Red for “Stop”: “Traffic-Light” Nutrition Labels Decrease Unhealthy Food Choices by Increasing Activity and Connectivity in the Frontal Lobe
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Materials and Nutrition Label Decision-Making Task
2.4. Measures
2.4.1. Restrained Eating Subscale of the DEBQ
2.4.2. Visual Analog Scale
2.5. fMRI Data Acquisition
2.6. Behavioral Data Analysis
2.7. fMRI Data Analysis
2.7.1. fMRI Data Analysis Processing
2.7.2. General Linear Model (GLM) Analyses
2.7.3. Psychophysiological Interaction (PPI) Analyses
3. Results
3.1. Effect of Label Condition on Decision-Making
3.2. Effect of Label Condition on Regional Brain Activity
3.3. Effect of Label Condition on the Functional Connectivity of the ACC
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Downs, J.S.; Loewenstein, G.; Wisdom, J. Strategies for promoting healthier food choices. Am. Econ. Rev. 2009, 99, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Just, D.R.; Payne, C.R. Obesity: Can behavioral economics help? Ann. Behav. Med. 2009, 38 (Suppl. 1), s47–s55. [Google Scholar] [CrossRef] [PubMed]
- Kiesel, K.; McCluskey, J.J.; Villas-Boas, S.B. Nutritional labeling and consumer choices. Annu. Rev. Resour. Econ. 2011, 3, 141–158. [Google Scholar] [CrossRef]
- Sonnenberg, L.; Gelsomin, E.; Levy, D.E.; Riis, J.; Barraclough, S.; Thorndike, A.N. A traffic light food labeling intervention increases consumer awareness of health and healthy choices at the point-of-purchase. Prev. Med. 2013, 57, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.; Doxey, J.; Hammond, D. Nutrition labels on pre-packaged foods: A systematic review. Public Health Nutr. 2011, 14, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.; Warin, L. Impact of food labelling systems on food choices and eating behaviours: A systematic review and meta-analysis of randomized studies. Obes. Rev. 2016, 17, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Jeffery, R.W. Predictors of nutrition label viewing during food purchase decision making: An eye tracking investigation. Public Health Nutr. 2012, 15, 189–197. [Google Scholar] [CrossRef]
- Miller, L.M.S.; Cassady, D.L. The effects of nutrition knowledge on food label use. A review of the literature. Appetite 2015, 92, 207–216. [Google Scholar] [CrossRef]
- Hersey, J.C.; Wohlgenant, K.C.; Arsenault, J.E.; Kosa, K.M.; Muth, M.K. Effects of front-of-package and shelf nutrition labeling systems on consumers. Nutr. Rev. 2013, 71, 1–14. [Google Scholar] [CrossRef]
