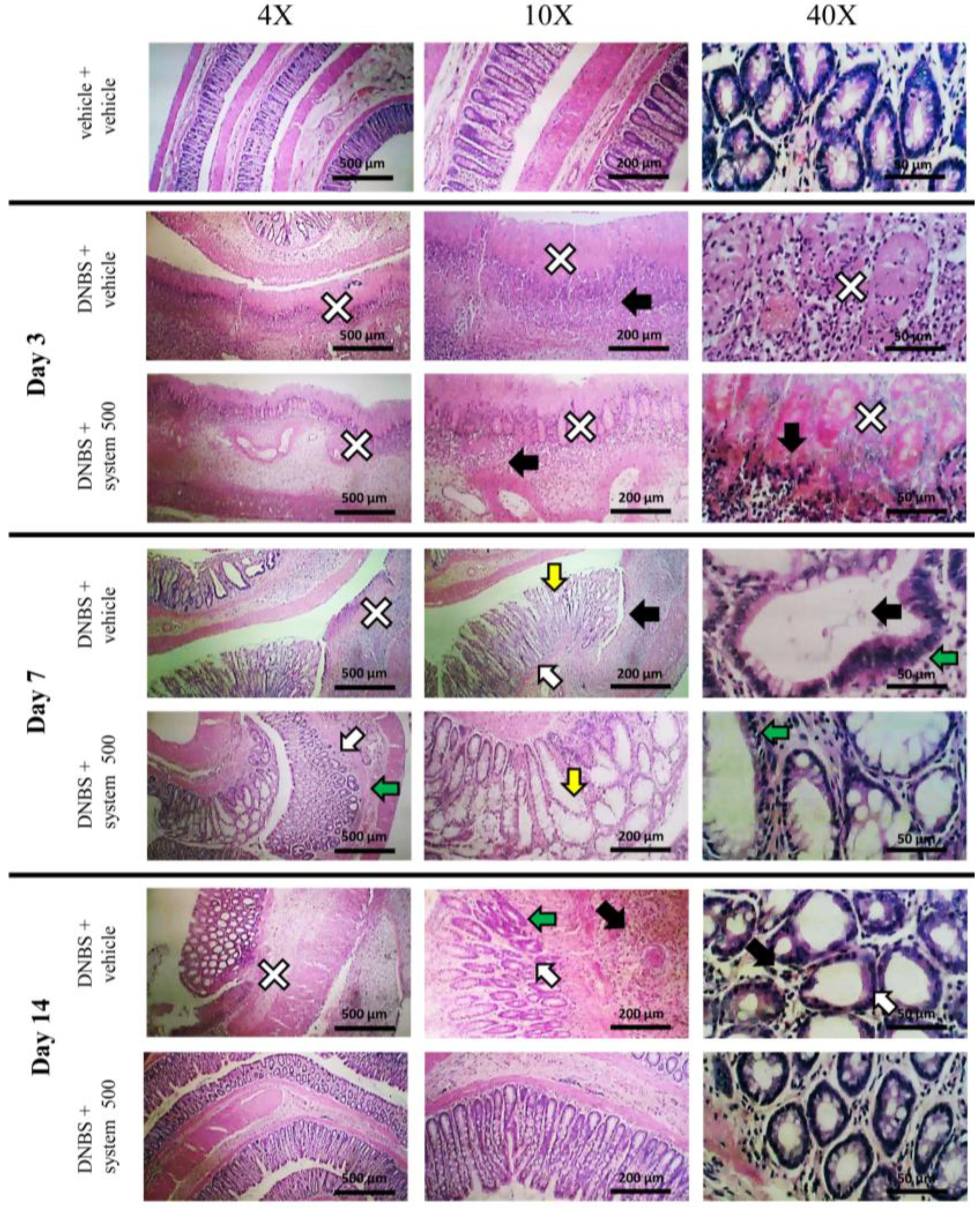
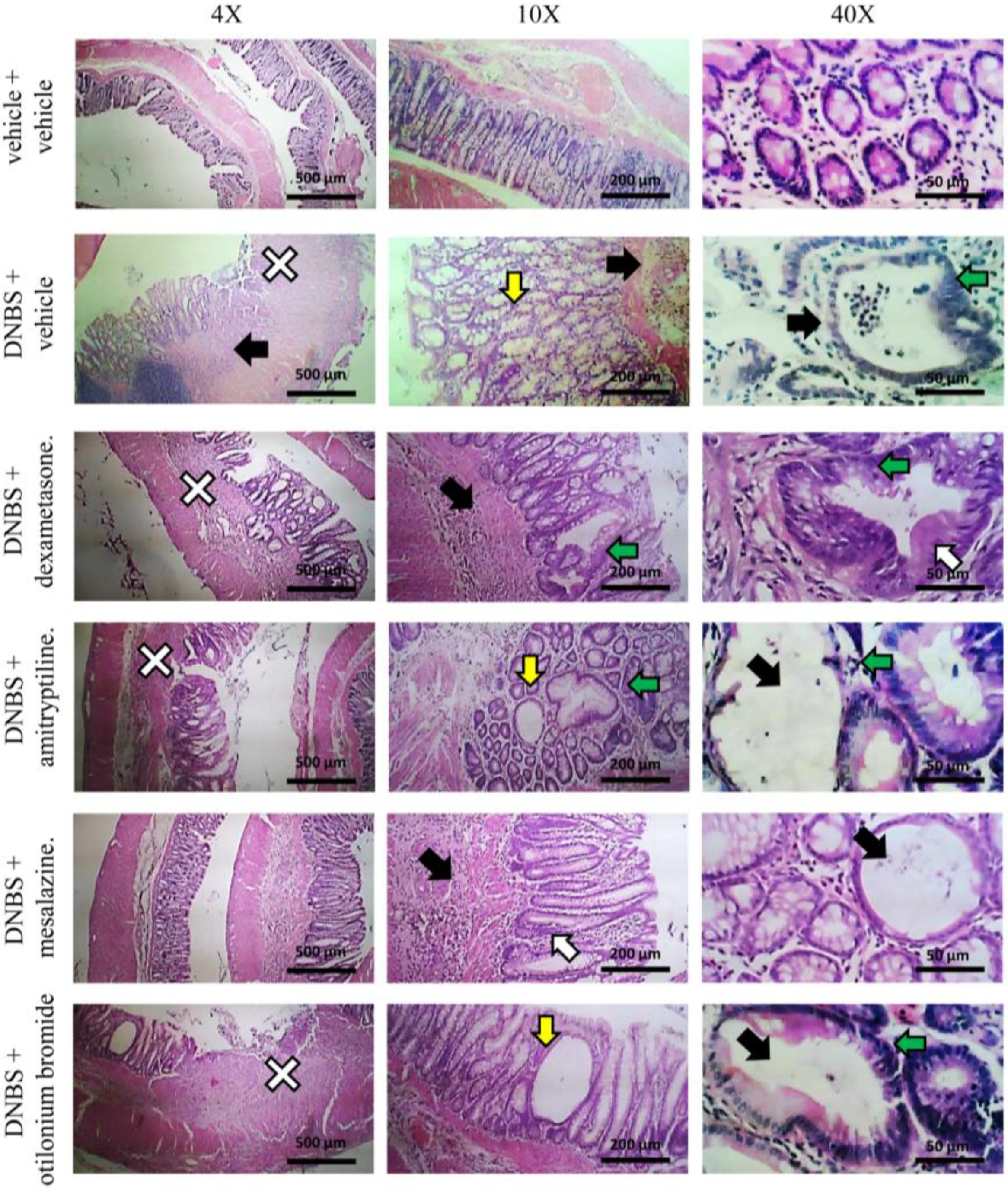
3.2. Effect of System Injection on Colon Damage
3.3. Effect of Reference Drugs Administration on Colon Damage
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Morales-Soto, W.; Gulbransen, B.D. Enteric glia: A new player in abdominal pain. Cell. Mol. Gastroenterol. Hepatol. 2018, 7, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Giamberardino, M.A.; Costantini, R.; Affaitati, G.; Fabrizio, A.; Lapenna, D.; Tafuri, E.; Mezzetti, A. Viscero–visceral hyperalgesia: Characterization in different clinical models. Pain 2010, 151, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Schirbel, A.; Reichert, A.; Roll, S.; Baumgart, D.C.; Büning, C.; Wittig, B.; Wiedenmann, B.; Dignass, A.; Sturm, A. Impact of pain on health-related quality of life in patients with inflammatory bowel disease. World J. Gastroenterol. 2010, 16, 3168–3177. [Google Scholar] [CrossRef]
- Pithadia, A.B.; Jain, S. Treatment of inflammatory bowel diseases (IBD). Pharmacol. Rep. 2011, 63, 629–642. [Google Scholar] [CrossRef]
- Srinath, A.I.; Walter, C.; Newara, M.C.; Szigethy, E.M. Pain management in patients with inflammatory bowel disease: Insights for the clinician. Ther. Adv. Gastroenterol. 2012, 5, 339–357. [Google Scholar] [CrossRef]
- Minderhoud, I.M.; Oldenburg, B.; Wismeijer, J.A.; Van Berge Henegouwen, G.P.; Smout, A.J. IBS-like symptoms in patients with inflammatory bowel disease in remission; relationships with quality of life and coping behavior. Dig. Dis. Sci. 2004, 49, 469–474. [Google Scholar] [CrossRef]
- Farrokhyar, F.; Marshall, J.K.; Easterbrook, B.; Irvine, E.J. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: Prevalence and impact on health. Inflamm. Bowel Dis. 2006, 12, 38–46. [Google Scholar] [CrossRef]
- Spiller, R.; Major, G. IBS and IBD—Separate entities or on a spectrum? Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 613–621. [Google Scholar] [CrossRef]
- Portincasa, P.; Lembo, A.; De Bari, O.; Di Palo, D.M.; Maggio, A.; Cataldo, I.; Calamita, G. The role of dietary approach in Irritable Bowel Syndrome. Curr. Med. Chem. 2017, 26, 3512–3520. [Google Scholar] [CrossRef]
