Glutathione Supplementation of Parenteral Nutrition Prevents Oxidative Stress and Sustains Protein Synthesis in Guinea Pig Model
Abstract
1. Introduction
2. Methods
2.1. Experimental Design
- (1)
- Reference group: Animals of the same age without any manipulation and fed with regular guinea pig food.
- (2)
- PN: Animals exclusively fed by PN, having free access to tap water. The PN was compounded with 10% (w/v) glucose, 2% (w/v) amino acids preparation (Primene, Baxter, Toronto, ON, Canada), 2% (w/v) lipid emulsion (Intralipid 20%, Fresenius Kabi, Mississauga, ON, Canada), electrolytes, 1% (v/v), multivitamin preparation (Multi-12, Sandoz, Boucherville, QC, Canada), and 1 U/mL heparin. PN solutions were freshly prepared daily and gradually administered at an average rate of 129 mL/kg/day, giving an average caloric intake of 85 kcal/kg/day. These values are close to the recommendations in paediatric parenteral nutrition [29].
- (3)
- PN + 10 µM GSSG: Intervention group, animals receiving PN enriched with 10 µM GSSG. GSSG was used because it has a better stability in the PN solution than GSH [30] and because its affinity for γ-glutamylcysteine transferase is similar to that of GSH [31]. The chosen concentration is the same as previously used with success to prevent pulmonary oxidative stress in neonatal guinea pigs [15].
2.2. Determinations
2.3. Statistical Analyses
3. Results
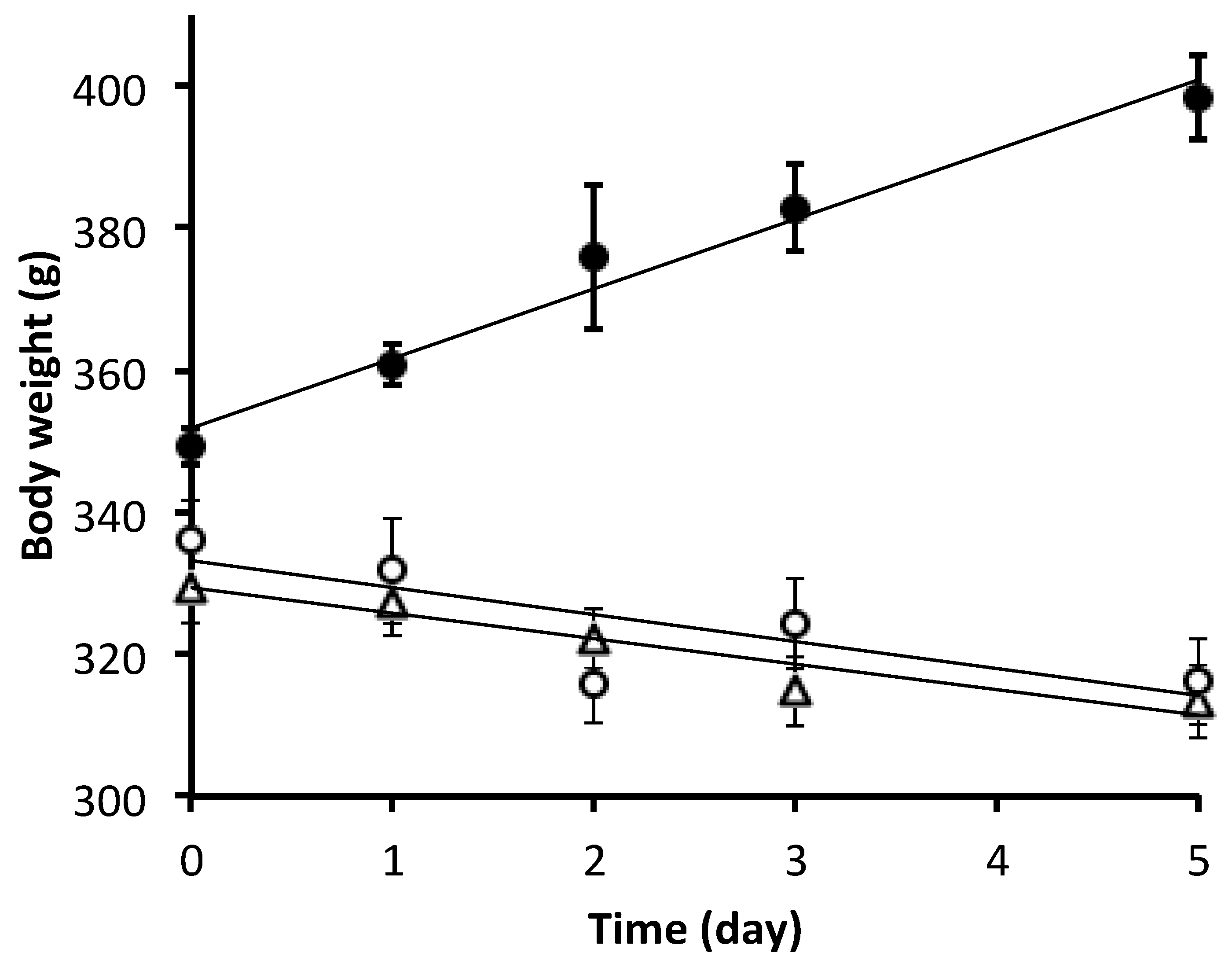
3.1. Animal Characterization
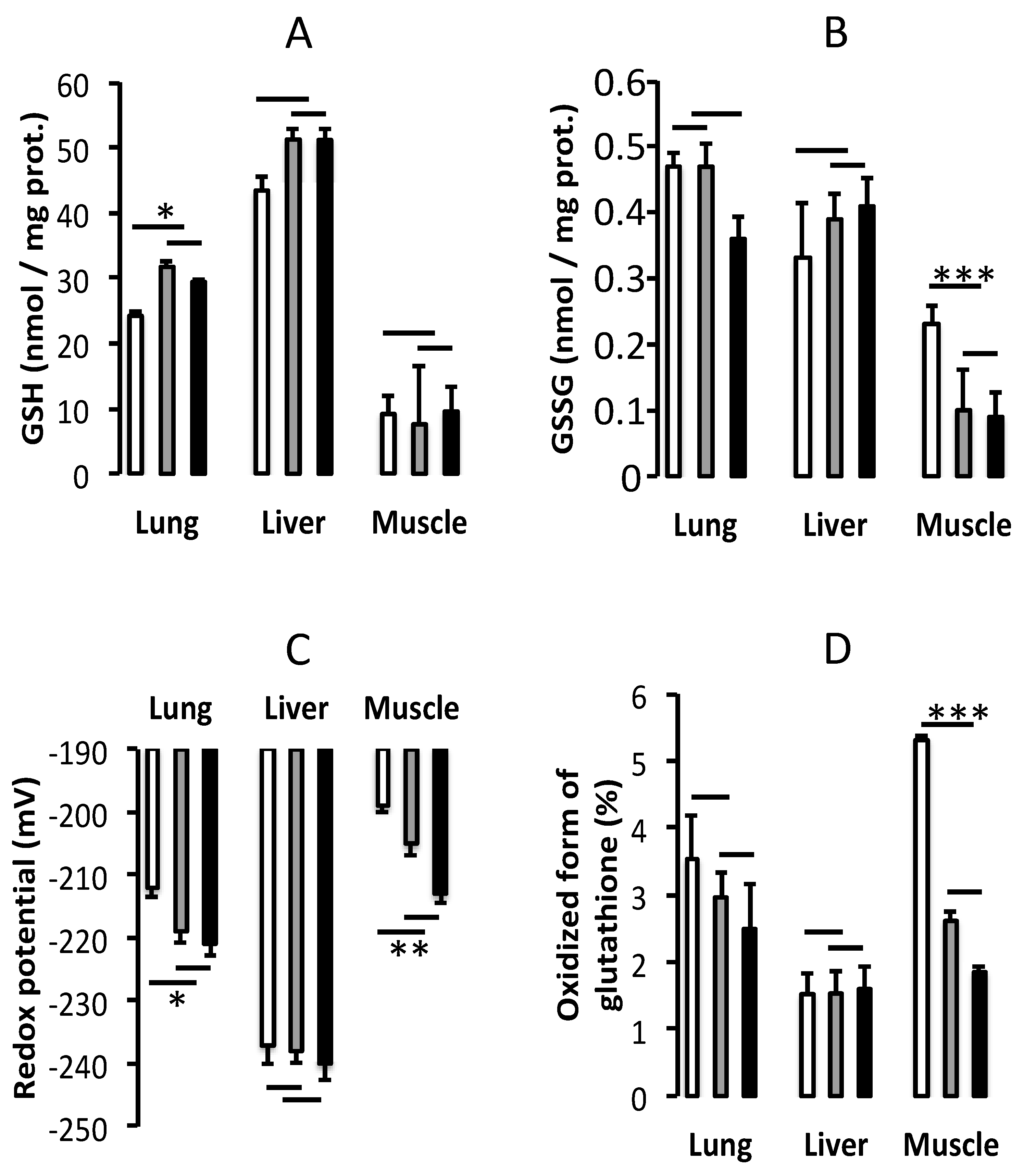
3.2. Oxidative Stress
3.3. Protein Synthesis Index
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Worthington, P.; Balint, J.; Bechtold, M.; Bingham, A.; Chan, L.N.; Durfee, S.; Jevenn, A.K.; Malone, A.; Mascarenhas, M.; Robinson, D.T.; et al. When is parenteral nutrition appropriate? J. Parenter. Enter. Nutr. JPEN 2017, 41, 324–377. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.C.; Chessex, P. Parenteral nutrition and oxidant stress in the newborn: A narrative review. Free Radic. Biol. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.C.; Bélanger, S.; Spalinger, M.; Chessex, P. Admixture of a multivitamin preparation to parenteral nutrition: The major contributor to in vitro generation of peroxides. Pediatrics 1997, 99, E61–E70. [Google Scholar] [CrossRef] [PubMed]
- Helbock, H.J.; Motchnik, P.A.; Ames, B.N. Toxic hydroperoxides in intravenous lipid emulsions used in preterm infants. Pediatrics 1993, 91, 83–88. [Google Scholar] [PubMed]
