Impact of Moderate Sodium Restriction and Hydrochlorothiazide on Iodine Excretion in Diabetic Kidney Disease: Data from a Randomized Cross-Over Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
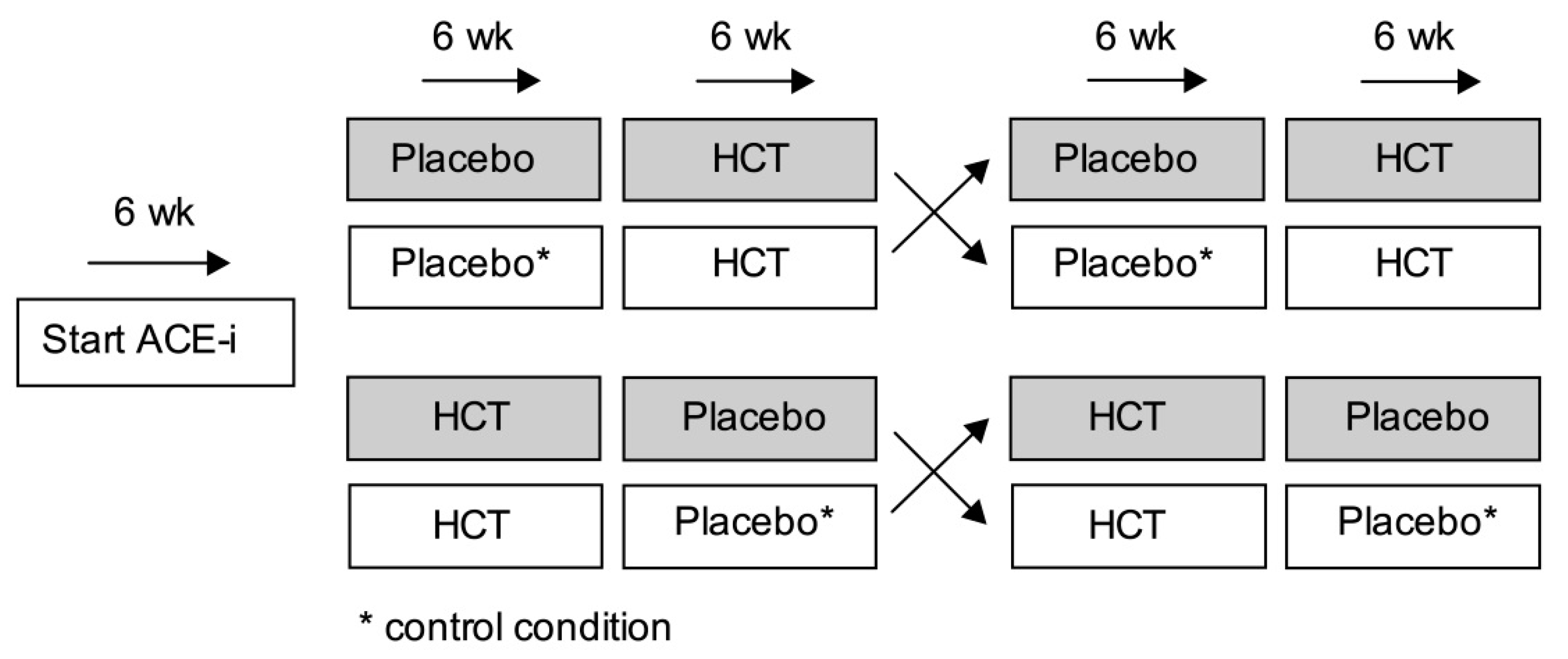
2.2. Trial Protocol
2.3. Sodium Restriction
2.4. Measurements
2.5. Statistical Analysis
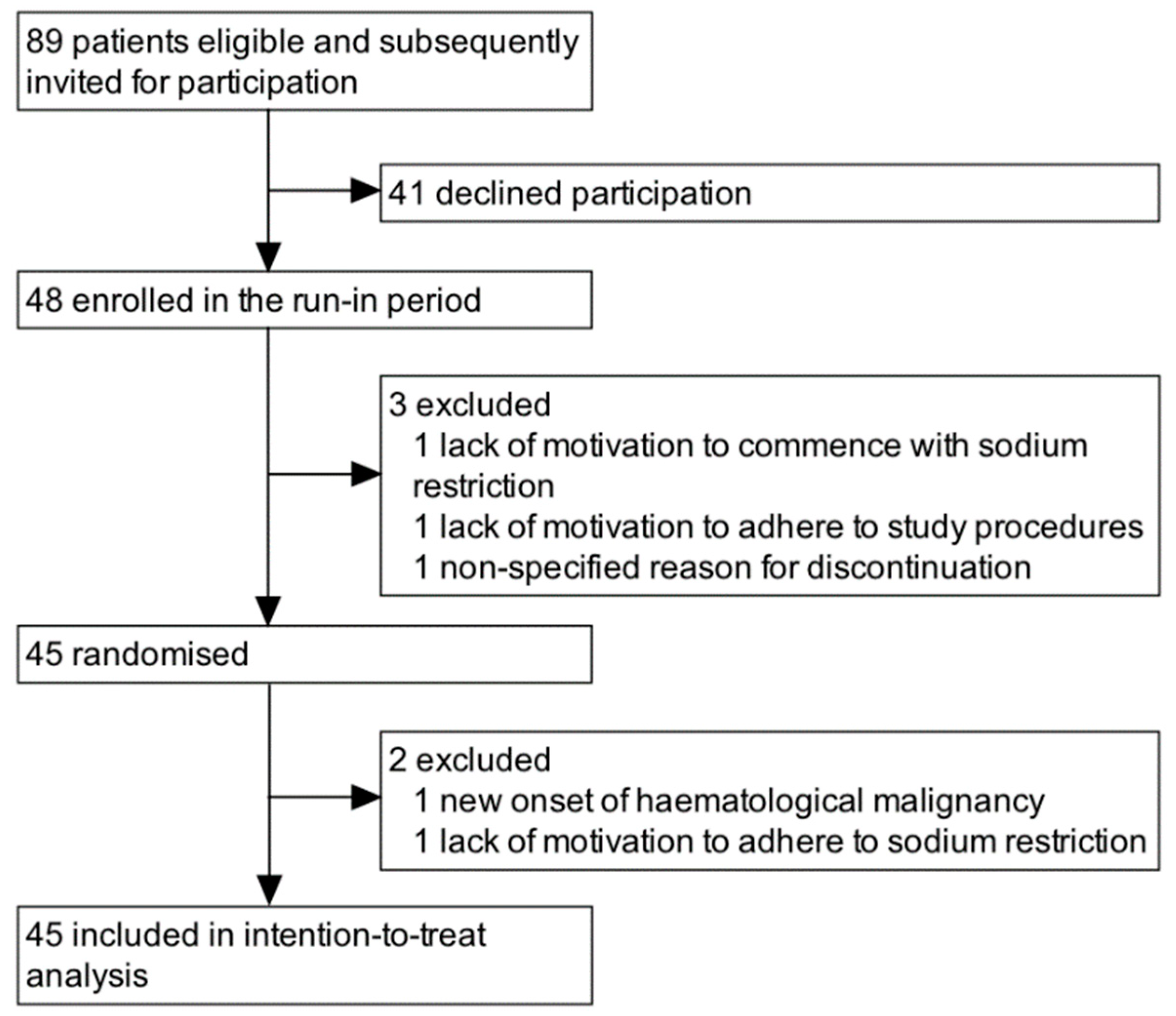
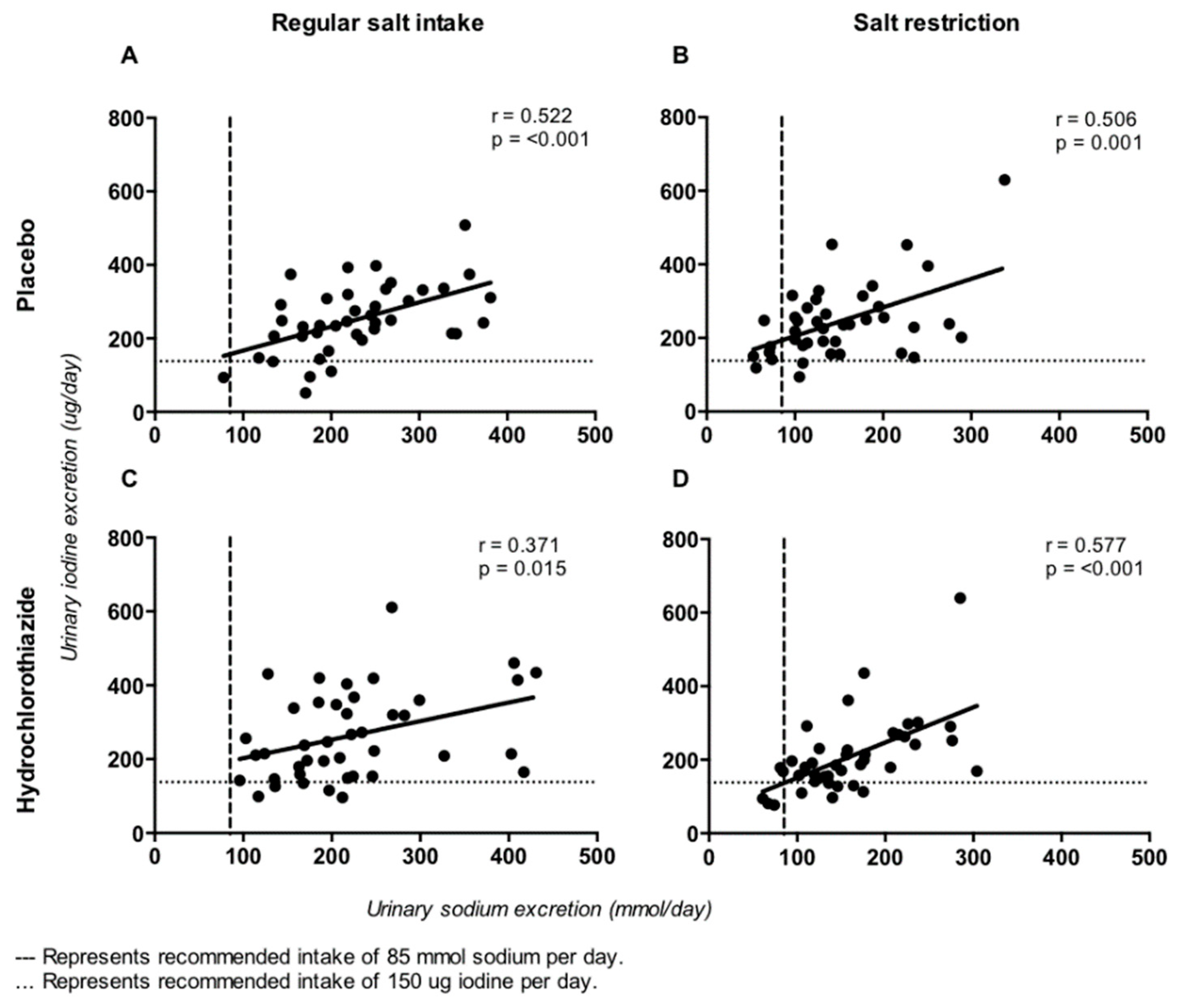
3. Results
4. Discussion
4.1. Sodium Restriction
4.2. Hydrochlorothiazide
4.3. Sodium Restriction with Hydrochlorothiazide
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Clinical trial registry
References
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012; pp. 1–46. [Google Scholar]
- Cogswell, M.E.; Mugavero, K.; Bowman, B.A.; Frieden, T.R. Dietary Sodium and Cardiovascular Disease Risk—Measurement Matters. N. Engl. J. Med. 2016, 375, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Graudal, N.; Jürgens, G.; Baslund, B.; Alderman, M.H. Compared With Usual Sodium Intake, Low- and Excessive-Sodium Diets Are Associated With Increased Mortality: A Meta-Analysis. Am. J. Hypertens. 2014, 27, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; O’Donnell, M.; Rangarajan, S.; Dagenais, G.; Lear, S.; McQueen, M.; Díaz, R.; Avezum, A.; López-Jaramillo, P.; Lanas, F.; et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet 2016, 388, 465–475. [Google Scholar] [CrossRef]
- Stolarz-Skrzypek, K.; Kuznetsova, T.; Thijs, L.; Tikhonoff, V.; Seidlerová, J.; Richart, T.; Jin, Y.; Olszanecka, A.; Malyutina, S.; Casiglia, E.; et al. Fatal and Nonfatal Outcomes, Incidence of Hypertension, and Blood Pressure Changes in Relation to Urinary Sodium Excretion. JAMA 2011, 305, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Alderman, M.H. Reducing dietary sodium: the case for caution. JAMA 2010, 303, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Hubeck-Graudal, T.; Jurgens, G.; Graudal, N.A. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst. Rev. 2017, 2017, CD004022. [Google Scholar]