- Trudel, R.; Murray, K.B.; Kim, S.; Chen, S. The impact of traffic light color-coding on food health perceptions and choice. J. Exp. Psychol. Appl. 2015, 21, 255. [Google Scholar] [CrossRef]
- Hawley, K.L.; Roberto, C.A.; Bragg, M.A.; Liu, P.J.; Schwartz, M.B.; Brownell, K.D. The science on front-of-package food labels. Public Health Nutr. 2013, 16, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Enax, L.; Hu, Y.; Trautner, P.; Weber, B. Nutrition labels influence value computation of food products in the ventromedial prefrontal cortex. Obesity 2015, 23, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Thorndike, A.N.; Sonnenberg, L.; Riis, J.; Barraclough, S.; Levy, D.E. A 2-phase labeling and choice architecture intervention to improve healthy food and beverage choices. Am. J. Public Health 2012, 102, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Emrich, T.E.; Qi, Y.; Lou, W.Y.; L’Abbe, M.R. Traffic-light labels could reduce population intakes of calories, total fat, saturated fat, and sodium. PLoS ONE 2017, 12, e0171188. [Google Scholar] [CrossRef] [PubMed]
- Sacks, G.; Veerman, J.L.; Moodie, M.; Swinburn, B. ‘Traffic-light’ nutrition labelling and ‘junk-food’ tax: A modelled comparison of cost-effectiveness for obesity prevention. Int. J. Obes. 2011, 35, 1001. [Google Scholar] [CrossRef]
- Becker, M.W.; Bello, N.M.; Sundar, R.P.; Peltier, C.; Bix, L. Front of pack labels enhance attention to nutrition information in novel and commercial brands. Food Policy 2015, 56, 76–86. [Google Scholar] [CrossRef]
- Bialkova, S.; Grunert, K.G.; Juhl, H.J.; Wasowicz-Kirylo, G.; Stysko-Kunkowska, M.; van Trijp, H.C. Attention mediates the effect of nutrition label information on consumers’ choice. Evidence from a choice experiment involving eye-tracking. Appetite 2014, 76, 66–75. [Google Scholar] [CrossRef]
- Enax, L.; Krajbich, I.; Weber, B. Salient nutrition labels increase the integration of health attributes in food decision-making. Judgm. Decis. Mak. 2016, 11, 460. [Google Scholar]
- Ng, J.; Stice, E.; Yokum, S.; Bohon, C. An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite 2011, 57, 65–72. [Google Scholar] [CrossRef]
- Wegman, J.; van Loon, I.; Smeets, P.A.; Cools, R.; Aarts, E. Top-down expectation effects of food labels on motivation. NeuroImage 2018, 173, 13–24. [Google Scholar] [CrossRef]