- Khan, I.; Samson, S.E.; Grover, A.K. Antioxidant supplements and gastrointestinal diseases: A critical appraisal. Med. Princ. Pract. 2017, 26, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Sue, W.; Lesley, R.; Andrea, R.; Pam, B.; Sukhdev, S. Prevalence of irritable bowel syndrome: A community survey. Br. J. Gen. Pract. 2004, 54, 495–502. [Google Scholar]
- Docherty, M.J.; Jones, R.C., III; Wallace, M.S. Managing pain in inflammatory bowel disease. Gastroenterol. Hepatol. 2011, 7, 592–601. [Google Scholar]
- Camilleri, M.; Boeckxstaens, G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 2017, 66, 966–974. [Google Scholar] [CrossRef]
- Su, H.; Li, Y.T.; Heitkemper, M.M.; Zia, J. Effects of low-FODMAPS diet on irritable bowel syndrome symptoms and gut microbiome. Gastroenterol. Nurs. 2019, 42, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, D.; Rancan, S.; Orso, G.; Dall’Acqua, S.; Brun, P.; Giron, M.C.; Carrara, M.; Castagliuolo, I.; Ragazzi, E.; Caparrotta, L.; et al. Boswellia serrata preserves intestinal epithelial barrier from oxidative and inflammatory damage. PLoS ONE 2015, 10, e0125375. [Google Scholar] [CrossRef]
- Cervero, F. Central sensitization and visceral hypersensitivity: Facts and fictions. Scand. J. Pain 2014, 5, 49–50. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Mijan, M.A.; Lim, B.O. Diets, functional foods, and nutraceuticals as alternative therapies for inflammatory bowel disease: Present status and future trends. World J. Gastroenterol. 2018, 24, 2673–2685. [Google Scholar] [CrossRef]
- Wallace, J.L.; Le, T.; Carter, L.; Appleyard, C.B.; Beck, P.L. Hapten-induced chronic colitis in the rat: alternatives to trinitrobenzene sulfonic acid. J. Pharmacol. Toxicol. Methods 1995, 33, 237–239. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; Mancuso, G.; Midiri, A.; Di Paola, R.; Cuzzocrea, S. Therapeutic potential of dinitrobenzene sulfonic acid (DNBS)-induced colitis in mice by targeting IL-1β and IL-18. Biochem. Pharmacol. 2018, 155, 150–161. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [PubMed]
- Fornai, M.; Blandizzi, C.; Antonioli, L.; Colucci, R.; Bernardini, N.; Segnani, C.; De Ponti, F.; Del Tacca, M. Differential role of cyclooxygenase 1 and 2 isoforms in the modulation of colonic neuromuscular function in experimental inflammation. J. Pharmacol. Exp. Ther. 2006, 317, 938–945. [Google Scholar] [CrossRef]