- Neuzil, J.; Darlow, B.A.; Inder, T.E.; Sluis, K.B.; Winterbourn, C.C.; Stocker, R. Oxidation of parenteral lipid emulsion by ambient and phototherapy lights: Potential toxicity of routine parenteral feeding. J. Pediatr. 1995, 26, 785–790. [Google Scholar] [CrossRef]
- Brawley, V.; Bathia, J.; Karp, B. Hydrogen peroxide generation in a model pediatric parenteral amino acid solution. Clin. Sci. 1993, 85, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Shattuck, K.E.; Bhatia, J.; Grinnell, C.; Rassin, D.K. The effects of light exposure on the in vitro hepatic response to an amino acid-vitamin solution. J. Parenter. Enter. Nutr. JPEN 1995, 19, 398–402. [Google Scholar] [CrossRef]
- Elremaly, W.; Mohamed, I.; Mialet-Marty, T.; Rouleau, T.; Lavoie, J.C. Ascorbylperoxide from parenteral nutrition induces oxidized redox potential of glutathione and loss of alveoli in newborn guinea pig lungs. Redox Biol. 2014, 2, 725–731. [Google Scholar] [CrossRef]
- Mohamed, I.; Elremaly, W.; Rouleau, T.; Lavoie, J.C. Ascorbylperoxide contaminating parenteral nutrition is associated with bronchopulmonary dysplasia or death in extremely preterm infants. J. Parenter. Enter. Nutr. JPEN 1017, 41, 1023–1029. [Google Scholar] [CrossRef]
- Lavoie, J.C.; Chessex, P. Gender and maturation affect glutathione status in human neonatal tissues. Free Radic. Biol. Med. 1997, 23, 648–657. [Google Scholar] [CrossRef]
- Elremaly, W.; Rouleau, T.; Lavoie, J.C. Inhibition of hepatic methionine adenosyltransferase by peroxides contaminating parenteral nutrition leads to a lower level of glutathione in newborn guinea pigs. Free Radic. Biol. Med. 2012, 53, 2250–2255. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Function of glutathione in kidney via the γ-glutamy cycle. Med. Clin. N. Am. 1975, 1, 649–666. [Google Scholar] [CrossRef]
- Meister, A. On the enzymology of amino acid transport. Transport in kidney and probably other tissues is mediated by a cycle enzymatic reactions involving glutathione. Science 1973, 180, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Neuhauser-Berthold, M.; Kuhfus, A.; Bassler, K.H. Utilization of Glutathione Disulfide as Cysteine Source During Long-Terrn Parenteral Nutrition in the Growing Rat. Metabolism 1988, 37, 796–801. [Google Scholar] [CrossRef]
- Elremaly, W.; Mohamed, I.; Rouleau, T.; Lavoie, J.C. Adding glutathione to parenteral nutrition prevents alveolar loss in newborn Guinea pig. Free Radic. Biol. Med. 2015, 87, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Deneke, S.M.; Fanburg, B.L. Regulation of cellular glutathione. Am. J. Physiol. 1989, 257, L163–L173. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, H.F. Molecular and cellular aspects of thioldisulfide exchange. Adv. Enzymol. Relat. Areas Mol. Biol. 1990, 63, 69–172. [Google Scholar] [PubMed]
