A Randomized, Placebo-Controlled, Double-Blind Crossover Study to Assess a Unique Phytosterol Ester Formulation in Lowering LDL Cholesterol Utilizing a Novel Virtual Tracking Tool
Abstract
:1. Introduction
2. Materials and Methods
- a)
- Screen for most efficacious formulations in lab tests;
- b)
- Test formulations in their final applications in in-vitro models;
- c)
- Substantiate the efficacy of the best in-vitro formulation in a clinical trial.
2.1. Screening for Most Efficacious Formulations in Lab Tests
2.2. In Vitro Model as First Means to Test the Effectiveness of New Formulations
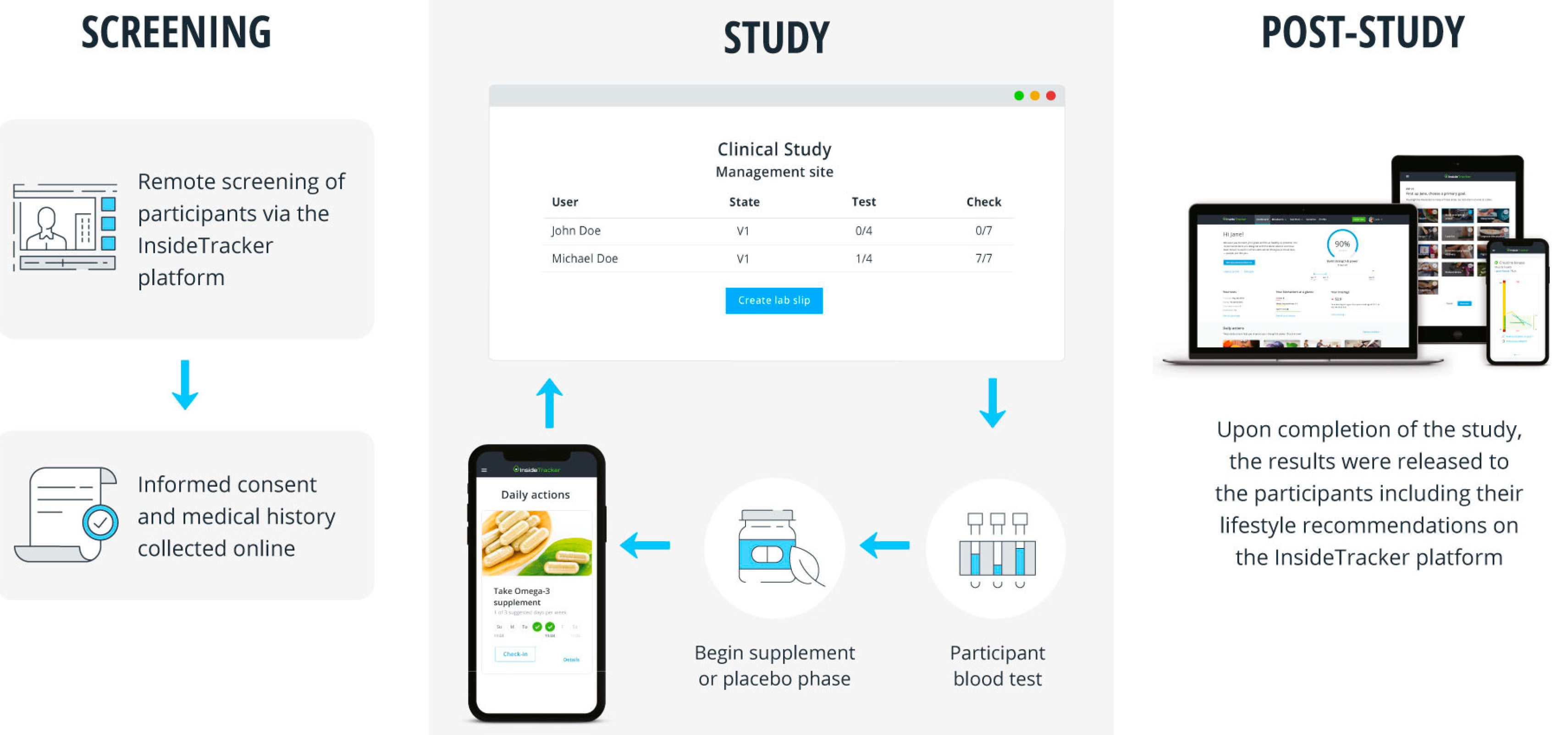
2.3. Clinical Trial Design
2.4. Statistical Analysis
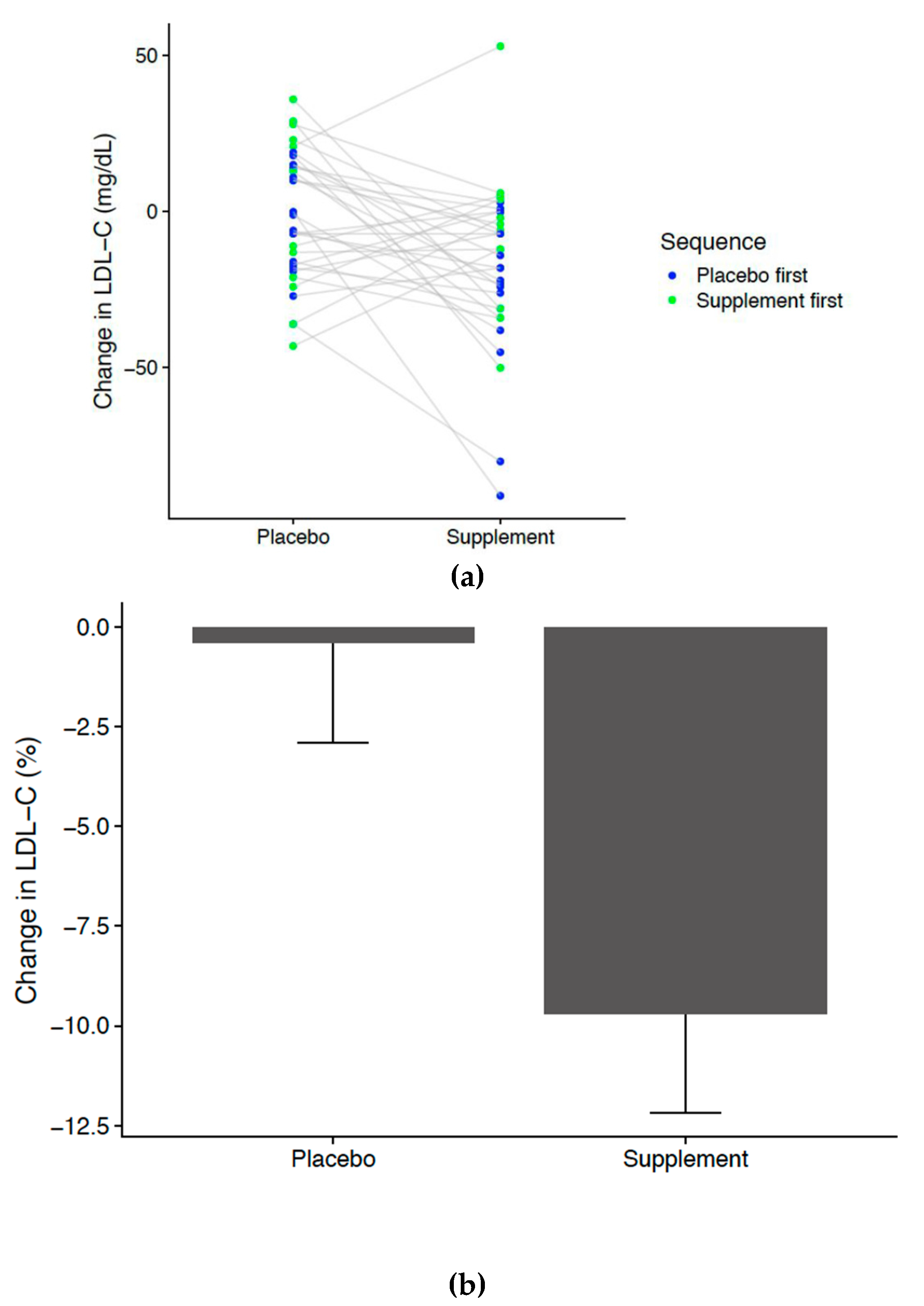
3. Results
3.1. Compliance
3.2. Safety
4. Discussion
Limitations
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Glasziou, P.P.; Irwig, L.; Kirby, A.C.; Tonkin, A.M.; Simes, R.J. Which lipid measurement should we monitor? An analysis of the LIPID study. BMJ. Open 2014, 4, e003512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Status Report on Non-Communicable Diseases; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Revista espanola de cardiologia (English ed.) 2017, 70, 115. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2014, 63, 2889–2934. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Marchie, A.; Jenkins, A.L.; Connelly, P.W.; Jones, P.J.; Vuksan, V. The Garden of Eden--plant based diets, the genetic drive to conserve cholesterol and its implications for heart disease in the 21st century. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 136, 141–151. [Google Scholar] [CrossRef]
- Gylling, H.; Simonen, P. Phytosterols, Phytostanols, and Lipoprotein Metabolism. Nutrients 2015, 7, 7965–7977. [Google Scholar] [CrossRef]
- Kendall, C.W.; Jenkins, D.J. A dietary portfolio: Maximal reduction of low-density lipoprotein cholesterol with diet. Curr. Atheroscler. Rep. 2004, 6, 492–498. [Google Scholar] [CrossRef]
- Ostlund, R.E., Jr. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002, 22, 533–549. [Google Scholar] [CrossRef]
- Demonty, I.; Ras, R.T.; van der Knaap, H.C.; Duchateau, G.S.; Meijer, L.; Zock, P.L.; Trautwein, E.A. Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake. J. Nutr. 2009, 139, 271–284. [Google Scholar] [CrossRef]