- Verkaik-Kloosterman, J.; Buurma-Rethans, E.J.M.; Dekkers, A.L.M.; Van Rossum, C.T.M. Decreased, but still sufficient, iodine intake of children and adults in the Netherlands. Br. J. Nutr. 2017, 117, 1020–1031. [Google Scholar] [CrossRef]
- Ershow, A.G.; Skeaff, S.A.; Merkel, J.M.; Pehrsson, P.R. Development of Databases on Iodine in Foods and Dietary Supplements. Nutrients 2018, 10, 100. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-deficiency disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef]
- Mannar, M.G.V.; Dunn, J.T. Salt Iodization for the Elimination of Iodine Deficiency. International Council for Control of Iodine Deficiency Disorders, 1995. Available online: http://www.ceecis.org/iodine/08_production/00_mp/SI_elim_IDD_1994venkatesh.pdf (accessed on 11 June 2019).
- Charlton, K.E.; Jooste, P.L.; Steyn, K.; Levitt, N.S.; Ghosh, A. A lowered salt intake does not compromise iodine status in Cape Town, South Africa, where salt iodization is mandatory. Nutrition 2013, 29, 630–634. [Google Scholar] [CrossRef]
- Land, M.A.; Webster, J.L.; Ma, G.; Li, M.; Su’A, S.A.F.; Ieremia, M.; Viali, S.; Faeamani, G.; Bell, A.C.; Quested, C.; et al. Salt intake and iodine status of women in Samoa. Asia Pac. J. Clin. Nutr. 2016, 25, 142–149. [Google Scholar]
- Verkaik-Kloosterman, J.; van’t Veer, P.; Ocké, M.C. Reduction of salt: will iodine intake remain adequate in The Netherlands? Br. J. Nutr. 2010, 104, 1712–1718. [Google Scholar] [CrossRef]
