Antidiabetic Properties of Curcumin I: Evidence from In Vitro Studies
Abstract
1. Introduction
1.1. Glucose Homeostasis: Role of Insulin
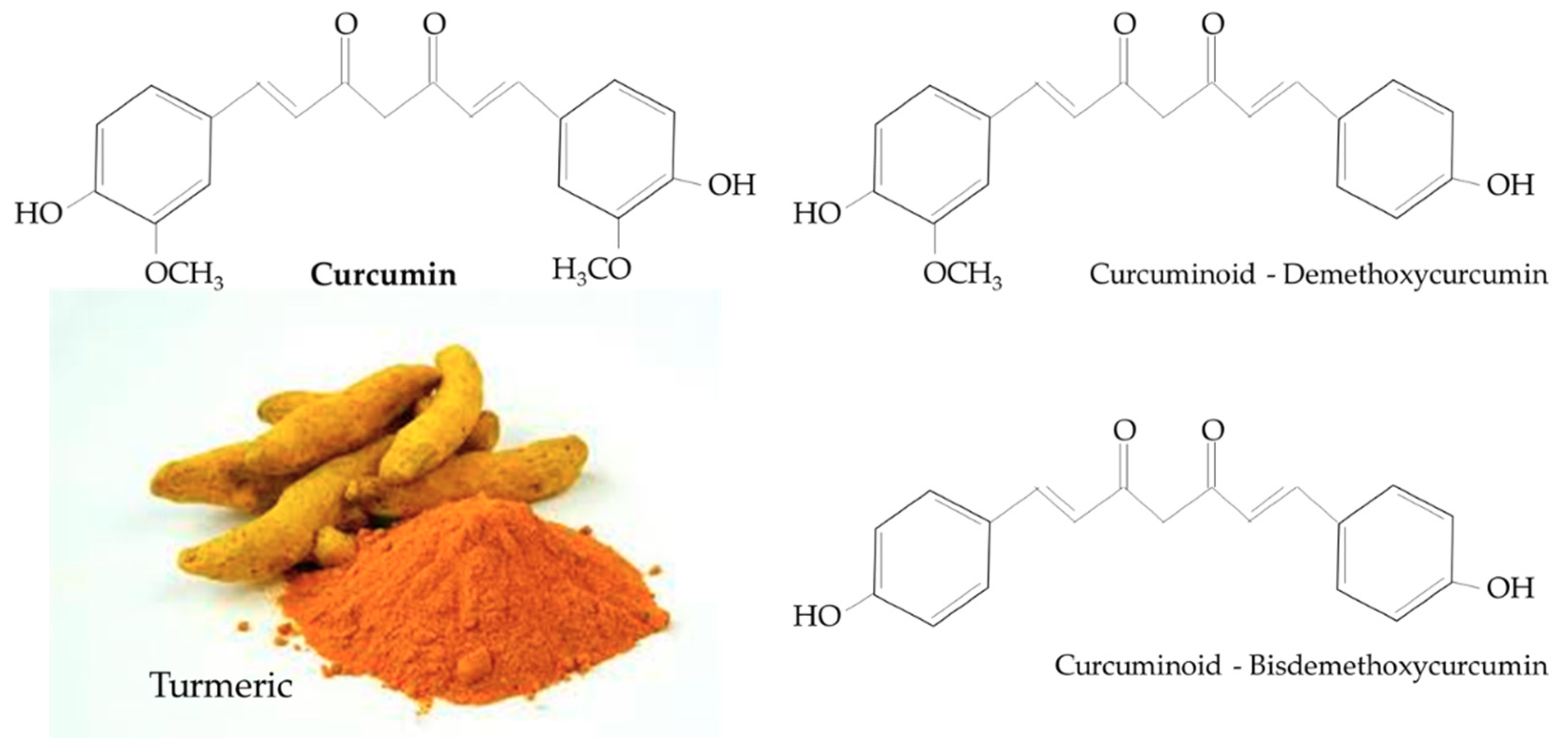
1.2. Curcumin
2. Antidiabetic Effects of Curcumin: In Vitro Studies
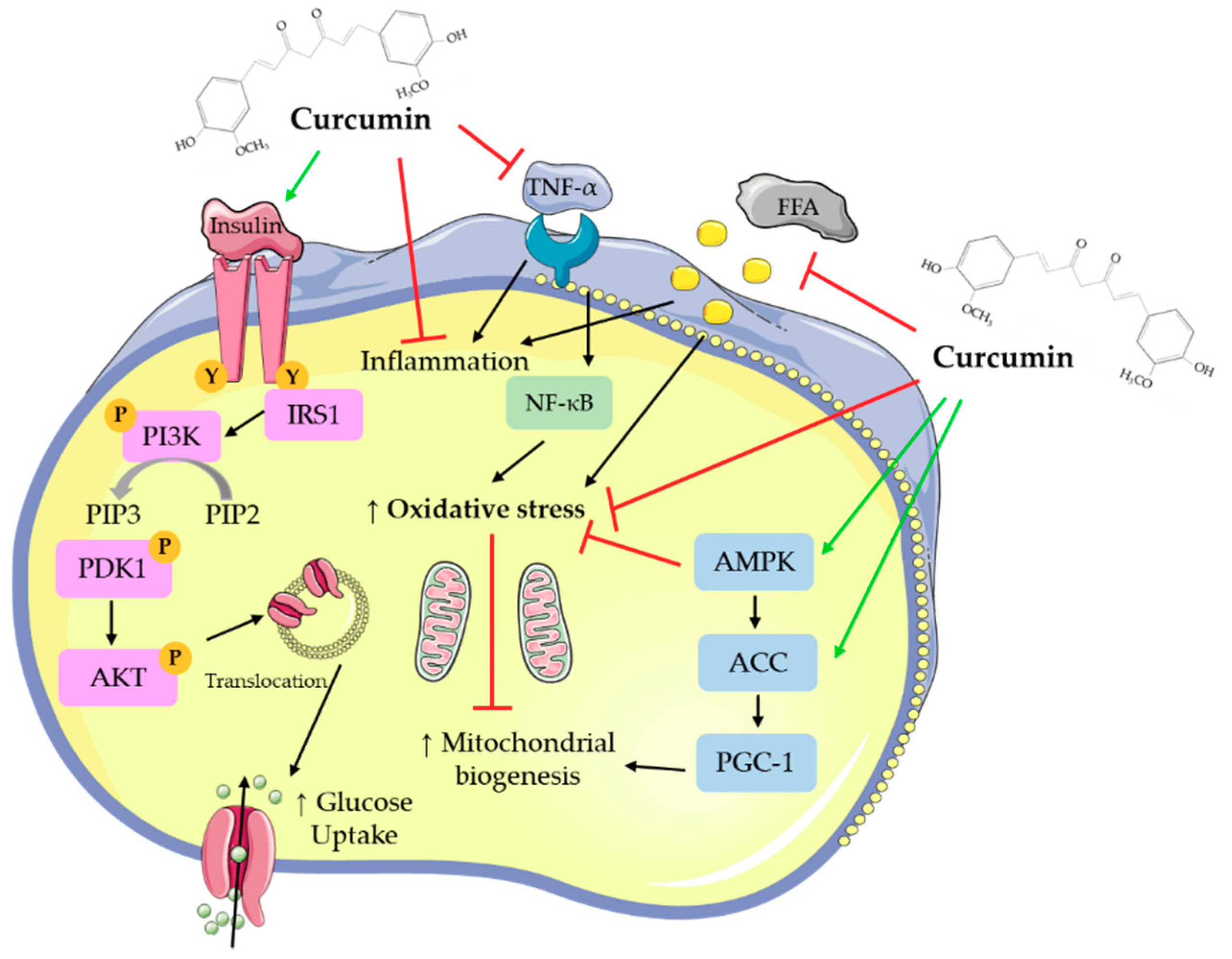
2.1. Effects of Curcumin: In Vitro Adipocyte Studies
2.2. Effects of Curcumin: In Vitro Hepatocyte Studies
2.3. Effects of Curcumin: In Vitro Muscle Cell Studies
2.4. Effects of Curcumin: In Vitro Pancreatic Cell Studies
3. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Sweet, I.R.; Cook, D.L.; DeJulio, E.; Wallen, A.R.; Khalil, G.; Callis, J.; Reems, J. Regulation of ATP/ADP in Pancreatic Islets. Diabetes 2004, 53, 401–409. [Google Scholar] [CrossRef]
- Tripathy, D.; Chavez, A.O. Defects in insulin secretion and action in the pathogenesis of type 2 diabetes mellitus. Curr. Diabetes Rep. 2010, 10, 184–191. [Google Scholar] [CrossRef]
- Saltiel, A.R. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 2001, 104, 517–529. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int. J. Clin. Pract. Suppl. 2004, 58, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Olson, P.; Hevener, A.; Mehl, I.; Chong, L.-W.; Olefsky, J.M.; Gonzalez, F.J.; Ham, J.; Kang, H.; Peters, J.M.; et al. PPAR regulates glucose metabolism and insulin sensitivity. Proc. Natl. Acad. Sci. USA 2006, 103, 3444–3449. [Google Scholar] [CrossRef] [PubMed]
- Defronzo, R.A. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Torres, N.; Tovar, A.R. The renin-angiotensin system in adipose tissue and its metabolic consequences during obesity. J. Nutr. Biochem. 2013, 24, 2003–2015. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Spiegelman, B.M. Tumor necrosis factor alpha: A key component of the obesity-diabetes link. Diabetes 1994, 43, 1271–1278. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Park, E.-J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta 2015, 1852, 1071–1113. [Google Scholar] [CrossRef]
- Anja, B.; Laura, R. The cost of diabetes in Canada over 10 years: Applying attributable health care costs to a diabetes incidence prediction model. Health Promot. Chronic Dis. Prev. Can. 2017, 37, 49–53. [Google Scholar]
- Vieira, A.R.; Abar, L.; Vingeliene, S.; Chan, D.S.M.; Aune, D.; Navarro-Rosenblatt, D.; Stevens, C.; Greenwood, D.; Norat, T. Fruits, vegetables and lung cancer risk: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Prevention of metabolic diseases: Fruits (including fruit sugars) vs. vegetables. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Häring, H.-U.; Schulze, M.B. Metabolically healthy obesity: The low-hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 2018, 6, 249–258. [Google Scholar] [CrossRef]
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2019, 11, 53. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- Yousef, M.; Vlachogiannis, I.A.; Tsiani, E. Effects of Resveratrol against Lung Cancer: In Vitro and In Vivo Studies. Nutrients 2017, 9, 1231. [Google Scholar] [CrossRef]
- Naimi, M.; Vlavcheski, F.; Shamshoum, H.; Tsiani, E. Rosemary Extract as a Potential Anti-Hyperglycemic Agent: Current Evidence and Future Perspectives. Nutrients 2017, 9, 968. [Google Scholar] [CrossRef] [PubMed]
- Dreosti, I.E. Antioxidant polyphenols in tea, cocoa, and wine. Nutrition 2000, 16, 692–694. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Châlons, P.; Aires, V.; Delmas, D. Polyphenol Extracts from Red Wine and Grapevine: Potential Effects on Cancers. Diseases 2018, 6, 106. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R.K. Turmeric and curcumin: Biological actions and medicinal applications. Curr. Sci. 2004, 87, 10. [Google Scholar]
- Reddy, A.C.; Lokesh, B.R. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol. Cell. Biochem. 1992, 111, 117–124. [Google Scholar]
- Singh, S.; Aggarwal, B.B. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef]
- Nirmala, C.; Puvanakrishnan, R. Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol. Cell. Biochem. 1996, 159, 85–93. [Google Scholar] [CrossRef]
- Arun, N.; Nalini, N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant Foods Hum. Nutr. 2002, 57, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Vajragupta, O.; Boonchoong, P.; Watanabe, H.; Tohda, M.; Kummasud, N.; Sumanont, Y. Manganese complexes of curcumin and its derivatives: Evaluation for the radical scavenging ability and neuroprotective activity. Free Radic. Biol. Med. 2003, 35, 1632–1644. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Dikshit, P.; Goswami, A.; Nukina, N. Inhibition of proteasomal function by curcumin induces apoptosis through mitochondrial pathway. J. Biol. Chem. 2004, 279, 11680–11685. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Sun, J.; Bi, C.; Chan, H.M.; Sun, S.; Zhang, Q.; Zheng, Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf. B Biointerfaces 2013, 111, 367–375. [Google Scholar] [CrossRef]