- Meister, A.; Anderson, M.E. Glutathione. Ann. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Tateishi, N.; Naruse, A.; Sakamoto, Y. A novel physiological role of liver glutathione as a reservoir of l-cysteine. J. Biochem. 1977, 182, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Vore, M. Canaiicular transport: Discovery of ATP dependent mechanisms. Toxicol. Appl. Pharmacol. 1993, 118, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Vento, M.; Garcia-Sala, F.; Puertes, I.; Gasco, E.; Sastre, J.; Asensi, M.; Pallardo, F. l-cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency. Am. J. Clin. Nutr. 1995, 61, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, S.H.; Anderson, G.H. The development of cystathionase activity during the first year of life. Pediatr. Res. 1982, 16, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.C.; Rebeuh, J.; Herzog, D.; Faure, C.; Rouleau, T. Oxidative stress in children on long-term parenteral nutrition. J. Hum. Nutr. Food Sci. 2017, 5, 1110. [Google Scholar]
- Liu, C.; Gamper, H.; Shtivelband, S.; Hauenstein, S.; Perona, J.J.; Hou, Y.M. Kinetic quality control of anticodon recognition by a eukaryotic aminoacyl-tRNA synthetase. J. Mol. Biol. 2007, 367, 1063–1078. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, M.M.; Iwamoto, T.; Forman, H.J. Gamma-Glutamylcysteine synthetase and GSH increase in quinone-induced oxidative stress in BPAEC. Am. J. Physiol. 1994, 267, L414–L421. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Chang, L.S.; Anderson, M.E.; Meister, A. Catalytic and regulatory properties of the heavy subunit of rat kidney Gamma-glutamylcysteine synthetase. J. Biol. Chem. 1993, 268, 19675–19680. [Google Scholar] [PubMed]
- Neelis, E.; Kouwenhoven, S.; Olieman, J.; Tabbers, M.; Jonkers, C.; Wells, J.; Fewtrell, M.; Wijnen, R.; Rings, E.; de Koning, B.; et al. Body composition using air displacement plethysmography in children with intestinal failure receiving long-term home parenteral nutrition. J. Parenter. Enter. Nutr. JPEN 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.C.; Rouleau, T.; Tsopmo, A.; Friel, J.; Chessex, P. Influence of lung oxidant and antioxidant status on alveolarization: Role of light-exposed total parenteral nutrition. Free Radic. Biol. Med. 2008, 45, 572–577. [Google Scholar] [CrossRef]
- Joosten, K.; Embleton, N.; Yan, W.; Senterre, T. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Energy. Clin. Nutr. 2018, 37, 2309–2314. [Google Scholar] [CrossRef]