- Grzesiuk, W.; Dabrowska, J.; Osikowska-Loksztejn, M.; Kondracka, A.; Kolasińska, K.; Bar-Andziak, E. Effectiveness of iodine prophylaxis in hypertensive patients on salt restricted diet. Pol. Arch. Intern. Med. 2005, 113, 147–154. [Google Scholar]
- Tayie, F.A.; Jourdan, K. Hypertension, Dietary Salt Restriction, and Iodine Deficiency among Adults. Am. J. Hypertens. 2010, 23, 1095–1102. [Google Scholar] [CrossRef]
- Charlton, K.; Ware, L.J.; Baumgartner, J.; Cockeran, M.; Schutte, A.E.; Naidoo, N.; Kowal, P. How will South Africa’s mandatory salt reduction policy affect its salt iodisation programme? A cross-sectional analysis from the WHO-SAGE Wave 2 Salt & Tobacco study. BMJ Open 2018, 8, e020404. [Google Scholar]
- He, F.J.; Ma, Y.; Feng, X.; Zhang, W.; Lin, L.; Guo, X.; Zhang, J.; Niu, W.; Wu, Y.; MacGregor, G.A. Effect of salt reduction on iodine status assessed by 24 hour urinary iodine excretion in children and their families in northern China: a substudy of a cluster randomised controlled trial. BMJ Open 2016, 6, e011168. [Google Scholar] [CrossRef]
- Musso, N.; Conte, L.; Carloni, B.; Campana, C.; Chiusano, M.C.; Giusti, M. Low-Salt Intake Suggestions in Hypertensive Patients Do not Jeopardize Urinary Iodine Excretion. Nutrients 2018, 10, 1548. [Google Scholar] [CrossRef]
- Suckling, R.J.; He, F.J.; MacGregor, G.A. Altered dietary salt intake for preventing and treating diabetic kidney disease. Cochrane Database Syst Rev. 2010, CD006763. [Google Scholar] [CrossRef]
- Kwakernaak, A.J.; Krikken, J.A.; Binnenmars, S.H.; Visser, F.W.; Hemmelder, M.H.; Woittiez, A.J.; Groen, H.; Laverman, G.D.; Navis, G. Effects of sodium restriction and hydrochlorothiazide on RAAS blockade efficacy in diabetic nephropathy: a randomised clinical trial. Lancet Diabetes Endocrinol. 2014, 2, 385–395. [Google Scholar] [CrossRef]