- Sharma, V.; Nehru, B.; Munshi, A.; Jyothy, A. Antioxidant potential of curcumin against oxidative insult induced by pentylenetetrazol in epileptic rats. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 227–232. [Google Scholar] [CrossRef]
- Suresh, D.; Srinivasan, K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J. Med. Res. 2010, 131, 682–691. [Google Scholar]
- Kuroda, M.; Mimaki, Y.; Nishiyama, T.; Mae, T.; Kishida, H.; Tsukagawa, M.; Takahashi, K.; Kawada, T.; Nakagawa, K.; Kitahara, M. Hypoglycemic effects of turmeric (Curcuma longa L. rhizomes) on genetically diabetic KK-Ay mice. Biol. Pharm. Bull. 2005, 28, 937–939. [Google Scholar] [CrossRef]
- Nishiyama, T.; Mae, T.; Kishida, H.; Tsukagawa, M.; Mimaki, Y.; Kuroda, M.; Sashida, Y.; Takahashi, K.; Kawada, T.; Nakagawa, K.; et al. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J. Agric. Food Chem. 2005, 53, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.-M.; Kang, J.-H.; Kawada, T.; Yoo, H.; Sung, M.-K.; Yu, R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007, 80, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.M.; Orlando, R.A. Curcumin and resveratrol inhibit nuclear factor-kappa B-mediated cytokine expression in adipocytes. Nutr. Metab. 2008, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef]
- Wang, S.-L.; Li, Y.; Wen, Y.; Chen, Y.-F.; Na, L.-X.; Li, S.-T.; Sun, C.-H. Curcumin, a potential inhibitor of up-regulation of TNF-alpha and IL-6 induced by palmitate in 3T3-L1 adipocytes through NF-kappaB and JNK pathway. Biomed. Environ. Sci. 2009, 22, 32–39. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lee, W.S.; Hwang, J.T.; Kwon, D.Y.; Surh, Y.J.; Park, O.J. Curcumin exerts antidifferentiation effect through AMPKalpha-PPAR-gamma in 3T3-L1 adipocytes and antiproliferatory effect through AMPKalpha-COX-2 in cancer cells. J. Agric. Food Chem. 2009, 57, 305–310. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Ha, T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. Am. J. Physiol. Cell Physiol. 2010, 298, C1510–C1516. [Google Scholar] [CrossRef]
- Dong, S.; Zhao, S.; Wu, Z.; Yang, J.; Xie, X.; Yu, B.; Nie, S. Curcumin promotes cholesterol efflux from adipocytes related to PPARgamma-LXRalpha-ABCA1 passway. Mol. Cell. Biochem. 2011, 358, 281–285. [Google Scholar] [CrossRef]
- Kim, C.Y.; Le, T.T.; Chen, C.; Cheng, J.-X.; Kim, K.-H. Curcumin inhibits adipocyte differentiation through modulation of mitotic clonal expansion. J. Nutr. Biochem. 2011, 22, 910–920. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, X.-B.; Ye, F.; Tian, W.-X. Suppression of fatty acid synthase, differentiation and lipid accumulation in adipocytes by curcumin. Mol. Cell. Biochem. 2011, 351, 19–28. [Google Scholar] [CrossRef]
- Kim, C.Y.; Bordenave, N.; Ferruzzi, M.G.; Safavy, A.; Kim, K.-H. Modification of curcumin with polyethylene glycol enhances the delivery of curcumin in preadipocytes and its antiadipogenic property. J. Agric. Food Chem. 2011, 59, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Kong, P.-R.; Wu, J.; Li, Y.; Li, Y. Curcumin attenuates lipolysis stimulated by tumor necrosis factor-α or isoproterenol in 3T3-L1 adipocytes. Phytomedicine 2012, 20, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ciardi, C.; Jenny, M.; Tschoner, A.; Ueberall, F.; Patsch, J.; Pedrini, M.; Ebenbichler, C.; Fuchs, D. Food additives such as sodium sulphite, sodium benzoate and curcumin inhibit leptin release in lipopolysaccharide-treated murine adipocytes in vitro. Br. J. Nutr. 2012, 107, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Cai, Y.; Huang, C.; Shi, Q.; Yang, H. Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation. Pharmacogn. Mag. 2012, 8, 202–208. [Google Scholar]
- Hirzel, E.; Lindinger, P.W.; Maseneni, S.; Giese, M.; Rhein, V.V.; Eckert, A.; Hoch, M.; Krähenbühl, S.; Eberle, A.N. Differential modulation of ROS signals and other mitochondrial parameters by the antioxidants MitoQ, resveratrol and curcumin in human adipocytes. J. Recept. Signal Transduct. Res. 2013, 33, 304–312. [Google Scholar] [CrossRef]
- Green, A.; Krause, J.; Rumberger, J.M. Curcumin is a direct inhibitor of glucose transport in adipocytes. Phytomedicine 2014, 21, 118–122. [Google Scholar] [CrossRef]
- Priyanka, A.; Anusree, S.S.; Nisha, V.M.; Raghu, K.G. Curcumin improves hypoxia induced dysfunctions in 3T3-L1 adipocytes by protecting mitochondria and down regulating inflammation. Biofactors 2014, 40, 513–523. [Google Scholar] [CrossRef]
- Roh, J.S.; Lee, H.; Woo, S.; Yoon, M.; Kim, J.; Park, S.D.; Shin, S.S.; Yoon, M. Herbal composition Gambigyeongsinhwan (4) from Curcuma longa, Alnus japonica, and Massa Medicata Fermentata inhibits lipid accumulation in 3T3-L1 cells and regulates obesity in Otsuka Long-Evans Tokushima Fatty rats. J. Ethnopharmacol. 2015, 171, 287–294. [Google Scholar]