- Christianson, J.A.; Gebhart, G.F. Assessment of colon sensitivity by luminal distension in mice. Nat. Protoc. 2007, 2, 2624–2631. [Google Scholar] [CrossRef]
- Antonioli, L.; Fornai, M.; Colucci, R.; Ghisu, N.; Da Settimo, F.; Natale, G.; Kastsiuchenka, O.; Duranti, E.; Virdis, A.; Vassalle, C.; et al. Inhibition of adenosine deaminase attenuates inflammation in experimental colitis. J. Pharmacol. Exp. Ther. 2007, 322, 435–442. [Google Scholar] [CrossRef]
- Qin, X. Etiology of inflammatory bowel disease: A unified hypothesis. World J. Gastroenterol. 2012, 18, 1708–1722. [Google Scholar] [CrossRef]
- Adam, B.; Liebregts, T.; Bertram, S.; Holtmann, G. Functional gastrointestinal disorders. Dtsch. Med. Wochenschr. 2006, 131, 2531–2540. [Google Scholar] [CrossRef]
- Lucarini, E.; Di Cesare Mannelli, L.; Micheli, L.; Trallori, E.; Antonioli, L.; Fornai, M.; Blandizzi, C.; Ghelardini, C. Post-inflammatory visceral pain induced by DNBS: Preclinical features for novel therapeutics. J. Crohn’s Colitis 2018, 12 (Suppl. 1), S123. [Google Scholar] [CrossRef][Green Version]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]
- Qin, X. The effect of dietary chemicals on gut bacteria and IBD demands further study. J. Crohn’s Colitis 2011, 5, 175. [Google Scholar] [CrossRef]
- Choi, E.J.; Lee, H.J.; Kim, W.J.; Han, K.I.; Iwasa, M.; Kobayashi, K.; Debnath, T.; Tang, Y.; Kwak, Y.S.; Yoon, J.H.; et al. Enterococcus faecalis EF-2001 protects DNBS-induced inflammatory bowel disease in mice model. PLoS ONE 2019, 14, e0210854. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, M.R.; Di Sabatino, A.; Cremon, C.; Giuffrida, P.; Fiorentino, M.; Altimari, A.; Bellacosa, L.; Stanghellini, V.; Barbara, G. Interferon-γ is increased in the gut of patients with irritable bowel syndrome and modulates serotonin metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G439–G447. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Cremon, C.; Pallotti, F.; De Giorgio, R.; Stanghellini, V.; Corinaldesi, R. Postinfectious irritable bowel syndrome. Gastroenterology 2009, 48 (Suppl. 2), S95–S97. [Google Scholar] [CrossRef]
- Barbara, G.; Cremon, C.; De Giorgio, R.; Dothel, G.; Zecchi, L.; Bellacosa, L.; Carini, G.; Stanghellini, V.; Corinaldesi, R. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr. Gastroenterol. Rep. 2011, 13, 308–315. [Google Scholar] [CrossRef]