- Grabenhorst, F.; Schulte, F.P.; Maderwald, S.; Brand, M. Food labels promote healthy choices by a decision bias in the amygdala. NeuroImage 2013, 74, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Prevost, M.; Hot, P.; Muller, L.; Ruffieux, B.; Cousin, E.; Pichat, C.; Baciu, M. Neural correlates of the healthiness evaluation processes of food labels. Nutr. Neurosci. 2018, 21, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Fayet, F.; Petocz, P.; Samman, S. Prevalence and correlates of dieting in college women: A cross sectional study. Int. J. Women’s Health 2012, 4, 405. [Google Scholar]
- Krain, A.L.; Wilson, A.M.; Arbuckle, R.; Castellanos, F.X.; Milham, M.P. Distinct neural mechanisms of risk and ambiguity: A meta-analysis of decision-making. NeuroImage 2006, 32, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Neubert, F.X.; Mars, R.B.; Sallet, J.; Rushworth, M.F. Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc. Natl. Acad. Sci. USA 2015, 112, E2695–E2704. [Google Scholar] [CrossRef]
- Rogers, R.D.; Ramnani, N.; Mackay, C.; Wilson, J.L.; Jezzard, P.; Carter, C.S.; Smith, S.M. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol. Psychiatry 2004, 55, 594–602. [Google Scholar] [CrossRef]
- Rushworth, M.F.; Kolling, N.; Sallet, J.; Mars, R.B. Valuation and decision-making in frontal cortex: One or many serial or parallel systems? Curr. Opin. Neurobiol. 2012, 22, 946–955. [Google Scholar] [CrossRef]
- Rushworth, M.F.; Noonan, M.P.; Boorman, E.D.; Walton, M.E.; Behrens, T.E. Frontal cortex and reward-guided learning and decision-making. Neuron 2011, 70, 1054–1069. [Google Scholar] [CrossRef]
- Botvinick, M.M. Conflict monitoring and decision making: Reconciling two perspectives on anterior cingulate function. Cogn. Affect. Behav. Neurosci. 2007, 7, 356–366. [Google Scholar] [CrossRef]
- Vassena, E.; Krebs, R.M.; Silvetti, M.; Fias, W.; Verguts, T. Dissociating contributions of ACC and vmPFC in reward prediction, outcome, and choice. Neuropsychologia 2014, 59, 112–123. [Google Scholar] [CrossRef]
- Walton, M.E.; Croxson, P.L.; Behrens, T.E.; Kennerley, S.W.; Rushworth, M.F. Adaptive decision making and value in the anterior cingulate cortex. Neuroimage 2007, 36, T142–T154. [Google Scholar] [CrossRef] [PubMed]
- Batterink, L.; Yokum, S.; Stice, E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: An fMRI study. Neuroimage 2010, 52, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Muggleton, N.G.; Tzeng, O.J.; Hung, D.L.; Juan, C.H. Control of prepotent responses by the superior medial frontal cortex. Neuroimage 2009, 44, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Floden, D.; Stuss, D.T. Inhibitory control is slowed in patients with right superior medial frontal damage. J. Cogn. Neurosci. 2006, 18, 1843–1849. [Google Scholar] [CrossRef]