- Zhu, L.; Han, M.B.; Gao, Y.; Wang, H.; Dai, L.; Wen, Y.; Na, L.X. Curcumin triggers apoptosis via upregulation of Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes. Mol. Med. Rep. 2015, 12, 1151–1156. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Ye, M.; Ding, Y.; Tang, Z.; Li, M.; Zhou, Y.; Wang, C. Interference with Akt signaling pathway contributes curcumin-induced adipocyte insulin resistance. Mol. Cell. Endocrinol. 2016, 429, 1–9. [Google Scholar] [CrossRef]
- Song, W.-Y.; Choi, J.-H. Korean Curcuma longa L. induces lipolysis and regulates leptin in adipocyte cells and rats. Nutr. Res. Pract. 2016, 10, 487–493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.W.; Choi, J.H.; Mukherjee, R.; Hwang, K.-C.; Yun, J.W. Proteomic identification of fat-browning markers in cultured white adipocytes treated with curcumin. Mol. Cell. Biochem. 2016, 415, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.S.; Nam, H.; Morrison, R.F. Curcumin Inhibits 3T3-L1 Preadipocyte Proliferation by Mechanisms Involving Post-transcriptional p27 Regulation. Biochem. Biophys. Rep. 2016, 5, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-S.; Chen, Y.-Y.; Lee, P.-S.; Kalyanam, N.; Ho, C.-T.; Liou, W.-S.; Yu, R.-C.; Pan, M.-H. Bisdemethoxycurcumin Inhibits Adipogenesis in 3T3-L1 Preadipocytes and Suppresses Obesity in High-Fat Diet-Fed C57BL/6 Mice. J. Agric. Food Chem. 2016, 64, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhao, D.; Yu, N.; An, T.; Miao, J.; Mo, F.; Gu, Y.; Zhang, D.; Gao, S.; Jiang, G. Curcumin improves glycolipid metabolism through regulating peroxisome proliferator activated receptor γ signalling pathway in high-fat diet-induced obese mice and 3T3-L1 adipocytes. R. Soc. Open Sci. 2017, 4, 170917. [Google Scholar] [CrossRef]
- Priyanka, A.; Shyni, G.L.; Anupama, N.; Raj, P.S.; Anusree, S.S.; Raghu, K.G. Development of insulin resistance through sprouting of inflammatory markers during hypoxia in 3T3-L1 adipocytes and amelioration with curcumin. Eur. J. Pharmacol. 2017, 812, 73–81. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, V.K.; Kumar, D.; Yadav, P.; Kumar, S.; Beg, M.; Shankar, K.; Varshney, S.; Rajan, S.; Srivastava, A.; et al. Curcumin-3,4-Dichloro Phenyl Pyrazole (CDPP) overcomes curcumin’s low bioavailability, inhibits adipogenesis and ameliorates dyslipidemia by activating reverse cholesterol transport. Metab. Clin. Exp. 2017, 73, 109–124. [Google Scholar] [CrossRef]
- Song, Z.; Revelo, X.; Shao, W.; Tian, L.; Zeng, K.; Lei, H.; Sun, H.-S.; Woo, M.; Winer, D.; Jin, T. Dietary Curcumin Intervention Targets Mouse White Adipose Tissue Inflammation and Brown Adipose Tissue UCP1 Expression. Obesity 2018, 26, 547–558. [Google Scholar] [CrossRef]
- Wang, T.; Yan, R.; Xu, X.; Li, X.; Cao, L.; Gao, L.; Liu, J.; Zhou, X.; Yu, H.; Wang, X.; et al. Curcumin represses adipogenic differentiation of human bone marrow mesenchymal stem cells via inhibiting kruppel-like factor 15 expression. Acta Histochem. 2019, 121, 253–259. [Google Scholar] [CrossRef]
- Xu, J.; Fu, Y.; Chen, A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G20–G30. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Hosokawa, M.; Zhou, X.; Fujimoto, S.; Fukuda, K.; Toyoda, K.; Nishi, Y.; Fujita, Y.; Yamada, K.; Yamada, Y.; et al. Curcumin inhibits glucose production in isolated mice hepatocytes. Diabetes Res. Clin. Pract. 2008, 80, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Davis, J.; Zhang, A.J.; He, X.; Mathews, S.T. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem. Biophys. Res. Commun. 2009, 388, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zheng, S.; Chen, A. Curcumin attenuates the effects of insulin on stimulating hepatic stellate cell activation by interrupting insulin signaling and attenuating oxidative stress. Lab. Investig. 2009, 89, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, A.I. Effects of curcumin on ethanol-induced hepatocyte necrosis and apoptosis: Implication of lipid peroxidation and cytochrome c. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 47–60. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, A. Curcumin prevents leptin raising glucose levels in hepatic stellate cells by blocking translocation of glucose transporter-4 and increasing glucokinase. Br. J. Pharmacol. 2010, 161, 1137–1149. [Google Scholar] [CrossRef]
- Schrader, C.; Schiborr, C.; Frank, J.; Rimbach, G. Curcumin induces paraoxonase 1 in cultured hepatocytes in vitro but not in mouse liver in vivo. Br. J. Nutr. 2011, 105, 167–170. [Google Scholar] [CrossRef]
- Zhao, S.-G.; Li, Q.; Liu, Z.-X.; Wang, J.-J.; Wang, X.-X.; Qin, M.; Wen, Q.-S. Curcumin attenuates insulin resistance in hepatocytes by inducing Nrf2 nuclear translocation. Hepatogastroenterology 2011, 58, 2106–2111. [Google Scholar] [CrossRef]
- Kuo, J.-J.; Chang, H.-H.; Tsai, T.-H.; Lee, T.-Y. Curcumin ameliorates mitochondrial dysfunction associated with inhibition of gluconeogenesis in free fatty acid-mediated hepatic lipoapoptosis. Int. J. Mol. Med. 2012, 30, 643–649. [Google Scholar] [CrossRef]
- Messner, D.J.; Rhieu, B.H.; Kowdley, K.V. Iron overload causes oxidative stress and impaired insulin signaling in AML-12 hepatocytes. Dig. Dis. Sci. 2013, 58, 1899–1908. [Google Scholar] [CrossRef]