- Gerova, V.A.; Stoynov, S.G.; Katsarov, D.S.; Svinarov, D.A. Increased intestinal permeability in inflammatory bowel diseases assessed by iohexol test. World J. Gastroenterol. 2011, 17, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Pizzoferrato, M.; Gerardi, V.; Lopetuso, L.; Gasbarrini, A. The gut barrier: New acquisitions and therapeutic approaches. J. Clin. Gastroenterol. 2012, 46, S12–S17. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Petito, V.; Zambrano, D.; Orlando, D.; Dal Lago, A.; Serrichhio, L.; Papa, A.; Gasbarrini, A.; Scaldaferri, F. Gut microbiota: A key modulator of intestinal healing in inflammatory bowel disease. Dig. Dis. 2016, 34, 202–209. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Peloquin, J.M.; Nguyen, D.D. The microbiota and inflammatory bowel disease: Insights from animal models. Anaerobe 2013, 24, 102–106. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Franceschi, F.; Gasbarrini, A. The gastrointestinal microbiome—Functional interference between stomach and intestine. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 995–1002. [Google Scholar] [CrossRef]
- Fasano, A. Intestinal permeability and its regulation by zonulin: Diagnostic and therapeutic implications. Clin. Gastroenterol. Hepatol. 2012, 10, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Matsuda, H.; Yoshikawa, M. A review of anti-inflammatory terpenoids from the incense gum resins frankincense and myrrh. J. Oleo Sci. 2017, 66, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Kiela, P.R.; Midura, A.J.; Kuscuoglu, N.; Jolad, S.D.; Sólyom, A.M.; Besselsen, D.G.; Timmermann, B.N.; Ghishan, F.K. Effects of Boswellia serrata in mouse models of chemically induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G798–G808. [Google Scholar] [CrossRef]
- Hartmann, R.M.; Martins, M.I.; Tieppo, J.; Fillmann, H.S.; Marroni, N.P. Effect of Boswellia serrata on antioxidant status in an experimental model of colitis rats induced by acetic acid. Dig. Dis. Sci. 2012, 57, 2038–2044. [Google Scholar] [CrossRef]
- Gayathri, B.; Manjula, N.; Vinaykumar, K.S.; Lakshmi, B.S.; Balakrishnan, A. Pure compound from Boswellia serrata extract exhibits anti-inflammatory property in human PBMCs and mouse macrophages through inhibition of TNFalpha, IL-1beta, NO and MAP kinases. Int. Immunopharmacol. 2007, 7, 473–482. [Google Scholar] [CrossRef]
