Inflammatory Dietary Pattern Predicts Dyslipidemia and Anemia in Middle-Aged and Older Taiwanese Adults with Declined Kidney Function: A Cross-Sectional Population Study from 2008 to 2010
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Clinical and Biochemical Data and Definition of the Diseases
2.3. Dietary Assessment and Other Covariates
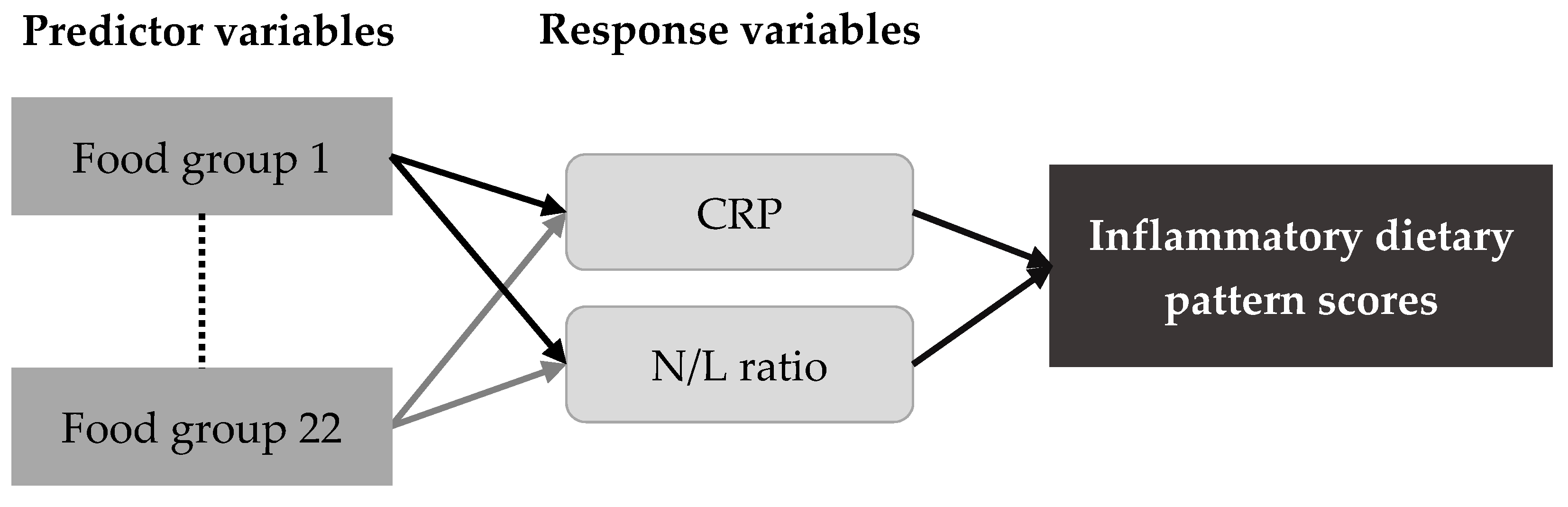
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
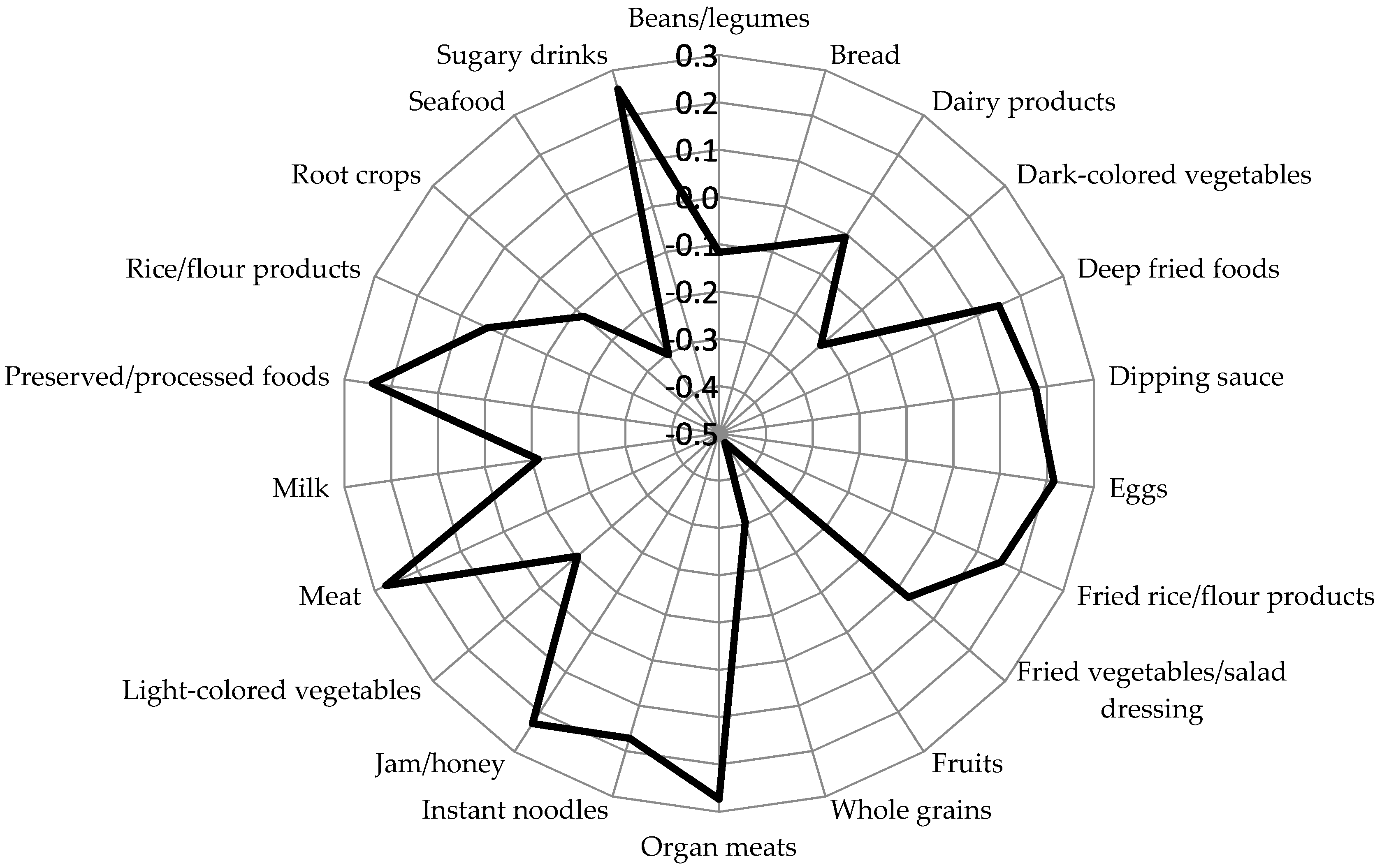
3.2. Inflammatory Dietary Pattern and Characteristics Across Quartiles of Dietary Pattern Scores
3.3. Association of Inflammatory Dietary Pattern with Dyslipidemia, Anemia, and Kidney Function Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saland, J.M.; Ginsberg, H.N. Lipoprotein metabolism in chronic renal insufficiency. Pediatr. Nephrol. 2007, 22, 1095–1112. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Chang, H.Y.; Tung, C.W.; Hsu, Y.C.; Lei, C.C.; Chang, H.H.; Yang, H.F.; Lu, L.C.; Jong, M.C.; Chen, C.Y.; et al. Hypertriglyceridemia: An independent risk factor of chronic kidney disease in taiwanese adults. Am. J. Med. Sci. 2009, 338, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Baragetti, A.; Norata, G.D.; Sarcina, C.; Rastelli, F.; Grigore, L.; Garlaschelli, K.; Uboldi, P.; Baragetti, I.; Pozzi, C.; Catapano, A.L. High density lipoprotein cholesterol levels are an independent predictor of the progression of chronic kidney disease. J. Intern. Med. 2013, 274, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Qiu, X.L.; Lv, L.S.; Wang, C.X.; Ye, Z.C.; Li, S.M.; Liu, Q.; Lou, T.; Liu, X. Correlation between serum lipid levels and measured glomerular filtration rate in chinese patients with chronic kidney disease. PLoS ONE 2016, 11, e0163767. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Hung, C.C.; Kuo, M.C.; Lee, J.J.; Chiu, Y.W.; Chang, J.M.; Hwang, S.J.; Chen, H.C. Association of dyslipidemia with renal outcomes in chronic kidney disease. PLoS ONE 2013, 8, e55643. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Coresh, J.; Levey, A.S.; Tonelli, M.; Muntner, P. Estimated GFR, albuminuria, and complications of chronic kidney disease. J. Am. Soc. Nephrol. 2011, 22, 2322–2331. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, M.E.; Fan, T. Prevalence of anemia in chronic kidney disease in the United States. PLoS ONE 2014, 9, e84943. [Google Scholar] [CrossRef]
- Levin, A.; Thompson, C.R.; Ethier, J.; Carlisle, E.J.; Tobe, S.; Mendelssohn, D.; Burgess, E.; Jindal, K.; Barrett, B.; Singer, J.; et al. Left ventricular mass index increase in early renal disease: Impact of decline in hemoglobin. Am. J. Kidney Dis. 1999, 34, 125–134. [Google Scholar] [CrossRef]