- Krikken, J.; Lely, A.; Bakker, S.; Navis, G. The effect of a shift in sodium intake on renal hemodynamics is determined by body mass index in healthy young men. Kidney Int. 2007, 71, 260–265. [Google Scholar] [CrossRef]
- Vejbjerg, P.; Knudsen, N.; Perrild, H.; Laurberg, P.; Andersen, S.; Rasmussen, L.B.; Ovesen, L.; Jørgensen, T. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid 2009, 19, 1281–1286. [Google Scholar] [CrossRef]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary Reference Intakes. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Kusek, J.W.; Coresh, J.; Feldman, H.I.; Eggers, P.; Van Lente, F.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- EMA. Committee for Medicinal Products for Human Use. Guideline on bioanalytical method validation. EMEA/CHMP/EWP/192217/2009 Rev 1 Corr 2. 2012, 44(July 2011):1-23. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 11 June 2019).
- Hendriksen, M.A.H.; van Raaij, J.M.A.; Geleijnse, J.M.; Wilson-van den Hooven, C.; Ocké, M.C.; van der, A.D.L. Monitoring salt and iodine intakes in Dutch adults between 2006 and 2010 using 24 h urinary sodium and iodine excretions. Public Health Nutr. 2014, 17, 1431–1438. [Google Scholar] [CrossRef]
- Hendriksen, M.; Etemad, Z.; van den Bogaard, C.H.; van der, A.D.L. Zout-, jodium- en kaliuminname 2015. Rijksinst voor Volksgezond en Milieu. Available online: https://www.rivm.nl/bibliotheek/rapporten/2016-0081.pdf (accessed on 11 June 2019).
- Fregly, M.J.; McCarthy, J.S. Effect of diuretics on renal iodide excretion by humans. Toxicol. Appl. Pharmacol. 1973, 25, 289–298. [Google Scholar] [CrossRef]