- Hartmann, R.M.; Fillmann, H.S.; Martins, M.I.; Meurer, L.; Marroni, N.P. Boswellia serrata has beneficial anti-inflammatory and antioxidant properties in a model of experimental colitis. Phytother. Res. 2014, 28, 1392–1398. [Google Scholar] [CrossRef]
- Park, C.H.; Nam, D.Y.; Son, H.U.; Lee, S.R.; Lee, H.J.; Heo, J.C.; Cha, T.Y.; Baek, J.H.; Lee, S.H. Polymer fraction of Aloe vera exhibits a protective activity on ethanol-induced gastric lesions. Int. J. Mol. Med. 2011, 27, 511–518. [Google Scholar]
- Azar, A.S.; Shilan, M.; Yara, S.; Maryam, B.; Reza, H.; Hamid Reza, M.E.; Mohammad, A. Benefit of Aloe vera and Matricaria recutita mixture in rat irritable bowel syndrome: Combination of antioxidant and spasmolytic effects. Chin. J. Integr. Med. 2012, 1–9. [Google Scholar]
- Zhang, L.J.; Huang, X.J.; Shi, X.D.; Chen, H.H.; Cui, S.W.; Nie, S.P. Protective effect of three glucomannans from different plants against DSS induced colitis in female BALB/c mice. Food Funct. 2019, 10, 1928–1939. [Google Scholar] [CrossRef]
- Valussi, M. Functional foods with digestion-enhancing properties. Int. J. Food Sci. Nutr. 2012, 63 (Suppl. 1), 82–89. [Google Scholar] [CrossRef]
- Vejdani, R.; Shalmani, H.R.M.; Mir-Fattahi, M. The efficacy of an herbal medicine, Carmint, on the relief of abdominal pain and bloating in patients with irritable bowel syndrome: A pilot study. Dig. Dis Sci. 2006, 51, 1501. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U.; Müller, V.; Schneider, B.; Stange, R. Efficacy and safety of a herbal medicinal product containing myrrh, chamomile and coffee charcoal for the treatment of gastrointestinal disorders: A non-interventional study. BMJ Open Gastroenterol. 2015, 1, e000015. [Google Scholar] [CrossRef] [PubMed]
- Maged, Y.; Peter, A.; Fernando, A.; Riccardo, C.; Metka, F.; Maria, J.F.; Pierre, G.; David, G.; Ursula, G.R.; Gunter, G.K.; et al. Safety of hydroxyanthracene derivatives for use in food. EFSA J. 2018, 16, 5090. [Google Scholar]
- Calapai, G.; Marisa, D. Assessment Report on Glycyrrhiza glabra L. and/or Glycyrrhiza inflata Bat. and/or Glycyrrhiza uralensis Fisch.; Report No. EMA/HMPC/571122/2010; Committee on Herbal Medicinal Products (HMPC), European Medicines Agency: London, UK, 2011. [Google Scholar]