- Elremaly, W.; Mohamed, I.; Rouleau, T.; Lavoie, J.C. Impact of glutathione supplementation of parenteral nutrition on hepatic methionine adenosyltransferase activity. Redox Biol. 2016, 8, 18–23. [Google Scholar] [CrossRef]
- Cotgreave, I.A.; Schuppe-Koistinen, I. A role for gamma-glutamyl transpeptidase in the transport of cysteine into human endothelial cells: Relationship to intracellular glutathione. Biochem. Biophys. Acta 1994, 1223, 375–382. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Woollard, A.C.S.; Wolff, S.P. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids 1991, 26, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Darmaun, D.; Mauras, N. Use of stable isotopes to assess protein and amino acid metabolism in children and adolescents: A brief review. Horm. Res. 2005, 64, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Prefontaine, A.; Calderone, A.; Dupuis, J. Role of endothelin receptors on basal and endothelin-1-stimulated lung myofibroblast proliferation. Can. J. Physiol. Pharmacol. 2008, 86, 337–342. [Google Scholar] [CrossRef]
- Fearon, W.R. The carbamido diacetyl reaction: A test for citrulline. Biochem. J. 1939, 33, 902–907. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Boyde, T.R. Improvements in the determination of urea using diacetyl monoxime; methods with and without deproteinisation. Clin. Chim. Acta 1980, 107, 3–9. [Google Scholar] [CrossRef]
- Quigley, E.M.M.; Arsh, M.N.; Shaffer, J.L.; Markin, R.S. Hepatobiliary complications of total parenteral nutrition. Gastroenterology 1993, 104, 286–301. [Google Scholar] [CrossRef]
- Lu, C.J.; Redmond, D.; Baggs, R.B.; Schecter, A.; Gasiewicz, T.A. Growth and hepatic composition in the guinea pig after long-term parenteral hyperalimentation. Am. J. Physiol. 1986, 251, R388–R397. [Google Scholar] [CrossRef]
- Reichman, B.L.; Chessex, P.; Putet, G.; Verellen, G.J.E.; Smith, J.M.; Heim, T.; Swyer, P.R. Partition of energy metabolism and energy cost of growth in the very low-birth-weight infant. Pediatrics 1982, 29, 446–451. [Google Scholar]
- Soghier, L.M.; Brion, L.P. Cysteine, cystine or N-acetylcysteine supplementation in parenterally fed neonates. Cochrane Database Syst. Rev. 2006, 18, CD004869. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Cantin, A.M.; North, S.L.; Hubbard, R.C.; Crystal, R.G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 1987, 63, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, G.; Mirabelli, F.; Richelmi, P.; Orrenius, S. Critical role of sulfhydryl group(s) in ATP-dependent Ca2+ sequestraation by the plasma membrane fraction from rat liver. FEBS Lett. 1983, 163, 136–139. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Gilliam, L.A.A.; Reid, M.B. L-2-Oxothiazolidine-4-carboxylate reverses glutathione oxidation and delays fatigue of skeletal muscle in vitro. J. Appl. Physiol. 2009, 107, 211–216. [Google Scholar] [CrossRef]