- Astor, B.C.; Coresh, J.; Heiss, G.; Pettitt, D.; Sarnak, M.J. Kidney function and anemia as risk factors for coronary heart disease and mortality: The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2006, 151, 492–500. [Google Scholar] [CrossRef]
- Chang, J.M.; Chen, S.C.; Huang, J.C.; Su, H.M.; Chen, H.C. Anemia and left ventricular hypertrophy with renal function decline and cardiovascular events in chronic kidney disease. Am. J. Med. Sci. 2014, 347, 183–189. [Google Scholar] [CrossRef]
- Gupta, J.; Mitra, N.; Kanetsky, P.A.; Devaney, J.; Wing, M.R.; Reilly, M.; Shah, V.O.; Balakrishnan, V.S.; Guzman, N.J.; Girndt, M.; et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012, 7, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nothlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Heimbürger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Kutuby, F.; Wang, S.; Desai, C.; Lerma, E.V. Anemia of chronic kidney disease. Dis. Mon. 2015, 61, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Okyay, G.U.; Inal, S.; Onec, K.; Er, R.E.; Paşaoğlu, O.; Paşaoğlu, H.; Derici, U.; Erten, Y. Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Ren. Fail. 2013, 35, 29–36. [Google Scholar] [CrossRef]
- Lu, X.X.; Wang, S.X.; Zhang, G.Z.; Xiong, R.F.; Li, H. High neutrophil-to-lymphocyte ratio is a significant predictor of cardiovascular and all-cause mortality in patients undergoing peritoneal dialysis. Kidney Blood Press. Res. 2018, 43, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.L.; Stampfer, M.J.; Hankinson, S.E.; Spiegelman, D.; Curhan, G.C. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann. Intern. Med. 2003, 138, 460–467. [Google Scholar] [CrossRef]
- de Oliveira Otto, M.C.; Mozaffarian, D.; Kromhout, D.; Bertoni, A.G.; Sibley, C.T.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary intake of saturated fat by food source and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2012, 96, 397–404. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Schwandt, A.; Denkinger, M.; Fasching, P.; Pfeifer, M.; Wagner, C.; Weiland, J.; Zeyfang, A.; Holl, R.W. Comparison of MDRD, CKD-EPI, and Cockcroft-Gault equation in relation to measured glomerular filtration rate among a large cohort with diabetes. J. Diabetes Complicat. 2017, 31, 1376–1383. [Google Scholar] [CrossRef]
- Chang, H.Y.; Yeh, W.T.; Chang, Y.H.; Tsai, K.S.; Pan, W.H. Prevalence of dyslipidemia and mean blood lipid values in Taiwan: Results from the Nutrition and Health Survey in Taiwan (NAHSIT, 1993-1996). Chin. J. Physiol. 2002, 45, 187–197. [Google Scholar] [PubMed]