- Tian, N.; Li, X.; Luo, Y.; Han, Z.; Li, Z.; Fan, C. Curcumin regulates the metabolism of low density lipoproteins by improving the C-to-U RNA editing efficiency of apolipoprotein B in primary rat hepatocytes. Mol. Med. Rep. 2014, 9, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Canfrán-Duque, A.; Pastor, O.; Quintana-Portillo, R.; Lerma, M.; de la Peña, G.; Martín-Hidalgo, A.; Fernández-Hernando, C.; Lasunción, M.A.; Busto, R. Curcumin promotes exosomes/microvesicles secretion that attenuates lysosomal cholesterol traffic impairment. Mol. Nutr. Food Res. 2014, 58, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.-H.; Chen, P.-K.; Chen, P.-Y.; Wu, M.-J.; Ho, C.-T.; Yen, J.-H. Curcumin enhances cell-surface LDLR level and promotes LDL uptake through downregulation of PCSK9 gene expression in HepG2 cells. Mol. Nutr. Food Res. 2014, 58, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Tang, S.; Li, D.; Zhao, K.; Xiao, X. Curcumin attenuates quinocetone-induced oxidative stress and genotoxicity in human hepatocyte L02 cells. Toxicol. Mech. Methods 2015, 25, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zeng, K.; Shao, W.; Yang, B.B.; Fantus, I.G.; Weng, J.; Jin, T. Short-Term Curcumin Gavage Sensitizes Insulin Signaling in Dexamethasone-Treated C57BL/6 Mice. J. Nutr. 2015, 145, 2300–2307. [Google Scholar] [CrossRef]
- Dai, C.; Li, B.; Zhou, Y.; Li, D.; Zhang, S.; Li, H.; Xiao, X.; Tang, S. Curcumin attenuates quinocetone induced apoptosis and inflammation via the opposite modulation of Nrf2/HO-1 and NF-kB pathway in human hepatocyte L02 cells. Food Chem. Toxicol. 2016, 95, 52–63. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J.-J.; Cho, W.-K.; Yim, N.-H.; Kim, H.-K.; Yun, B.; Ma, J.Y. KBH-1, an herbal composition, improves hepatic steatosis and leptin resistance in high-fat diet-induced obese rats. BMC Complementary Altern. Med. 2016, 16, 355. [Google Scholar] [CrossRef]
- Zeng, K.; Tian, L.; Sirek, A.; Shao, W.; Liu, L.; Chiang, Y.-T.; Chernoff, J.; Ng, D.S.; Weng, J.; Jin, T. Pak1 mediates the stimulatory effect of insulin and curcumin on hepatic ChREBP expression. J. Mol. Cell. Biol. 2017, 9, 384–394. [Google Scholar] [CrossRef]
- Zeng, K.; Tian, L.; Patel, R.; Shao, W.; Song, Z.; Liu, L.; Manuel, J.; Ma, X.; McGilvray, I.; Cummins, C.L.; et al. Diet Polyphenol Curcumin Stimulates Hepatic Fgf21 Production and Restores Its Sensitivity in High-Fat-Diet-Fed Male Mice. Endocrinology 2017, 158, 277–292. [Google Scholar] [CrossRef][Green Version]
- Geng, S.; Wang, S.; Zhu, W.; Xie, C.; Li, X.; Wu, J.; Zhu, J.; Jiang, Y.; Yang, X.; Li, Y.; et al. Curcumin attenuates BPA-induced insulin resistance in HepG2 cells through suppression of JNK/p38 pathways. Toxicol. Lett. 2017, 272, 75–83. [Google Scholar] [CrossRef]
- Lu, C.; Xu, W.; Zheng, S. Nrf2 activation is required for curcumin to induce lipocyte phenotype in hepatic stellate cells. Biomed. Pharmacother. 2017, 95, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, Y.; Zhang, X.; Aa, J.; Wang, G.; Xie, Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed. Pharmacother. 2018, 105, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, D.; She, L.; Zhang, Y.; Wei, Q.; Aa, J.; Wang, G.; Liu, B.; Xie, Y. Curcumin restrains hepatic glucose production by blocking cAMP/PKA signaling and reducing acetyl CoA accumulation in high-fat diet (HFD)-fed mice. Mol. Cell. Endocrinol. 2018, 474, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.-Q.; Wu, W.-Y.; Jiao, R.-Q.; Gu, T.-T.; Xu, Q.; Pan, Y.; Kong, L.-D. Curcumin and allopurinol ameliorate fructose-induced hepatic inflammation in rats via miR-200a-mediated TXNIP/NLRP3 inflammasome inhibition. Pharmacol. Res. 2018, 137, 64–75. [Google Scholar] [CrossRef]
- Lee, E.J.; Hwang, J.S.; Kang, E.S.; Lee, S.B.; Hur, J.; Lee, W.J.; Choi, M.-J.; Kim, J.T.; Seo, H.G. Nanoemulsions improve the efficacy of turmeric in palmitate- and high fat diet-induced cellular and animal models. Biomed. Pharmacother. 2019, 110, 181–189. [Google Scholar] [CrossRef]
- Wu, B.; Xiao, Z.; Zhang, W.; Chen, H.; Liu, H.; Pan, J.; Cai, X.; Liang, G.; Zhou, B.; Shan, X.; et al. A novel resveratrol-curcumin hybrid, a19, attenuates high fat diet-induced nonalcoholic fatty liver disease. Biomed. Pharmacother. 2019, 110, 951–960. [Google Scholar] [CrossRef]
- Kang, C.; Kim, E. Synergistic effect of curcumin and insulin on muscle cell glucose metabolism. Food Chem. Toxicol. 2010, 48, 2366–2373. [Google Scholar] [CrossRef]
- Deng, Y.-T.; Chang, T.-W.; Lee, M.-S.; Lin, J.-K. Suppression of free fatty acid-induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J. Agric. Food Chem. 2012, 60, 1059–1066. [Google Scholar] [CrossRef]
- Peleli, M.; Aggeli, I.-K.; Matralis, A.N.; Kourounakis, A.P.; Beis, I.; Gaitanaki, C. Evaluation of two novel antioxidants with differential effects on curcumin-induced apoptosis in C2 skeletal myoblasts; involvement of JNKs. Bioorg. Med. Chem. 2015, 23, 390–400. [Google Scholar] [CrossRef]