- Seabold, J.E.; Ben-Haim, S.; Pettit, W.A.; Gurli, N.J.; Rojeski, M.T.; Flanigan, M.J.; Ponto, J.A.; Bricker, J.A. Diuretic-enhanced I-131 clearance after ablation therapy for differentiated thyroid cancer. Radiology 1993, 187, 839–842. [Google Scholar] [CrossRef]
- McCarthy, J.S.; Fregly, M.J.; Nechay, B.R. Effect of diuretics on renal iodide excretion by rats and dogs. J. Pharmacol. Exp. Ther. 1967, 158, 294–303. [Google Scholar]
- Beyer, K.H.; Fehr, D.M.; Gelarden, R.T.; White, W.J.; Lang, C.M.; Vesell, E.S. Hydrochlorothiazide-induced 131I excretion facilitated by salt and water. J. Clin. Pharmacol. 1981, 21, 201–212. [Google Scholar] [CrossRef]
- Barakat, R.M.; Ingbar, S.H. The effect of acute iodide depletion on thyroid function in man. J. Clin. Invest. 1965, 44, 1117–1124. [Google Scholar] [CrossRef]
- Kapucu, L.O.; Azizoglu, F.; Ayvaz, G.; Karakoc, A.; Azizoğlu, F. Effects of diuretics on iodine uptake in non-toxic goitre: comparison with low-iodine diet. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1270–1272. [Google Scholar] [CrossRef]
- Maruca, J.; Santner, S.; Miller, K.; Santen, R.J. Prolonged iodine clearance with a depletion regimen for thyroid carcinoma: concise communication. J. Nucl. Med. 1984, 25, 1089–1093. [Google Scholar]
- Matovic, M.D.; Jankovic, S.M.; Jeremic, M.; Tasic, Z.; Vlajkovic, M. Unexpected effect of furosemide on radioiodine urinary excretion in patients with differentiated thyroid carcinomas treated with iodine 131. Thyroid 2009, 19, 843–848. [Google Scholar] [CrossRef]
- Sheffer, M. Concise International Chemical Assessment Document 72 iodine and inorganic iodides: Human health aspects. World Health Organzation, 2009. Available online: https://apps.who.int/iris/bitstream/handle/10665/43579/9789241530729_eng.pdf?sequence=1&isAllowed=y (accessed on 11 June 2019).