- World Health Organization. Iron Deficiency Anemia: Assessment, Prevention and Control. A Guide for Programme Managers; World Health Organization: Geneva, Switzerland, 2001; pp. 21–42. [Google Scholar]
- Muga, M.A.; Owili, P.O.; Hsu, C.Y.; Rau, H.H.; Chao, J.C.J. Association between dietary patterns and cardiovascular risk factors among middle-aged and elderly adults in Taiwan: A population-based study from 2003 to 2012. PLoS ONE 2016, 11, e0157745. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, A.L.; Hsu, C.Y.; Rau, H.H.; Lin, L.Y.; Chao, J.C. Association of kidney function-related dietary pattern, weight status, and cardiovascular risk factors with severity of impaired kidney function in middle-aged and older adults with chronic kidney disease: A cross-sectional population study. Nutr. J. 2019, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nothlings, U.; Boeing, H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Weikert, C.; Schulze, M.B. Evaluating dietary patterns: The role of reduced rank regression. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Paterson, E.N.; Neville, C.E.; Silvestri, G.; Montgomery, S.; Moore, E.; Silvestri, V.; Cardwell, C.R.; MacGillivray, T.J.; Maxwell, A.P.; Woodside, J.V.; et al. Dietary patterns and chronic kidney disease: A cross-sectional association in the Irish Nun Eye Study. Sci. Rep. 2018, 8, 6654. [Google Scholar] [CrossRef]
- Lin, J.L.; Fung, T.T.; Hu, F.B.; Curhan, G.C. Association of dietary patterns with albuminuria and kidney function decline in older white women: A subgroup analysis from the Nurses’ Health Study. Am. J. Kidney Dis. 2011, 57, 245–254. [Google Scholar] [CrossRef]
- Poggio, R.; Elorriaga, N.; Gutierrez, L.; Irazola, V.; Rubinstein, A.; Danaei, G. Associations between dietary patterns and serum lipids, apo and C-reactive protein in an adult population: Evidence from a multi-city cohort in South America. Br. J. Nutr. 2017, 117, 548–555. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S.; Roberts, S.; Haskell, W. Efficacy of aerobic exercise and a prudent diet for improving selected lipids and lipoproteins in adults: A meta-analysis of randomized controlled trials. BMC Med. 2011, 9, 74. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Warady, B.A. Anemia in chronic kidney disease. Pediatr. Nephrol. 2017, 33, 227–238. [Google Scholar] [CrossRef]
- Babitt, J.L.; Lin, H.Y. Mechanisms of anemia in CKD. J. Am. Soc. Nephrol. 2012, 23, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Schulze, M.B.; Fung, T.T.; Meigs, J.B.; Rifai, N.; Manson, J.E.; Hu, F.B. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2004, 80, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; Steffen, L.M.; Mayer-Davis, E.J.; Jenny, N.S.; Jiang, R.; Herrington, D.M.; Jacobs, D.R., Jr. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2006, 83, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Binnetoğlu, E.; Şengül, E.; Halhallı, G.; Dindar, S.; Şen, H. Is neutrophil lymphocyte ratio an indicator for proteinuria in chronic kidney disease? J. Clin. Lab. Anal. 2014, 28, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Amdur, R.L.; Feldman, H.I.; Gupta, J.; Yang, W.; Kanetsky, P.; Shlipak, M.; Rahman, M.; Lash, J.P.; Townsend, R.R.; Ojo, A.; et al. Inflammation and progression of CKD: The CRIC study. Clin. J. Am. Soc. Nephrol. 2016, 11, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Sacks, F.; Pfeffer, M.; Jhangri, G.S.; Curhan, G. Cholesterol and Recurrent Events (CARE) Trial Investigators. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005, 68, 237–245. [Google Scholar] [CrossRef]
- Lew, Q.J.; Jafar, T.H.; Koh, H.W.; Jin, A.; Chow, K.Y.; Yuan, J.M.; Koh, W.P. Red meat intake and risk of ESRD. J. Am. Soc. Nephrol. 2017, 28, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Crews, D.C.; Wesson, D.E.; Tilea, A.M.; Saran, R.; Rios-Burrows, N.; Williams, D.E.; Powe, N.R.; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. High dietary acid load predicts ESRD among adults with CKD. J. Am. Soc. Nephrol. 2015, 26, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Anderson, C.A. Dietary acid load: A novel nutritional target in chronic kidney disease? Adv. Chronic Kidney Dis. 2013, 20, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, G.; Jalili, T.; Metos, J.; Giri, A.; Cho, M.E.; Boucher, R.; Greene, T.; Beddhu, S. The associations of plant protein intake with all-cause mortality in CKD. Am. J. Kidney Dis. 2016, 67, 423–430. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Appel, L.J.; Wolf, M.; Yang, W.; Zhang, X.M.; Sozio, S.M.; Miller, E.R., 3rd; Bazzano, L.A.; Cuevas, M.; Glenn, M.J.; et al. Plant protein intake is associated with fibroblast growth factor 23 and serum bicarbonate levels in patients with chronic kidney disease: The Chronic Renal Insufficiency Cohort study. J. Ren. Nutr. 2012, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Pitsavos, C.; Panagiotakos, D.; Skoumas, J.; Lazaros, G.; Oikonomou, E.; Galiatsatos, N.; Striggou, M.; Xynogala, M.; Stefanadis, C. Long-term fish intake preserves kidney function in elderly individuals: The Ikaria study. J. Ren. Nutr. 2013, 23, e75–e82. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Guo, L.; Zhang, L.; Li, Y.; He, R.; Cheng, G. Inflammatory potential of diet and risk of cardiovascular disease or mortality: A meta-analysis. Sci. Rep. 2017, 7, 6367. [Google Scholar] [CrossRef]