- Mirza, K.A.; Luo, M.; Pereira, S.; Voss, A.; Das, T.; Tisdale, M.J. In vitro assessment of the combined effect of eicosapentaenoic acid, green tea extract and curcumin C3 on protein loss in C2C12 myotubes. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 838–845. [Google Scholar] [CrossRef]
- Sadeghi, A.; Rostamirad, A.; Seyyedebrahimi, S.; Meshkani, R. Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NF-kB pathway and ROS production. Inflammopharmacology 2018, 26, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Tamrakar, A.K.; Mahajan, S.; Prasad, G.B.K.S. Chitosan encapsulated nanocurcumin induces GLUT-4 translocation and exhibits enhanced anti-hyperglycemic function. Life Sci. 2018, 213, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Best, L.; Elliott, A.C.; Brown, P.D. Curcumin induces electrical activity in rat pancreatic beta-cells by activating the volume-regulated anion channel. Biochem. Pharmacol. 2007, 73, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, S.; Akhov, L.; Selvaraj, G.; Wang, M.; Alam, J. Regulation of heme oxygenase-1 expression by demethoxy curcuminoids through Nrf2 by a PI3-kinase/Akt-mediated pathway in mouse beta-cells. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E645–E655. [Google Scholar] [CrossRef] [PubMed]
- Meghana, K.; Sanjeev, G.; Ramesh, B. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: A prophylactic and protective role. Eur. J. Pharmacol. 2007, 577, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kanitkar, M.; Bhonde, R.R. Curcumin treatment enhances islet recovery by induction of heat shock response proteins, Hsp70 and heme oxygenase-1, during cryopreservation. Life Sci. 2008, 82, 182–189. [Google Scholar] [CrossRef]
- Kanitkar, M.; Gokhale, K.; Galande, S.; Bhonde, R.R. Novel role of curcumin in the prevention of cytokine-induced islet death in vitro and diabetogenesis in vivo. Br. J. Pharmacol. 2008, 155, 702–713. [Google Scholar] [CrossRef]
- Balamurugan, A.N.; Akhov, L.; Selvaraj, G.; Pugazhenthi, S. Induction of antioxidant enzymes by curcumin and its analogues in human islets: Implications in transplantation. Pancreas 2009, 38, 454–460. [Google Scholar] [CrossRef]
- Abdel Aziz, M.T.; El-Asmar, M.F.; El Nadi, E.G.; Wassef, M.A.; Ahmed, H.H.; Rashed, L.A.; Obaia, E.M.; Sabry, D.; Hassouna, A.A.; Abdel Aziz, A.T. The effect of curcumin on insulin release in rat-isolated pancreatic islets. Angiology 2010, 61, 557–566. [Google Scholar] [CrossRef]
- Rouse, M.; Younès, A.; Egan, J.M. Resveratrol and curcumin enhance pancreatic β-cell function by inhibiting phosphodiesterase activity. J. Endocrinol. 2014, 223, 107–117. [Google Scholar] [CrossRef]
- Song, Z.; Wang, H.; Zhu, L.; Han, M.; Gao, Y.; Du, Y.; Wen, Y. Curcumin improves high glucose-induced INS-1 cell insulin resistance via activation of insulin signaling. Food Funct. 2015, 6, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Rashid, K.; Sil, P.C. Curcumin enhances recovery of pancreatic islets from cellular stress induced inflammation and apoptosis in diabetic rats. Toxicol. Appl. Pharmacol. 2015, 282, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Kang, J.; Cao, Y.; Fan, S.; Yang, H.; An, Y.; Pan, Y.; Tie, L.; Li, X. Curcumin attenuates palmitate-induced apoptosis in MIN6 pancreatic β-cells through PI3K/Akt/FoxO1 and mitochondrial survival pathways. Apoptosis 2015, 20, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Oh, J.; Ihm, S.-H.; Lee, M. Peptide micelle-mediated curcumin delivery for protection of islet β-cells under hypoxia. J. Drug Target. 2016, 24, 618–623. [Google Scholar] [CrossRef]
- Kim, S.S.; Jang, H.J.; Oh, M.Y.; Lee, J.H.; Kang, K.S. Tetrahydrocurcumin Enhances Islet Cell Function and Attenuates Apoptosis in Mouse Islets. Transplant. Proc. 2018, 50, 2847–2853. [Google Scholar] [CrossRef]
- Kose, T.; Vera-Aviles, M.; Sharp, P.A.; Latunde-Dada, G.O. Curcumin and (−)- Epigallocatechin-3-Gallate Protect Murine MIN6 Pancreatic Beta-Cells Against Iron Toxicity and Erastin-Induced Ferroptosis. Pharmaceuticals 2019, 12, 26. [Google Scholar] [CrossRef]
- Bruni, A.; Gala-Lopez, B.; Pepper, A.R.; Abualhassan, N.S.; Shapiro, A.M.J. Islet cell transplantation for the treatment of type 1 diabetes: Recent advances and future challenges. Diabetes Metab Syndr Obes. 2014, 7, 211–223. [Google Scholar]
- Wal, P.; Saraswat, N.; Pal, R.S.; Wal, A.; Chaubey, M. A Detailed Insight of the Anti-inflammatory Effects of Curcumin with the Assessment of Parameters, Sources of ROS and Associated Mechanisms. Open Med. J. 2019, 6. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar] [CrossRef]
- Katsiki, N.; Manes, C. Is there a role for supplemented antioxidants in the prevention of atherosclerosis? Clin. Nutr. 2009, 28, 3–9. [Google Scholar] [CrossRef]
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Br. J. Clin. Pharmacol. 2013, 75, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Q. Antioxidants may not always be beneficial to health. Nutrition 2014, 30, 131–133. [Google Scholar] [CrossRef] [PubMed]