- Hampshire, A.; Chamberlain, S.R.; Monti, M.M.; Duncan, J.; Owen, A.M. The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage 2010, 50, 1313–1319. [Google Scholar] [CrossRef]
- Sharp, D.J.; Bonnelle, V.; De Boissezon, X.; Beckmann, C.F.; James, S.G.; Patel, M.C.; Mehta, M.A. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. USA 2010, 107, 6106–6111. [Google Scholar] [CrossRef]
- Swann, N.C.; Cai, W.; Conner, C.R.; Pieters, T.A.; Claffey, M.P.; George, J.S.; Tandon, N. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. NeuroImage 2012, 59, 2860–2870. [Google Scholar] [CrossRef]
- Swick, D.; Ashley, V.; Turken, U. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008, 9, 102. [Google Scholar] [CrossRef]
- Kong, F.C.; Zhang, Y.; Chen, H.; Shi, M.L.; Todd, J.; Gao, X. The cognitive bias in food cues for restricted eaters: Evidences from behavioral and neuropsychological studies. Adv. Psychol. Sci. 2011, 19, 1355–1362. [Google Scholar]
- Tapper, K.; Pothos, E.M.; Fadardi, J.S.; Ziori, E. Restraint, disinhibition and food-related processing bias. Appetite 2008, 51, 335–338. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Chen, H.; Gu, Y.; Xu, W. General and food-specific inhibitory control as moderators of the effects of the impulsive systems on food choices. Front. Psychol. 2017, 8, 802. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, X.; Chen, H.; Kong, F. High-disinhibition restrained eaters are disinhibited by self-regulatory depletion in the food-related inhibitory control. Eat. Behav. 2017, 26, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Luo, Y.; Liu, Y.; Yang, C.; Chen, H. Lack of conflict during food choice is associated with the failure of restrained eating. Eat. Behav. 2019, 34, 101309. [Google Scholar] [CrossRef] [PubMed]
- Van Strien, T.; Frijters, J.E.; Bergers, G.P.; Defares, P.B. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 1986, 5, 295–315. [Google Scholar] [CrossRef]
- Yan, C.G.; Wang, X.D.; Zuo, X.N.; Zang, Y.F. DPABI: Data processing & analysis for (resting-state) brain imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar]
- Chumbley, J.R.; Friston, K.J. False discovery rate revisited: FDR and topological inference using Gaussian random fields. NeuroImage 2009, 44, 62–70. [Google Scholar] [CrossRef]