- Kim, Y.H.; Pham, T.D.; Zheng, W.; Hong, S.; Baylis, C.; Pech, V.; Beierwaltes, W.H.; Farley, D.B.; Braverman, L.E.; Verlander, J.W.; et al. Role of pendrin in iodide balance: Going with the flow. Am. J. Physiol. Physiol. 2009, 297, F1069–F1079. [Google Scholar] [CrossRef][Green Version]
- Quentin, F.; Chambrey, R.; Trinh-Trang-Tan, M.M.; Fysekidis, M.; Cambillau, M.; Paillard, M.; Aronson, P.S.; Eladari, D. The Cl−/HCO3− exchanger pendrin in the rat kidney is regulated in response to chronic alterations in chloride balance. Am. J. Physiol. Physiol. 2004, 287, F1179–F1188. [Google Scholar] [CrossRef]
- Vallet, M.; Picard, N.; Loffing-Cueni, D.; Fysekidis, M.; Bloch-Faure, M.; Deschènes, G.; Breton, S.; Meneton, P.; Loffing, J.; Aronson, P.S.; et al. Pendrin Regulation in Mouse Kidney Primarily Is Chloride-Dependent. J. Am. Soc. Nephrol. 2006, 17, 2153–2163. [Google Scholar] [CrossRef]
- Walser, M.; Rahill, W.J. Renal Tubular Reabsorption of Iodide as Compared with Chloride. J. Clin. Investig. 1965, 44, 1371–1381. [Google Scholar] [CrossRef]
- Zahedi, K.; Barone, S.; Xu, J.; Soleimani, M. Potentiation of the Effect of Thiazide Derivatives by Carbonic Anhydrase Inhibitors: Molecular Mechanisms and Potential Clinical Implications. PLoS ONE 2013, 8, e79327. [Google Scholar] [CrossRef]
- Vogt, L.; Waanders, F.; Boomsma, F.; De Zeeuw, D.; Navis, G. Effects of Dietary Sodium and Hydrochlorothiazide on the Antiproteinuric Efficacy of Losartan. J. Am. Soc. Nephrol. 2008, 19, 999–1007. [Google Scholar] [CrossRef]
- Slagman, M.C.; Waanders, F.; Hemmelder, M.H.; Woittiez, A.J.; Janssen, W.M.; Heerspink, H.J.; Navis, G.; Laverman, G.D. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: Randomised controlled trial. BMJ 2011, 343, d4366. [Google Scholar] [CrossRef]
- Campbell, N.R.C.; He, F.J.; Tan, M.; Cappuccio, F.P.; Neal, B.; Woodward, M.; Cogswell, M.E.; McLean, R.; Arcand, J.; MacGregor, G.; et al. The International Consortium for Quality Research on Dietary Sodium/Salt (TRUE) position statement on the use of 24-hour, spot, and short duration (<24 hours) timed urine collections to assess dietary sodium intake. J. Clin. Hypertens. 2019, 21, 700–709. [Google Scholar] [CrossRef]
| All Subjects 1 | |
|---|---|
| Demographics | |
| Age (years) | 65 ± 9 |
| Male gender | 38 (84%) |
| Caucasian race | 45 (100%) |
| Diabetes duration (years) | 9 (range 5–19) |
| Macrovascular disease | 21 (47%) |