- Nissinen, M.; Gylling, H.; Vuoristo, M.; Miettinen, T.A. Micellar distribution of cholesterol and phytosterols after duodenal plant stanol ester infusion. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G1009–G1015. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.J.; Raeini-Sarjaz, M.; Ntanios, F.Y.; Vanstone, C.A.; Feng, J.Y.; Parsons, W.E. Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters. J. Lipid Res. 2000, 41, 697–705. [Google Scholar]
- Normen, L.; Dutta, P.; Lia, Å.; Andersson, H. Soy sterol esters and beta-sitostanol ester as inhibitors of cholesterol absorption in human small bowel. Am. J. Clin. Nutr. 2000, 71, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, E.A.; Vermeer, M.; Hiemstra, H.; Ras, R. LDL-Cholesterol Lowering of Plant Sterols and Stanols-Which Factors Influence Their Efficacy? Nutrients 2018, 10, 1262. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.B.; Grundy, S.M.; Jones, P.; Law, M.; Miettinen, T.; Paoletti, R.; Participants, S.W. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2003; Volume 78, pp. 965–978. [Google Scholar] [CrossRef]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [CrossRef]
- Jones, P.J.H.; Shamloo, M.; MacKay, D.S.; Rideout, T.C.; Myrie, S.B.; Plat, J.; Roullet, J.B.; Baer, D.J.; Calkins, K.L.; Davis, H.R.; et al. Progress and perspectives in plant sterol and plant stanol research. Nutrition reviews 2018, 76, 725–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plat, J.; Baumgartner, S.; Mensink, R.P. Mechanisms Underlying the Health Benefits of Plant Sterol and Stanol Ester Consumption. J. AOAC Int. 2015, 98, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M. The TNO Gastro-Intestinal Model (TIM). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, K., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 37–46. [Google Scholar] [Green Version]
- Westerman, K.; Reaver, A.; Roy, C.; Ploch, M.; Sharoni, E.; Nogal, B.; Blander, G. Longitudinal analysis of biomarker data from a personalized nutrition platform in healthy subjects. Sci. Rep. 2018, 8, 14685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amir Shaghaghi, M.; Abumweis, S.S.; Jones, P.J. Cholesterol-lowering efficacy of plant sterols/stanols provided in capsule and tablet formats: Results of a systematic review and meta-analysis. J. Acad. Nutr. Diet. 2013, 113, 1494–1503. [Google Scholar] [CrossRef]