- Case, A.; Menendez, A. Sex differences in obesity rates in poor countries: Evidence from South Africa. Econ. Hum. Biol. 2009, 7, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, V.; Begin, C.; Corneau, L.; Dodin, S.; Lemieux, S. Gender differences in dietary intakes: What is the contribution of motivational variables? J. Hum. Nutr. Diet. 2015, 28, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.; Malorni, W. Gender-related differences in dietary habits. Clin. Manag. Issues 2017, 11, 59–62. [Google Scholar] [CrossRef]
- Pizarro, F.; Olivares, M.; Hertrampf, E.; Mazariegos, D.I.; Arredondo, M. Heme-iron absorbtion is saturable by heme-iron dose in women. J. Nutr. 2003, 133, 2214–2217. [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Kourlaba, G.; Grammatikaki, E.; Androutsos, O.; Ioannou, E.; Roma-Giannikou, E. Comparison of two methods for identifying dietary patterns associated with obesity in preschool children: The GENESIS study. Eur. J. Clin. Nutr. 2010, 64, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, C.; Hoffmann, K.; Spranger, J.; Klipstein-Grobusch, K.; Möhlig, M.; Pfeiffer, A.; Boeing, H.; European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study Cohort. A dietary pattern protective against type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study cohort. Diabetologia 2005, 48, 1126–1134. [Google Scholar] [CrossRef]
| Dietary Pattern Scores | ||||||
|---|---|---|---|---|---|---|
| Males | Females | |||||
| Q1 (Low) | Q4 (High) | pb | Q1 (Low) | Q4 (High) | pb | |
| n | 4592 | 6300 | 5696 | 3968 | ||
| Age (years) | 55.3 ± 10.2 | 48.9 ± 9.0 | <0.001 | 55.4 ± 9.6 | 49.7 ± 9.0 | <0.001 |
| Smoking, current | 727 (16.5) | 2591 (42.2) | <0.001 | 63 (1.2) | 269 (7.0) | <0.001 |
| Alcohol drinking, yes | 1017 (23.8) | 1758 (29.5) | <0.001 | 215 (4.3) | 262 (7.2) | <0.001 |
| Physical inactivity | 2511 (62.2) | 3908 (70.7) | <0.001 | 3256 (66.9) | 2692 (79.0) | <0.001 |
| Family income | <0.001 | <0.001 | ||||
| <800,000 NTD | 1668 (40.0) | 2066 (35.4) | 2804 (54.9) | 1788 (49.0) | ||
| >1.61 M NTD | 853 (20.5) | 1263 (21.6) | 681 (13.3) | 554 (15.2) | ||
| Education | <0.001 | <0.001 | ||||
| <high school | 889 (19.7) | 862 (13.9) | 2085 (37.3) | 1010 (25.8) | ||
| >high school | 1692 (37.5) | 2486 (40.0) | 1263 (22.6) | 1082 (27.6) | ||
| Marital status, married | 4031 (93.2) | 5308 (88.5) | <0.001 | 4031 (76.1) | 2766 (74.2) | <0.001 |
| Dyslipidemia | 1550 (34.5) | 2551 (42.1) | <0.001 | 1449 (25.7) | 922 (23.6) | 0.04 |
| Anemia | 162 (3.5) | 154 (2.4) | <0.001 | 665 (11.7) | 584 (14.7) | <0.001 |
| BMI (kg/m2) | 24.4 ± 2.9 | 25.0 ± 3.3 | <0.001 | 23.0 ± 3.2 | 23.3 ± 3.7 | <0.001 |
| Inflammatory markers | ||||||
| N/L ratio | 1.8 ± 0.8 | 1.9 ± 0.9 | <0.001 | 1.8 ± 0.8 | 1.9 ± 1.0 | <0.001 |