| Medication | |
| Insulin treatment | 25 (53%) |
| Biguanide | 32 (71%) |
| Sulfonylurea derivative | 17 (38%) |
| DPP4-inhibitor | 1 (2%) |
| Thyroid hormone replacement therapy | 2 (4%) |
| Non-trial antihypertensive medication | |
| 0 | 8 (18%) |
| 1 | 17 (38%) |
| 2 | 17 (38) |
| 3 | 3 (6%) |
| Type of non-trial antihypertensive medication | |
| α blockade | 4 (9%) |
| β blockade | 27 (45%) |
| Calcium-channel blockade | 27 (45%) |
| Clinical measurements | |
| Systolic blood pressure (mmHg) | 147 ± 2 |
| Diastolic blood pressure (mmHg) | 82 ± 1 |
| BMI (kg/m2) | 32 ± 5 |
| Overweight | 13 (29%) |
| Obesity | 30 (67%) |
| Laboratory values | |
| Serum HbA1c (mmol/mol) | 54 ± 9 |
| eGFR (ml/min/1.73 m2) | 65 ± 27 |
| Urinary albumin excretion (mg/24 h) | 648 [230–2008] |
| Variable | Beta | F | p-Value 1 |
|---|---|---|---|
| Sodium excretion | 0.416 | 15.864 | <0.001 |
| Gender [male] | −5.722 | 0.033 | 0.9 |
| [female] | 0 | ||
| Age | 0.361 | 0.065 | 0.8 |
| BMI | 2.900 | 1.178 | 0.3 |
| Creatinine clearance | 0.250 | 1.015 | 0.3 |
| Values After Six Weeks of Intervention 1 | Treatment Effect 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control: Reg/Plac | SR/Plac | Reg/HCT | SR/HCT | SR/Plac vs. Control | p | Reg/HCT vs. Control | p-Value | SR/HCT vs. Control | p | |
| Clinical parameters | ||||||||||
| Systolic blood pressure (mmHg) | 147 ± 16 | 141 ± 16 | 135 ± 16 | 129 ± 14 | −5 (−9,−1) | 0.01 | −12 (−15,−8) | <0.001 | −17 (−21,−13) | <0.001 |
| Diastolic blood pressure (mmHg) | 82 ± 10 | 79 ± 10 | 76 ± 9 | 72 ± 8 | −3 (−7,1) | 0.1 | −6 (−9,−4) | 0.001 | −10 (−12,−7) | <0.001 |
| Weight (kg) | 101.8 ± 18.2 | 99.5 ± 18.1 | 100.1 ± 17.5 | 98.1 ± 17.8 | −1.7 (−2.5,−0.9) | <0.001 | −1.7 (−2.5,−0.9) | <0.001 | −3.0 (−4.0,−2.1) | <0.001 |
| Serum and plasma measurements | ||||||||||
| Sodium (mmol/L) | 140 ± 3 | 140 ± 3 | 140 ± 3 | 138 ± 4 | −0.7 (−1.4,0.1) | 0.08 | −0.9 (−1.6,−0.2) | 0.02 | −2.3 (−3.2,−1.3) | <0.001 |
| Potassium (mmol/L) | 4.4 ± 0.4 | 4.5 ± 0.5 | 4.3 ± 0.5 | 4.5 ± 0.5 | 0.1 (0.04,0.2) | 0.04 | −0.1 (−0.2,0.04) | 0.2 | 0.06 (−0.1,0.2) | 0.4 |
| Chloride (mmol/L) | 105 ± 3 | 104 ± 4 | 103 ± 4 | 101 ± 5 | −1 (−2,0.4) | 0.2 | −2 (−3,−1) | 0.003 | −4 (−5,−2) | <0.001 |
| Urea (mmol/L) | 8.5 ± 3.1 | 9.2 ± 4.4 | 10.1 ± 3.8 | 11.3 ± 5.5 | 0.6 (−0.3,1.5) | 0.2 | 1.5 (0.6,2.4) | 0.001 | 2.9 (2.0,3.8) | <0.001 |
| Creatinine (umol/L) | 111 ± 41 | 114 ± 51 | 122 ± 47 | 125 ± 52 | 2 (−4,9) | 0.5 | 11 (4,17) | 0.001 | 15 (8,21) | <0.001 |