- Xia, R.; Stangler, T.; Abramson, J.J. Skeletal muscle ryanodine receptor is a redox sensor with a well defined redox potential that is sensitive to channel modulators. J. Biol. Chem. 2000, 275, 36555–36561. [Google Scholar] [CrossRef]
- Nicotera, P.; Moore, M.; Bellomo, G.; Mirabelli, F.; Orrenius, S. Demonstration and partial characterization of glutathione disulfide-stimulated ATPase activity in the plasma membrane fraction from rat hepatocytes. J. Biol. Chem. 1985, 260, 1999–2002. [Google Scholar]
- Aebi, S.; Assereto, R.; Lauterburg, B.H. High-dose intravenous glutathione in man. Pharmacokinetics and effects on cyst(e)ine in plasma and urine. Eur. J. Clin. Investig. 1991, 21, 103–110. [Google Scholar] [CrossRef]


| Mean Daily Caloric Intake (kcal/kg) | |
|---|---|
| Day 1 | 60 ± 3 |
| Day 2 | 69 ± 4 |
| Day 3 | 84 ± 4 |
| Day 4 | 83 ± 5 |
| Day 5 | 93 ± 6 |
| PN | PN+10 µM GSSG | Reference Group | |
|---|---|---|---|
| Hb at d0 (g/L) | 198 ± 3 | 188 ± 4 | 192 ± 3 |
| Hb at d5 (g/L) | 213 ± 4 ** | 199 ± 5 | 191 ± 3 |
| Urea at d5 (mg/L) | 34.7 ± 1.3 | 34.3 ± 2.9 | 33.8 ± 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morin, G.; Guiraut, C.; Perez Marcogliese, M.; Mohamed, I.; Lavoie, J.-C. Glutathione Supplementation of Parenteral Nutrition Prevents Oxidative Stress and Sustains Protein Synthesis in Guinea Pig Model. Nutrients 2019, 11, 2063. https://doi.org/10.3390/nu11092063
Morin G, Guiraut C, Perez Marcogliese M, Mohamed I, Lavoie J-C. Glutathione Supplementation of Parenteral Nutrition Prevents Oxidative Stress and Sustains Protein Synthesis in Guinea Pig Model. Nutrients. 2019; 11(9):2063. https://doi.org/10.3390/nu11092063
Chicago/Turabian StyleMorin, Guillaume, Clémence Guiraut, Marisol Perez Marcogliese, Ibrahim Mohamed, and Jean-Claude Lavoie. 2019. "Glutathione Supplementation of Parenteral Nutrition Prevents Oxidative Stress and Sustains Protein Synthesis in Guinea Pig Model" Nutrients 11, no. 9: 2063. https://doi.org/10.3390/nu11092063
APA StyleMorin, G., Guiraut, C., Perez Marcogliese, M., Mohamed, I., & Lavoie, J.-C. (2019). Glutathione Supplementation of Parenteral Nutrition Prevents Oxidative Stress and Sustains Protein Synthesis in Guinea Pig Model. Nutrients, 11(9), 2063. https://doi.org/10.3390/nu11092063