| CRP (nmol/L) | 21.2 ± 43.8 | 25.0 ± 46.1 | <0.001 | 20.4 ± 39.9 | 23.1 ± 53.9 | <0.001 |
| Blood lipids | ||||||
| TG (mmol/L) | 1.5 ± 1.0 | 1.8 ± 1.3 | <0.001 | 1.2 ± 0.7 | 1.2 ± 0.8 | 0.08 |
| TC (mmol/L) | 5.2 ± 0.9 | 5.3 ± 0.9 | <0.001 | 5.3 ± 0.9 | 5.3 ± 0.9 | 0.32 |
| HDL-C (mmol/L) | 1.4 ± 0.3 | 1.3 ± 0.3 | <0.001 | 1.7 ± 0.4 | 1.7 ± 0.4 | 0.16 |
| LDL-C (mmol/L) | 3.1 ± 0.8 | 3.2 ± 0.8 | <0.001 | 3.1 ± 0.8 | 3.1 ± 0.8 | 0.22 |
| TC/HDL-C ratio | 3.9 ± 0.9 | 4.1 ± 0.9 | <0.001 | 3.3 ± 0.8 | 3.2 ± 0.8 | 0.016 |
| Anemic biomarkers | ||||||
| RBC (×106/µL) | 5.0 ± 0.5 | 5.1 ± 0.5 | <0.001 | 4.5 ± 0.4 | 4.5 ± 0.4 | <0.001 |
| Hemoglobin (g/dL) | 15.0 ± 1.1 | 15.2 ± 1.1 | <0.001 | 13.2 ± 1.1 | 13.0 ± 1.2 | <0.001 |
| Hematocrit (%) | 44.5 ± 3.2 | 45.2 ± 3.2 | <0.001 | 39.4 ± 3.1 | 39.0 ± 3.4 | <0.001 |
| MCV (fL) | 89.8 ± 6.0 | 89.2 ± 6.3 | <0.001 | 88.5 ± 6.6 | 87.1 ± 7.5 | <0.001 |
| MCH (pg) | 30.3 ± 2.4 | 30.0 ± 2.4 | <0.001 | 29.8 ± 2.6 | 29.2 ± 3.0 | <0.001 |
| MCHC (g/dL) | 33.7 ± 0.7 | 33.6 ± 0.7 | <0.001 | 33.6 ± 0.8 | 33.5 ± 0.8 | <0.001 |
| RDW (%) | 13.9 ± 1.1 | 14.0 ± 1.1 | <0.001 | 13.8 ± 1.3 | 14.1 ± 1.6 | <0.001 |
| Iron (µg/dL) | 101.4 ± 34.7 | 102.4 ± 36.0 | 0.65 | 84.8 ± 31.0 | 81.7 ± 35.4 | <0.001 |
| Kidney function biomarkers | ||||||
| BUN (mmol/L) | 5.4 ± 1.4 | 5.3 ± 1.4 | <0.001 | 5.0 ± 1.5 | 4.8 ± 1.4 | <0.001 |
| CRE (µmol/L) | 100.5 ± 16.9 | 100.6 ± 17.8 | 0.053 | 77.4 ± 20.7 | 76.9 ± 14.2 | 0.89 |
| eGFR (mL/min/1.73 m2) | 73.0 ± 9.9 | 74.5 ± 9.4 | <0.001 | 73.5 ± 10.0 | 75.1 ± 9.3 | <0.001 |
| Proteinuria | 0.47 | 0.54 | ||||
| +1 | 4375 (95.3) | 5952 (94.5) | 5514 (96.8) | 3828 (96.5) | ||
| +2 | 119 (2.6) | 204 (3.2) | 102 (1.8) | 82 (2.0) | ||
| ≥+3 | 98 (2.1) | 144 (2.3) | 80 (1.4) | 58 (1.5) | ||
| Dietary Pattern Score | Model 1 a | Model 2 b | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Males | ||||
| Q1 (low) | Reference | |||
| Q2 (mild) | 1.09 (0.99, 1.19) | 0.057 | 1.06 (0.95, 1.17) | 0.29 |
| Q3 (moderate) | 1.13 (1.04, 1.23) | 0.004 | 1.10 (0.99, 1.21) | 0.07 |
| Q4 (high) | 1.29 (1.18, 1.40) | <0.001 | 1.21 (1.10, 1.34) | <0.001 |
| Females | ||||
| Q1 (low) | Reference | |||
| Q2 (mild) | 1.00 (0.92, 1.10) | 0.90 | 0.94 (0.84, 1.06) | 0.32 |
| Q3 (moderate) | 1.12 (1.02, 1.23) | 0.016 | 1.12 (0.99, 1.26) | 0.052 |
| Q4 (high) | 1.10 (0.99, 1.22) | 0.06 | 1.11 (0.98, 1.26) | 0.11 |
| Dietary Pattern Score | Model 1 a | Model 2 b | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Males | ||||
| Q1 (low) | Reference | |||
| Q2 (mild) | 1.29 (1.04, 1.61) | 0.021 | 1.29 (0.97, 1.69) | 0.07 |
| Q3 (moderate) | 1.26 (1.01, 1.57) | 0.044 | 1.35 (1.00, 1.80) | 0.045 |