- Higashi, T.; Iohara, D.; Motoyama, K.; Arima, H. Supramolecular pharmaceutical sciences: A novel concept combining pharmaceutical sciences and supramolecular chemistry with a focus on cyclodextrin-based supermolecules. Chem. Pharm. Bull. 2018, 66, 207–216. [Google Scholar] [CrossRef] [PubMed]
- David, K.S. A supramolecular approach to medicinal chemistry: Medicine beyond the molecule. J. Chem. Educ. 2005, 82, 393. [Google Scholar]
- Sardi, C.; Garetto, S.; Capone, L.; Galbiati, V.; Racchi, M.; Govoni, S.; Giovagnoni, E.; Lucci, J. Experimental paradigm for the assessment of the non-pharmacological mechanism of action in medical device classification: The example of glycerine as laxative. Front. Pharmacol. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisio, C.; Lucarini, E.; Micheli, L.; Toti, A.; Di Cesare Mannelli, L.; Antonini, G.; Panizzi, E.; Maidecchi, A.; Giovagnoni, E.; Lucci, J.; et al. Researching New Therapeutic Approaches for Abdominal Visceral Pain Treatment: Preclinical Effects of an Assembled System of Molecules of Vegetal Origin. Nutrients 2020, 12, 22. https://doi.org/10.3390/nu12010022
Parisio C, Lucarini E, Micheli L, Toti A, Di Cesare Mannelli L, Antonini G, Panizzi E, Maidecchi A, Giovagnoni E, Lucci J, et al. Researching New Therapeutic Approaches for Abdominal Visceral Pain Treatment: Preclinical Effects of an Assembled System of Molecules of Vegetal Origin. Nutrients. 2020; 12(1):22. https://doi.org/10.3390/nu12010022
Chicago/Turabian StyleParisio, Carmen, Elena Lucarini, Laura Micheli, Alessandra Toti, Lorenzo Di Cesare Mannelli, Giulia Antonini, Elena Panizzi, Anna Maidecchi, Emiliano Giovagnoni, Jacopo Lucci, and et al. 2020. "Researching New Therapeutic Approaches for Abdominal Visceral Pain Treatment: Preclinical Effects of an Assembled System of Molecules of Vegetal Origin" Nutrients 12, no. 1: 22. https://doi.org/10.3390/nu12010022
APA StyleParisio, C., Lucarini, E., Micheli, L., Toti, A., Di Cesare Mannelli, L., Antonini, G., Panizzi, E., Maidecchi, A., Giovagnoni, E., Lucci, J., & Ghelardini, C. (2020). Researching New Therapeutic Approaches for Abdominal Visceral Pain Treatment: Preclinical Effects of an Assembled System of Molecules of Vegetal Origin. Nutrients, 12(1), 22. https://doi.org/10.3390/nu12010022