- Cavanagh, K.V.; Kruja, B.; Forestell, C.A. The effect of brand and caloric information on flavor perception and food consumption in restrained and unrestrained eaters. Appetite 2014, 82, 1–7. [Google Scholar] [CrossRef][Green Version]
- Prochaska, J.O.; DiClemente, C.C. Stage of change in the modification of problem behaviors. Prog. Behav. Modif. 1992, 28, 184–218. [Google Scholar]
- Stroebe, W.; Van Koningsbruggen, G.M.; Papies, E.K.; Aarts, H. Why most dieters fail but some succeed: A goal conflict model of eating behavior. Psychol. Rev. Am. Psychol. Assoc. 2013, 120, 110–118. [Google Scholar] [CrossRef]


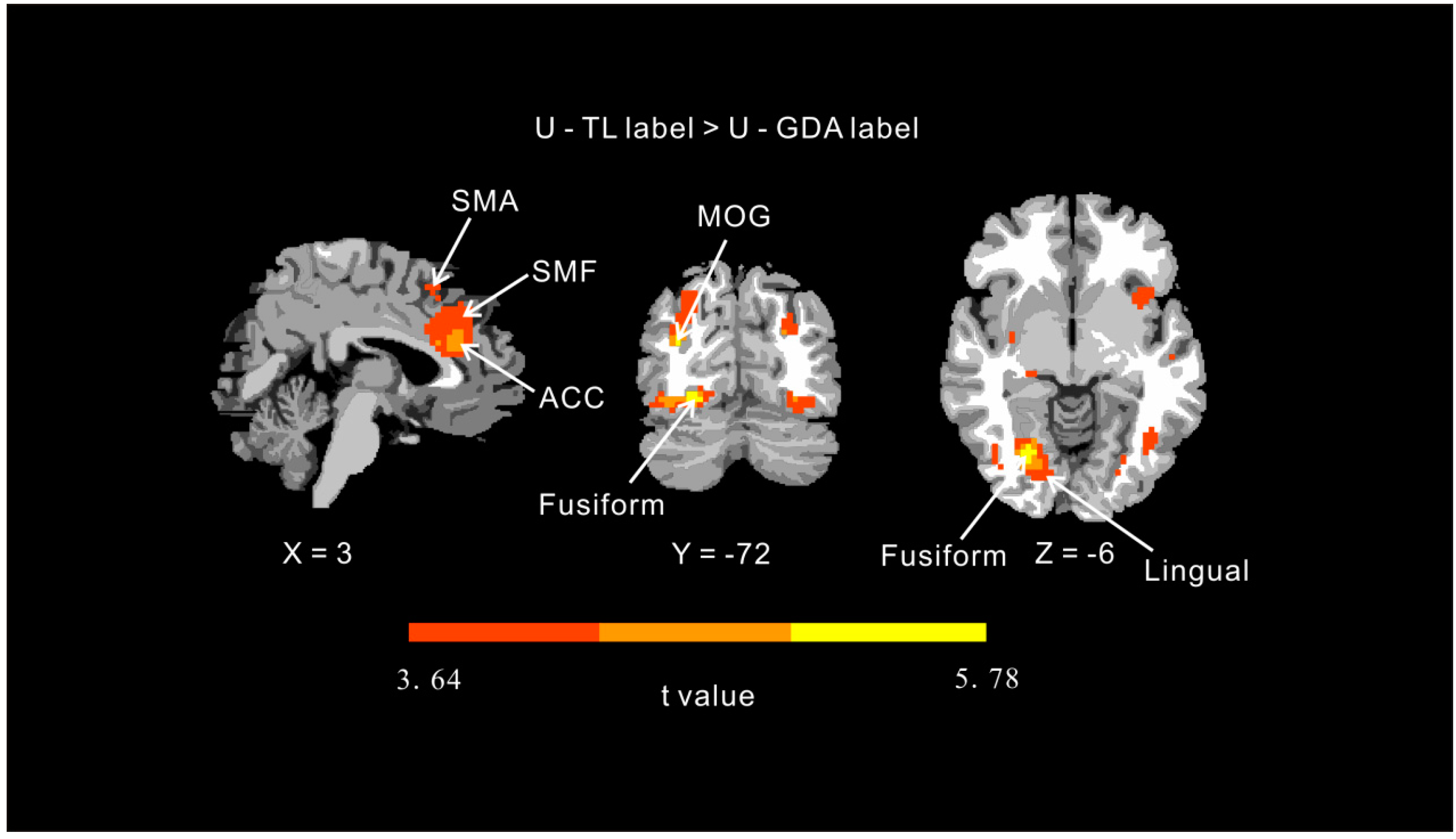
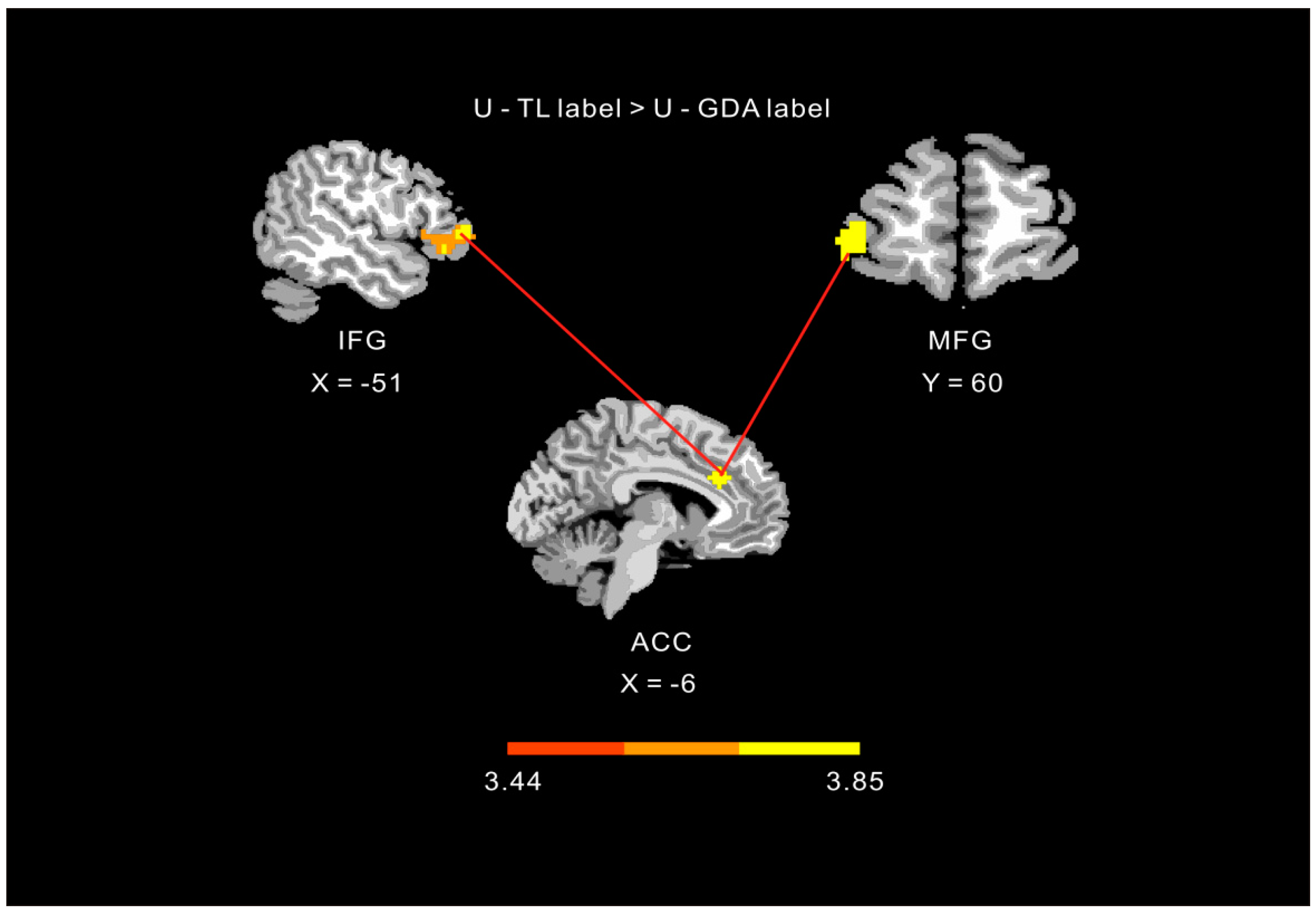
| Effect | Brain Area | Laterality | Cluster Size | x | y | z | t |
|---|---|---|---|---|---|---|---|
| U-TL label > U-GDA label a | Anterior cingulate cortex | L | 98 | −6 | 27 | 27 | 4.13 |
| Medial frontal gyrus | L | 65 | −3 | 30 | 42 | 3.66 | |
| Supplementary motor area | 25 | 0 | 18 | 48 | 3.87 | ||
| Fusiform gyrus | R | 112 | 24 | −72 | −9 | 5.78 | |
| Lingual gyrus | R | 64 | 12 | −78 | 0 | 3.64 | |
| Middle occipital gyrus | R | 147 | 30 | −84 | 17 | 3.98 | |
| PPI: H-TL label > H-GDA label | Inferior frontal gyrus | L | 104 | −51 | 45 | 3 | 3.85 |
| (seed area: Anterior cingulate cortex) b | Middle frontal gyrus | R | 22 | 39 | 60 | 0 | 3.51 |
| Middle temporal gyrus | R | 213 | 60 | −24 | −6 | 3.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, Y.; Gu, Y.; Wang, S.; Chen, H. Red for “Stop”: “Traffic-Light” Nutrition Labels Decrease Unhealthy Food Choices by Increasing Activity and Connectivity in the Frontal Lobe. Nutrients 2020, 12, 128. https://doi.org/10.3390/nu12010128
Zhang X, Liu Y, Gu Y, Wang S, Chen H. Red for “Stop”: “Traffic-Light” Nutrition Labels Decrease Unhealthy Food Choices by Increasing Activity and Connectivity in the Frontal Lobe. Nutrients. 2020; 12(1):128. https://doi.org/10.3390/nu12010128
Chicago/Turabian StyleZhang, Xuemeng, Yong Liu, Yan Gu, Shaorui Wang, and Hong Chen. 2020. "Red for “Stop”: “Traffic-Light” Nutrition Labels Decrease Unhealthy Food Choices by Increasing Activity and Connectivity in the Frontal Lobe" Nutrients 12, no. 1: 128. https://doi.org/10.3390/nu12010128
APA StyleZhang, X., Liu, Y., Gu, Y., Wang, S., & Chen, H. (2020). Red for “Stop”: “Traffic-Light” Nutrition Labels Decrease Unhealthy Food Choices by Increasing Activity and Connectivity in the Frontal Lobe. Nutrients, 12(1), 128. https://doi.org/10.3390/nu12010128