| Creatinine clearance (ml/min) | 101 ± 47 | 99 ± 48 | 97 ± 47 | 88 ± 42 | −2 (−10,6) | 0.6 | −5 (−13,4) | 0.3 | −15 (−23,−7) | 0.001 |
| 24-h urinary measurements | ||||||||||
| Volume (ml/24 h) | 2071 ± 605 | 1973 ± 692 | 2044 ± 646 | 2038 ± 766 | −88 (−216,41) | 0.2 | −26 (−218,167) | 0.8 | −29 (−180,123) | 0.7 |
| Sodium (mmol/24 h) | 224 ± 76 | 148 ± 65 | 224 ± 88 | 164 ± 71 | −75 (−96,−54) | <0.001 | −1 (−22,20) | 0.9 | −59 (−81,−37) | <0.001 |
| Chloride (mmol/24 h) | 187 ± 71 | 148 ± 71 | 181 ± 82 | 140 ± 67 | −41 (−67,−15) | 0.003 | −5 (−27,16) | 0.6 | −42 (−62,−23) | <0.001 |
| Iodine (ug/24 h) | 252 ± 94 | 244 ± 103 | 264 ± 120 | 208 ± 103 | −8 (−38,22) | 0.6 | 14 (−24,52) | 0.5 | −37 (−67,−7) | 0.02 |
| Iodine intake < RDA (n) | 5 | 3 | 5 | 9 | 0.3 3 | |||||
| Ln albumin (mg/24 h) | 6.6 ± 0.2 | 6.1 ± 0.2 | 6.0 ± 0.2 | 5.7 ± 0.2 | −0.5 (−0.8,−0.3) | <0.001 | −0.5 (−0.8,−0.3) | <0.001 | −0.9 (−1.2,−0.6) | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binnenmars, S.H.; Corpeleijn, E.; Kwakernaak, A.J.; Touw, D.J.; Kema, I.P.; Laverman, G.D.; Bakker, S.J.L.; Navis, G. Impact of Moderate Sodium Restriction and Hydrochlorothiazide on Iodine Excretion in Diabetic Kidney Disease: Data from a Randomized Cross-Over Trial. Nutrients 2019, 11, 2204. https://doi.org/10.3390/nu11092204
Binnenmars SH, Corpeleijn E, Kwakernaak AJ, Touw DJ, Kema IP, Laverman GD, Bakker SJL, Navis G. Impact of Moderate Sodium Restriction and Hydrochlorothiazide on Iodine Excretion in Diabetic Kidney Disease: Data from a Randomized Cross-Over Trial. Nutrients. 2019; 11(9):2204. https://doi.org/10.3390/nu11092204
Chicago/Turabian StyleBinnenmars, S. Heleen, Eva Corpeleijn, Arjan J. Kwakernaak, Daan J. Touw, Ido P. Kema, Gozewijn D. Laverman, Stephan J. L. Bakker, and Gerjan Navis. 2019. "Impact of Moderate Sodium Restriction and Hydrochlorothiazide on Iodine Excretion in Diabetic Kidney Disease: Data from a Randomized Cross-Over Trial" Nutrients 11, no. 9: 2204. https://doi.org/10.3390/nu11092204
APA StyleBinnenmars, S. H., Corpeleijn, E., Kwakernaak, A. J., Touw, D. J., Kema, I. P., Laverman, G. D., Bakker, S. J. L., & Navis, G. (2019). Impact of Moderate Sodium Restriction and Hydrochlorothiazide on Iodine Excretion in Diabetic Kidney Disease: Data from a Randomized Cross-Over Trial. Nutrients, 11(9), 2204. https://doi.org/10.3390/nu11092204