| Cell | Curcumin Concentration/Duration | Effect | Reference |
|---|---|---|---|
| Human adipocytes | 20 µM; 14 days | ↑ Adipocyte differentiation ↑ Glucose elevation levels ↑ PPAR-γ activity | [40] |
| Human adipocytes | 20 µM; 14 days | ↑ Adipocyte differentiation ↑ Glucose elevation levels ↑ PPAR-γ activity | [41] |
| Raw 264.7 macrophages and 3T3-L1 adipocytes | 10 µM; 24 h | ↓ Macrophage migration ↓ MCP-1 ↓ TNF-α ↓ NO | [42] |
| 3T3-L1 adipocytes | 20 µM; 20 min and 62 h | ↓ TNFα-activated NF-κB signaling ↓ IκB degradation ↓ NF-κB nuclear transition ↓ TNFα, IL-1β and IL-6 mRNA ↓ COX-2 mRNA ↓ IL-6 and PGE2 secretion | [43] |
| 3T3-L1 adipocytes | 5–20 µM; 24 h | ↓ Adipogenesis ↓ Adipocyte differentiation ↓ Apoptosis ↑ Phospho-AMPK) ↑ Phospho-ACC ↓ GPAT-1 mRNA ↑ CPT-1 mRNA | [44] |
| 3T3-L1 adipocytes | 5–20 µM; 24 h | ↑ Glucose uptake ↓ TNF-α mRNA ↓ IL-6 mRNA ↓ NF-κBp65 protein ↓ Phospho-JNK ↓ Phospho-ERK ↓ Phospho-p38 | [45] |
| 3T3-L1 adipocytes | 10–50 µM; 8 days | ↓ Adipocyte differentiation ↑ Phospho-AMPK ↓ PPARγ activity | [46] |
| 3T3-L1 adipocytes | 10–25 µM; 48 h | ↓ Adipocyte differentiation ↓ Phospho-JNK ↓ Phospho-ERK ↓ Phospho-p38 ↑ β-catenin nuclear translocation ↓ CK1α, GSK-3β and Axin mRNA ↓ AP2 mRNA ↓ Wnt10b, Fz2 and LRP5 mRNA ↑ c-Myc and cyclin D1 mRNA | [47] |
| Rabbit subcutaneous adipocytes | 5–20 µM; 24 h | ↑ Cholesterol efflux ↑ PPARγ, LXRα and ABCA1 mRNA | [48] |
| 3T3-L1 adipocytes and human primary preadipocytes | 5–30 µM; 6 days | ↓ Adipocyte differentiation ↓ Lipid accumulation ↓ C/EBPβ, C/EBPα, PPARγ, leptin, adiponectin and resistin mRNA ↓ MCE ↓ S to G2/M phase transition ↓ Cyclin A and CDK2 protein | [49] |
| 3T3-L1 adipocytes | 20 µM; 48 h | ↓ Adipocyte differentiation ↓ Lipid accumulation ↓ Fatty acid synthase ↓ Acetyl-CoA and malonyl-CoA ↓ PPARγ and CD36 mRNA | [50] |
| 3T3-L1 adipocytes and preadipocytes | 20 µM; 48 h | ↓ Adipocyte differentiation ↓ C/EBPβ, C/EBPα, PPARγ, leptin, adiponectin and resistin mRNA | [51] |
| 3T3-L1 adipocytes | 10–20 µM; 24 h | ↓ Lipolysis ↓ Phospho-ERK1/2 ↓ Phospho-perilipin ↑ Perilipin ↓ Hormone-sensitive lipase translocation | [52] |
| 3T3-L1 adipocytes | 10 and 50 µM; 24 h | ↓ LPS-induced leptin levels ↓ LPS-induced IL-6 secretion | [53] |
| Bone marrow mesenchymal stem cells (MSCs) | 15 µM; 10 days | ↓ Adipocyte differentiation ↓ PPARγ2 mRNA ↓ C/EBPα mRNA | [54] |
| Human adipocytes | 15 µM; 24 h | ↓ Oxidative stress ↓ ROS production | [55] |
| Primary rat adipocytes | 20 µM; 45 min | ↓ Insulin-stimulated glucose uptake | [56] |
| 3T3-L1 adipocytes | 20 µM; 24 h | ↓ Cell injury ↓ ROS production ↓ HIF1α mRNA ↓ Lactate release ↓ Glycerol release ↓ Lipid peroxidation ↓ Protein oxidation ↑ SOD and CAT activities ↓ TNFα, IL-6, IL-1β and IFN-γ ↑ Mito biogenesis ↑ Mito membrane potential ↓ Mito superoxide production ↓ Transition pore permeability | [57] |
| 3T3-L1 adipocytes | 10 µM; 48 h | ↓ Lipid accumulation ↓ PPARγ, aP2 and C/EBPα mRNA ↑ CPT-1, MCAD and VLCAD mRNA ↑ ACOX and thiolase mRNA ↑ PPARα reporter mRNA | [58] |
| 3T3-L1 adipocytes | 10–100 µM; 24–72 h | ↓ Cell viability ↑ Apoptosis ↑ Bax ↓ Bcl-2 ↑ Cytochrome c release ↑ PARP cleavage | [59] |
| 3T3-L1 adipocytes | 0–50 µM; 0–24 h | ↓ Glucose uptake ↓ Akt protein ↓ Glucose transporter (GLUT4) plasma membrane expression ↓ Trypsin-like peptidase activities ↓ p62 protein ↑ LC3-II protein ↑ LC3-II/LC3-I ratio | [60] |
| 3T3-L1 adipocytes | 10 µg/mL; 24 h | ↑ Lipolysis ↓ Lipid accumulation ↑ Free fatty acid levels ↑ Glycerol levels ↑ Adipose triglyceride lipase mRNA ↑ Hormone-sensitive lipase mRNA ↓ Leptin levels | [61] |
| Primary adipocytes | 20 µM; 8 days | ↓ Adipogenesis ↓ Lipogenesis ↓ Protein levels associated with lipolysis ↓ Protein levels associated with fatty acid β-oxidation ↓ Protein levels associated with energy expenditure ↓ ALDH2, ATP5B and PRDX5 ↑ ACCA2, ACO2, ATP5H, CS, DLD, GLUD1, HADHA, HSPD1, Hsp10, HSPE1 and VDAC1 ↓ ANXA2 and HSP90 protein ↑ ATP5B, HSL, CA3 and MDH protein ↑ Ucp1, Ucp2 and Ucp3 mRNA | [62] |
| 3T3-L1 adipocytes | 20 µM; 72 h | ↓ Adipocyte differentiation ↓ Ubiquitin proteasome activity ↓ Skp2 protein accumulation ↑ p27 protein accumulation ↑ G1 arrest ↓ p27 mRNA | [63] |
| 3T3-L1 adipocytes | 25 µM; 30 min | ↓ Lipid accumulation ↓ MCE ↓ C/EBPα and PPARγ protein ↑ Cell cycle arrest ↓ Cyclin A, cyclin B and p21 protein ↓ Phospho-ERK ↓ Phospho-JNK ↓ Phospho-p38 | [64] |
| 3T3-L1 and primary adipocytes | 20 µM; 6 days | ↑ Brown fat phenotype ↑ Mitochondrial biogenesis ↑ Mitochondrial density ↑ PGC-1α, PPARγ, PRDM16 and Ucp1 protein ↑ PGC-1α mRNA ↑ Mito CPT1 protein ↑ Cytochrome c protein ↑ Phospho-AMPK ↑ AMPK | [65] |
| 3T3-L1 adipocytes | 20 µM; 24 h | ↑ Glucose metabolism ↑ Lipid metabolism ↓ Glycerol release ↑ Glucose uptake ↑ PPARγ and C/EBPα mRNA ↑ PPARγ and C/EBPα protein | [66] |