- Musa-Veloso, K.; Poon, T.H.; Elliot, J.A.; Chung, C. A comparison of the LDL-cholesterol lowering efficacy of plant stanols and plant sterols over a continuous dose range: Results of a meta-analysis of randomized, placebo-controlled trials. Prostaglandins Leukot. Essent. Fatty Acids 2011, 85, 9–28. [Google Scholar] [CrossRef]
- Gould, A.L.; Davies, G.M.; Alemao, E.; Yin, D.D.; Cook, J. Cholesterol reduction yields clinical benefits: Meta-analysis including recent trials. Clin. Ther. 2007, 29, 778–794. [Google Scholar] [CrossRef]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegård, L.; Jessup, W.; Silbernagel, G. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Jiao, J.; Xu, J.; Zimmermann, D.; Actis-Goretta, L.; Guan, L.; Qin, L. Effects of plant stanol or sterol-enriched diets on lipid profiles in patients treated with statins: Systematic review and meta-analysis. Sci. Rep. 2016, 6, 31337. [Google Scholar] [CrossRef] [PubMed]
- Scholle, J.M.; Baker, W.L.; Talati, R.; Coleman, C.I. The effect of adding plant sterols or stanols to statin therapy in hypercholesterolemic patients: Systematic review and meta-analysis. J. Am. Coll. Nutr. 2009, 28, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Rideout, T.C.; Harding, S.V.; Mackay, D.; Abumweis, S.S.; Jones, P.J. High basal fractional cholesterol synthesis is associated with nonresponse of plasma LDL cholesterol to plant sterol therapy. Am. J. Clin. Nutr. 2010, 92, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weingartner, O.; Bogeski, I.; Kummerow, C.; Schirmer, S.H.; Husche, C.; Vanmierlo, T.; Laufs, U. Plant sterol ester diet supplementation increases serum plant sterols and markers of cholesterol synthesis, but has no effect on total cholesterol levels. J. Steroid Biochem. Mol. Biol. 2017, 169, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Gerber, A.; Evers, T.; Haverkamp, H.; Lauterbach, K.W. Cost-benefit analysis of a plant sterol containing low-fat margarine for cholesterol reduction. Eur. J. Health Econ. 2006, 7, 247–254. [Google Scholar] [CrossRef]