| Q4 (high) | 1.15 (0.91, 1.44) | 0.25 | 1.24 (0.93, 1.67) | 0.14 |
| Females | ||||
| Q1 (low) | Reference | |||
| Q2 (mild) | 1.06 (0.94, 1.91) | 0.32 | 1.11 (0.96, 1.27) | 0.16 |
| Q3 (moderate) | 1.19 (1.06, 1.34) | 0.003 | 1.28 (1.11, 1.48) | 0.001 |
| Q4 (high) | 1.30 (1.15, 1.47) | <0.001 | 1.47 (1.26, 1.72) | <0.001 |
| Dietary Pattern Score | |||||||
|---|---|---|---|---|---|---|---|
| Q1 (Ref) | Q2 (Mild) | Q3 (Moderate) | Q4 (High) | ||||
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | ||
| Males | |||||||
| Inflammatory markers | |||||||
| N/L ratio | 0 | 0.05 (0.01, 0.09) | 0.02 | 0.06 (0.02, 0.10) | 0.005 | 0.12 (0.08, 0.16) | <0.001 |
| CRP (nmol/L) | 0 | 1.75 (−0.70, 4.20) | 0.16 | 2.68 (0.25, 5.12) | 0.031 | 3.82 (1.38, 6.26) | 0.002 |
| Blood lipids | |||||||
| TG (mmol/L) | 0 | 0.03 (−0.02, 0.08) | 0.28 | 0.04 (−0.01, 0.09) | 0.13 | 0.09 (0.04, 0.14) | 0.001 |
| TC (mmol/L) | 0 | 0.05 (0.01, 0.09) | 0.013 | 0.10 (0.06, 0.14) | <0.001 | 0.12 (0.08, 0.16) | <0.001 |
| HDL-C (mmol/L) | 0 | −0.00 (−0.02, 0.01) | 0.72 | 0.00 (−0.01, 0.01) | 0.92 | −0.01 (−0.02, 0.00) | 0.13 |
| LDL-C (mmol/L) | 0 | 0.04 (−0.00, 0.07) | 0.07 | 0.08 (0.04, 0.12) | <0.001 | 0.09 (0.05, 0.12) | <0.001 |
| TC/HDL-C ratio | 0 | 0.04 (−0.01, 0.08) | 0.09 | 0.06 (0.02, 0.11) | 0.002 | 0.11 (0.07, 0.15) | <0.001 |
| Anemic biomarkers b | |||||||
| RBC (×106/µL) | 0 | 0.01 (−0.02, 0.03) | 0.53 | 0.01 (−0.01, 0.04) | 0.23 | 0.01 (−0.01, 0.03) | 0.55 |
| Hemoglobin (g/dL) | 0 | −0.02 (−0.07, 0.03) | 0.41 | −0.03 (−0.08, 0.02) | 0.27 | −0.06 (−0.11, −0.01) | 0.024 |
| Hematocrit (%) | 0 | −0.05 (−0.19, 0.10) | 0.50 | −0.05 (−0.19, 0.10) | 0.50 | −0.12 (−0.26, 0.02) | 0.11 |
| MCV (fL) | 0 | −0.22 (−0.51, 0.07) | 0.14 | −0.31 (−0.60, −0.02) | 0.034 | −0.35 (−0.63, −0.06) | 0.017 |
| MCH (pg) | 0 | −0.08 (−0.20, 0.03) | 0.14 | −0.13 (−0.24, −0.01) | 0.028 | −0.15 (−0.26, −0.04) | <0.001 |
| MCHC (g/dL) | 0 | −0.01 (−0.04, 0.02) | 0.54 | −0.02 (−0.06, 0.01) | 0.16 | −0.04 (−0.07, −0.01) | 0.024 |
| RDW (%) | 0 | 0.03 (−0.02, 0.08) | 0.31 | 0.08 (0.03, 0.13) | 0.003 | 0.08 (0.03, 0.13) | 0.003 |
| Iron (µg/dL) | 0 | −2.22 (−4.07, −0.36) | 0.019 | −1.71 (−3.56, 0.14) | 0.07 | −2.40 (−4.25, −0.54) | 0.011 |
| Kidney function biomarkers | |||||||
| BUN (mmol/L) | 0 | 0.11 (0.04, 0.17) | 0.001 | 0.09 (0.03, 0.15) | 0.004 | 0.09 (0.03, 0.15) | 0.004 |
| CRE (µmol/L) | 0 | 1.12 (0.22, 2.03) | 0.015 | 1.41 (0.51, 2.30) | 0.002 | 1.51 (0.62, 2.40) | 0.001 |
| eGFR (mL/min/1.73 m2) | 0 | −0.52 (−0.94, −0.10) | 0.015 | −0.62 (−1.03, −0.20) | 0.004 | −0.85 (−1.26, −0.43) | <0.001 |
| Females | |||||||
| Inflammatory markers | |||||||
| N/L ratio | 0 | 0.02 (−0.02, 0.06) | 0.36 | 0.07 (0.03, 0.11) | 0.001 | 0.13 (0.09, 0.17) | <0.001 |