| 3T3-L1 adipocytes | 20 µM; 24 h | ↓ Glucose uptake ↑ Insulin sensitivity ↓ Leptin levels ↑ Adiponectin levels ↓ Resistin mRNA ↓ MCP-1 levels ↓ TLR-4 mRNA ↓ NF-kB p65 nuclear fraction ↓ Phospho-JNK1/2 ↓ Phospho-IRS-1(S) ↑ IRS-2 protein ↓ GLUT1 mRNA and protein ↑ GLUT4 mRNA and protein ↓ MMP-2, MMP-9 and VEGF protein ↓ Angpt4 protein level | [67] |
| 3T3-L1 adipocytes | 20 µM; 7 days | ↓ Adipocyte differentiation ↓ Lipid content accumulation ↓ C/EBPα, αP2, SREBP1c and PPARγ mRNA ↓ FAS protein ↑ UCP1 and PGC-1α protein ↑ Oxygen consumption rate ↑ Cell cycle arrest ↓ Cyclin D1, cyclin D3, CDK2, CDK4 and CDK6 protein | [68] |
| Primary and mouse brown adipocyte cell (mBAC) adipocytes | 2 µM; 10 h | ↓ WAT inflammation ↑ UCP1, PPARα and PGC-1α mRNA (primary) ↑ UCP1, PPARα, PPARγ and PGC-1α mRNA (mBAC) | [69] |
| Human bone marrow MSCs | 10 µM; 10 days | ↓ Adipocyte differentiation ↓ Lipid content accumulation ↓ PPARγ, C/EBPα, FABP4 and KLF15 mRNA and protein | [70] |
| Cell | Curcumin Concentration/Duration | Effect | Reference |
|---|---|---|---|
| Hepatic stellate cells | 30 µM; 2 h | ↓ Cell proliferation ↑ Apoptosis ↓ α1(I) collagen, α-SMA and fibronectin mRNA ↑ p21 and p27 protein ↑ Caspase-3 activity ↓ Bcl-2 mRNA ↑ NF-κB activity | [71] |
| Primary mice isolated hepatocytes | 25 µM; 120 min | ↓ Hepatic glycogenolysis ↓ Hepatic gluconeogenesis ↓ G6Pase activity ↓ PEPCK activity ↑ Phospho-AMPK | [72] |
| H4IIE rat hepatoma and Hep3B human hepatoma cells | 2–50 µM; 30 min | ↓ Hepatic gluconeogenesis ↓ PEPCK activity ↓ G6Pase activity ↑ AMPK activation | [73] |
| Hepatic stellate cells | 0–30 µM; 1 h | ↓ HSC activation ↑ HSC apoptosis ↓ PDGF-βR and EGFR ↓ α-SMA and α-II-collagen ↑ PPARγ ↓ Bcl-2 ↑ Bax ↓ ROS levels ↑ GSH protein ↑ GSH/GSSG ratio ↑ GCL activity ↓ Phospho-InsR, -ERK1/2, -JNK, -PI3K and -Akt | [74] |
| Isolated rat hepatocytes | 1 and 10 µM; 30 min | 1 µM ↓ Lipid peroxidation ↓ Cytochrome c translocation 10 µM ↑ Oxidative stress ↑ Hepatocytoxicity ↑ Necrosis ↑ Apoptosis ↑ Glutathione depletion ↑ Caspase-3 activity | [75] |
| Rat HSCs and immortalized human hepatocytes | 20 µM; 1 h | ↓ Glucose levels ↓ GLUT4 membrane translocation ↑ Glucokinase activity ↑ Glucose-6-phosphate levels ↓ Phospho-IRS-1 ↓ Phospho-PI3K ↓ Phospho-Akt | [76] |
| Huh7 cells | 20 µM; 48 h | ↑ PON1 transactivation ↑ PON1 mRNA and protein | [77] |
| L02 hepatocytes | - | ↓ Oxidative stress ↓ Insulin resistance ↓ ROS production ↓ Glutathione depletion ↓ MDA levels ↓ LDH activity ↓ AST activity | [78] |
| Primary rat hepatocytes | 10 µM; 12 h | ↓ Lipo-apoptosis ↓ ROS production ↓ ATP depletion ↓ PEPCK levels ↓ G6Pase levels ↑ Mitochondrial DNA ↑ PGC1α, NRF1 and Tfam mRNA ↑ MMP ↓ NF-kB p65 mRNA | [79] |
| AML-12 cells | 50 µM; 2 h | ↑ Insulin response ↑ Phospho-PKB ↓ ROS levels ↓ MDA levels ↓ Phospho-JNK ↓ Phospho-p38 | [80] |
| Primary rat hepatocytes | 15 µM; 24 h | ↓ Lipid formation ↑ APOBEC-1 mRNA and protein ↑ ApoB mRNA editing ↓ Apo-B lipid formation | [81] |
| HepG2 cells | 30 µM; 2 h | ↓ Cholesterol cell content ↓ Endosome/lysosome size and localization ↑ Cholesterol β-hexosaminidase enzyme level ↑ Lysosomal β-hexosaminidase enzyme level ↑ Flotillin-2-positive granules ↑ CD63-positive granules | [82] |
| HepG2 cells | 5–20 µM; 24 h | ↑ Hypolipidemic activity ↑ LDL uptake ↑ LDL receptor expression ↑ LDL receptor activity ↓ PCSK9 mRNA and protein ↓ PCSK9 promoter activity ↓ HNF-1α mRNA and protein ↓ HNF-1α nuclear promoter | [83] |
| L02 cells | 2.5 and 5 µM; 2 h | ↓ Oxidative stress ↑ Cell viability ↓ DNA-fragments ↓ Micronuclei formation ↓ ROS production ↑ SOD activity ↑ GSH levels | [84] |
| Primary mice hepatocytes | 25 µM; 6 h | ↓ Glucose production ↑ FGF21 protein ↑ Phospho-Akt | [85] |
| L02 cells | 5 µM; 2 h | ↓ QCT-induced inflammatory response ↓ QCT-induced oxidative stress ↓ Mitochondrial dysfunction ↓ Apoptosis ↓ iNOS activity ↓ NO production ↓ NF-κB mRNA ↑ Nrf2 and HO-1 mRNA | [86] |
| HepG2 cells | 30 µM; 24 h | ↓ Hepatic steatosis ↑ Leptin resistance ↓ Lipid deposition ↓ SREBP-1c, SCD-1 and CD36 mRNA ↑ ACC, ACOX1, CPT-1 and PPARα mRNA ↑ Phospho-AMPK ↑ Phospho-ACC | [87] |
| Primary mouse hepatocytes | 1 µM; 4 h | ↑ Lipogenesis ↑ ChREBP activation ↑ ChREBP mRNA ↑ Lpk, Fas, Acc1, Scd1 and Me1 mRNA ↑ ChREBP-LUC activity ↓ Oct-1-ChREBP binding | [88] |
| HepG2 and primary mouse hepatocytes | 2 µM; 6 h | ↓ Fgf21 resistance ↑ Fgf21 activity ↑ Fgf21 mRNA ↑ PPARα mRNA ↑ Fgf21-LUC activity ↑ PPARα-Fgf21 binding | [89] |
| HepG2 cells | 5 µM; 5 days | ↓ Insulin resistance ↑ Glucose consumption ↓ Oxidative stress ↑ Phospho-IR ↑ Phospho-Akt ↓ MDA levels ↓ IL-6 levels ↓ TNF-α levels ↓ Phospho-JNK and p38 ↓ Phospho-IKKβ and IκB-α | [90] |
| Human LX-2 HSCs | 40 µM; 24 h | ↓ Fibrotic myofibroblastic phenotype ↑ Lipocyte phenotype ↑ Lipid droplet abundance ↑ Lipid metabolism ↑ Triglyceride content ↑ Nrf2 protein and nuclear translocation ↑ C/EBPα, PPARγ, RXRα and RXRβ protein ↓ PPARα protein | [91] |
| Primary mice hepatocytes | 10 µM; 24 h | ↓ Palmitate-induced steatosis ↑ CYP7A and CYP3A protein ↓ SREBP-1c protein | [92] |