- Shanahan, C.; de Lorimier, R. Health Care Cost Savings Resulting from the Targeted Use of Dietary Supplement. An Economic Case for Promoting Increased Intake of Key Dietary Complementary medicines as a Means to Combat Unsustainable Health Care Cost Growth in the United States; Frost & Sullivan: Santa Clara, CA, USA, 2013. [Google Scholar]
- Schulz, K.F.; Grimes, D.A. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet (London, England) 2002, 359, 781–785. [Google Scholar] [CrossRef]
- Edwards, P.J.; Roberts, I.; Clarke, M.J.; DiGuiseppi, C.; Wentz, R.; Kwan, I.; Pratap, S. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst. Rev. 2009, 3, Mr000008. [Google Scholar] [CrossRef]
- Brueton, V.C.; Tierney, J.; Stenning, S.; Harding, S.; Meredith, S.; Nazareth, I.; Rait, G. Strategies to improve retention in randomised trials. Cochrane Database Syst. Rev. 2013, 12, Mr000032. [Google Scholar] [CrossRef]
- Osterberg, L.; Blaschke, T. Adherence to medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef]
- Zullig, L.L.; Ramos, K.; Bosworth, H.B. Improving Medication Adherence in Coronary Heart Disease. Curr. Cardiol. Rep. 2017, 19, 113. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reaver, A.; Hewlings, S.; Westerman, K.; Blander, G.; Schmeller, T.; Heer, M.; Rein, D. A Randomized, Placebo-Controlled, Double-Blind Crossover Study to Assess a Unique Phytosterol Ester Formulation in Lowering LDL Cholesterol Utilizing a Novel Virtual Tracking Tool. Nutrients 2019, 11, 2108. https://doi.org/10.3390/nu11092108
Reaver A, Hewlings S, Westerman K, Blander G, Schmeller T, Heer M, Rein D. A Randomized, Placebo-Controlled, Double-Blind Crossover Study to Assess a Unique Phytosterol Ester Formulation in Lowering LDL Cholesterol Utilizing a Novel Virtual Tracking Tool. Nutrients. 2019; 11(9):2108. https://doi.org/10.3390/nu11092108
Chicago/Turabian StyleReaver, Ashley, Susan Hewlings, Kenneth Westerman, Gil Blander, Thorsten Schmeller, Marianne Heer, and Dietrich Rein. 2019. "A Randomized, Placebo-Controlled, Double-Blind Crossover Study to Assess a Unique Phytosterol Ester Formulation in Lowering LDL Cholesterol Utilizing a Novel Virtual Tracking Tool" Nutrients 11, no. 9: 2108. https://doi.org/10.3390/nu11092108