| HepG2 cells and primary mice hepatocytes | 10 µM; 30 and 120 min | ↓ Glucose production ↓ Mitochondrial complex I activity ↓ cAMP accumulation ↑ PDE4B induction ↑ Phospho-AMPK ↓ Acetyl CoA accumulation ↓ PDK4 mRNA ↓ PDH activity ↓ PC protein | [93] |
| BRL-3A and HepG2 cells | 2.5 µM; 8 and 12 h | ↓ Fructose-induced inflammation ↓ NLRP3 inflammasome activity ↑ miR-200a level ↓ NLRP3 and ASC protein ↓ Caspase-1 and IL-1β protein ↓ TXNIP protein ↓ ROS production ↓ H2O2 levels | [94] |
| HepG2 cells | 10 µM; 24 h | ↓ Lipotoxicity ↓ Lipid vacuole accumulation ↓ ROS production ↓ SREBP-1, PPAR-γ2 and PARP levels ↓ Cleaved caspase 3 levels | [95] |
| HepG2 cells | 10 µM; 1 h | ↓ Palmitate-induced inflammatory injury ↓ Palmitate-induced fibrosis ↓ TNF-α, IL-6, IL-1β and COX-2 mRNA ↓ ICAM, VCAM-1 and MCP-1 mRNA↓ α-SMA, COL-1, COL-4 and TGF-β mRNA ↓ α-SMA protein | [96] |
| Cell | Curcumin Concentration/Duration | Effect | Reference |
|---|---|---|---|
| C2C12 cells | 40 µM; 1 h | ↑ Glucose uptake ↑ GLUT4 translocation ↑ Phospho-AMPK ↑ Phospho-ACC ↑ Insulin-induced phospho-Akt | [97] |
| C2C12 cells | 20 µM; 2 h | ↑ Glucose uptake ↓ Phospho-IRS-1 ↓ Phospho-ACC ↑ Phospho-Akt ↑ Phospho-ERK1/2 ↑ Phospho-p38 | [98] |
| C2 murine myoblasts | 50 µM; 24 h | ↑ Apoptosis ↓ Cell viability ↑ PARP fragmentation ↑ Phospho-JNK | [99] |
| C2C12 cells | 10 µM; 24 h | ↓ Protein degradation ↓ Chymotrypsin-like enzyme activity ↑ Protein synthesis ↑ Myotube diameter | [100] |
| C2C12 cells | 40 µM; 1 h | ↓ Inflammation ↓ IL-6 mRNA and levels ↓ TNF-α mRNA and levels ↓ Phospho-IKKα-IKKβ ↓ Phospho-JNK ↓ ROS production | [101] |
| L6myc skeletal muscle cells | 25 µM; 16 h | ↑ GLUT4 translocation ↑ Phospho-Akt ↑ Phospho-GSK-3β ↓ TNF-α, IL-6 and MCP-1 levels ↑ IL-10 levels | [102] |
| Cell | Curcumin Concentration/Duration | Effect | Reference |
|---|---|---|---|
| Sprague–Dawley rat pancreatic islets | 10 µM; 30 min | ↑ Insulin release ↑ Volume-regulated anion channel opening ↑ Depolarization ↑ Electrical activity | [103] |
| MIN6 cells and BALB/c mouse pancreatic islets | 20 µM; 24 h | ↓ Pancreatic cell death ↑ HO-1 mRNA and promoter ↑ GCLM mRNA ↑ NQO1 mRNA ↑ Nrf2 nuclear translocation | [104] |
| C57/BL6J mice pancreatic islets | 10 µM; 24 h | ↑ Islet viability ↑ Insulin secretion ↓ ROS production ↓ MDA levels ↑ SOD levels ↓ Peroxynitrite levels ↓ NO levels ↓ Activated PARP | [105] |
| Swiss albino mice pancreatic cells | 10 µM; 24 h | ↑ Islet recovery ↑ Glucose responsiveness ↑ Insulin secretion ↑ Morphology ↓ ROS production ↑ Hsp70 level ↑ HO-1 level | [106] |
| C57/BL6J mice pancreatic islets | 10 µM; 24 h | ↓ Islet death and dysfunction ↑ Insulin mRNA and level ↓ ROS production ↓ NF-κB translocation ↓ Phospho-IκBα | [107] |
| Human isolated islets | 40 µM; 24 h | ↑ Insulin secretion ↑ HO-1 ↑ NQO1 ↑ GCLM | [108] |
| Rat pancreatic cells | 10 mM; 4 h | ↑ Insulin secretion ↑ HO-1 mRNA and activity | [109] |
| β-Min6 and HP62 β-cells | 100 pM; 2 h | ↑ Insulin secretion ↑ cAMP levels ↓ Pde3b, Pde8a and Pde10a mRNA ↓ PDE activity | [110] |
| INS-1 cells | 15 µM; 24 h | ↑ Insulin secretion ↑ Insulin mRNA ↑ GLUT2 ↑ Phospho-IRS1, PI3Kp85, AKT protein ↑ PI3K/IRS-1 association ↑ PDX-1 protein ↑ GCK protein | [111] |
| Wistar rat pancreatic islets | 20 µM; 24 h | ↑ Cell viability ↑ GLUT2 ↓ Nuclear NF-κB ↑ Phospho-PI3Kp85 ↑ Phospho-Akt ↑ Phospho-GSK3β ↑ Nuclear Nrf-2 ↑ HO-1 ↓ NO production ↓ Cleaved caspase-12, -3, -8 and -9 ↑ Bcl-2 ↓ Bax | [112] |
| MIN6 cells | 10 µM; 24 h | ↑ Insulin secretion ↓ Apoptosis ↓ Caspase-3 and caspase-9 activity ↑ Bcl-2/Bax ratio ↓ MDA protein ↑ SOD, catalase, GPx and glutathione activities ↑ Phospho-Akt ↑ Phospho-FoxO1 | [113] |
| INS-1 cells | 10:3 weight ratio (22 mg/L); 24 h | ↑ Curcumin cellular uptake ↑ Cell viability ↓ Apoptosis ↓ ROS production | [114] |
| Balb/c mice pancreatic islet cells | 12.5 µM; 24 h | ↑ Insulin secretion ↑ Glutathione levels ↑ NO production ↓ Apoptosis ↓ Active caspase 3 ↓ Active caspase 9 ↓ Bax protein ↑ Bcl-2 protein | [115] |
| MIN6 cells | 20 µM; 24 h | ↑ Cell viability ↓ Ferroptosis ↓ Iron levels ↑ Glutathione levels ↓ GPX4 degradation ↓ Lipid peroxidation | [116] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Den Hartogh, D.J.; Gabriel, A.; Tsiani, E. Antidiabetic Properties of Curcumin I: Evidence from In Vitro Studies. Nutrients 2020, 12, 118. https://doi.org/10.3390/nu12010118
Den Hartogh DJ, Gabriel A, Tsiani E. Antidiabetic Properties of Curcumin I: Evidence from In Vitro Studies. Nutrients. 2020; 12(1):118. https://doi.org/10.3390/nu12010118
Chicago/Turabian StyleDen Hartogh, Danja J., Alessandra Gabriel, and Evangelia Tsiani. 2020. "Antidiabetic Properties of Curcumin I: Evidence from In Vitro Studies" Nutrients 12, no. 1: 118. https://doi.org/10.3390/nu12010118
APA StyleDen Hartogh, D. J., Gabriel, A., & Tsiani, E. (2020). Antidiabetic Properties of Curcumin I: Evidence from In Vitro Studies. Nutrients, 12(1), 118. https://doi.org/10.3390/nu12010118