| CRP (nmol/L) | 0 | 0.63 (−1.45, 2.71) | 0.55 | 0.92 (−1.23, 3.08) | 0.40 | 2.30 (−0.03, 4.62) | 0.053 |
| Blood lipids | |||||||
| TG (mmol/L) | 0 | 0.02 (−0.02, 0.05) | 0.31 | 0.03 (−0.00, 0.06) | 0.09 | 0.06 (0.02, 0.09) | 0.001 |
| TC (mmol/L) | 0 | 0.02 (−0.02, 0.07) | 0.21 | 0.06 (0.02, 0.11) | 0.002 | 0.08 (0.04, 0.13) | <0.001 |
| HDL-C (mmol/L) | 0 | −0.00 (−0.02, 0.02) | 0.80 | −0.00 (−0.02, 0.02) | 0.74 | 0.01 (−0.01, 0.03) | 0.44 |
| LDL-C (mmol/L) | 0 | 0.02 (−0.01, 0.06) | 0.19 | 0.06 (0.02, 0.09) | 0.003 | 0.05 (0.01, 0.09) | 0.019 |
| TC/HDL-C ratio | 0 | 0.02 (−0.02, 0.05) | 0.34 | 0.05 (0.02, 0.09) | 0.005 | 0.04 (−0.00, 0.08) | 0.06 |
| Anemic biomarkers b | |||||||
| RBC (×106/µL) | 0 | −0.01 (−0.03, 0.01) | 0.21 | −0.01 (−0.03, 0.01) | 0.19 | 0.02 (−0.00, 0.04) | 0.13 |
| Hemoglobin (g/dL) | 0 | −0.05 (−0.10, 0.00) | 0.07 | −0.09 (−0.14, −0.03) | 0.002 | −0.16 (−0.22, −0.10) | <0.001 |
| Hematocrit (%) | 0 | −0.15 (−0.29, −0.01) | 0.041 | −0.19 (−0.34, −0.04) | 0.012 | −0.38 (−0.54, −0.22) | <0.001 |
| MCV (fL) | 0 | −0.14 (−0.46, 0.17) | 0.37 | −0.22 (−0.55, 0.11) | 0.19 | −1.14 (−1.49, −0.79) | <0.001 |
| MCH (pg) | 0 | −0.04 (−0.17, 0.09) | 0.53 | −0.05 (−0.19, 0.08) | 0.41 | −0.44 (−0.58, −0.30) | <0.001 |
| MCHC (g/dL) | 0 | 0.01 (−0.03, 0.04) | 0.71 | 0.02 (−0.02, 0.06) | 0.28 | −0.08 (−0.12, −0.04) | <0.001 |
| RDW (%) | 0 | 0.05 (−0.02, 0.12) | 0.16 | 0.04 (−0.03, 0.11) | 0.24 | 0.15 (0.08, 0.23) | <0.001 |
| Iron (µg/dL) | 0 | 0.50 (−1.17, 2.14) | 0.58 | 0.95 (−0.77, 2.67) | 1.08 | −2.45 (−4.31, −0.60) | 0.01 |
| Kidney function biomarkers | |||||||
| BUN (mmol/L) | 0 | 0.04 (−0.02, 0.10) | 0.18 | 0.16 (0.09, 0.22) | <0.001 | 0.10 (0.03, 0.16) | 0.005 |
| CRE (µmol/L) | 0 | −0.12 (−0.91, 0.67) | 0.77 | 1.05 (0.23, 1.86) | 0.012 | 0.61 (−0.26, 1.48) | 0.17 |
| eGFR (mL/min/1.73 m2) | 0 | −0.03 (−0.44, 0.38) | 0.89 | −0.54 (−0.96, −0.11) | 0.013 | −0.53 (−0.98, −0.08) | 0.022 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurniawan, A.L.; Hsu, C.-Y.; Rau, H.-H.; Lin, L.-Y.; Chao, J.C.-J. Inflammatory Dietary Pattern Predicts Dyslipidemia and Anemia in Middle-Aged and Older Taiwanese Adults with Declined Kidney Function: A Cross-Sectional Population Study from 2008 to 2010. Nutrients 2019, 11, 2052. https://doi.org/10.3390/nu11092052
Kurniawan AL, Hsu C-Y, Rau H-H, Lin L-Y, Chao JC-J. Inflammatory Dietary Pattern Predicts Dyslipidemia and Anemia in Middle-Aged and Older Taiwanese Adults with Declined Kidney Function: A Cross-Sectional Population Study from 2008 to 2010. Nutrients. 2019; 11(9):2052. https://doi.org/10.3390/nu11092052
Chicago/Turabian StyleKurniawan, Adi Lukas, Chien-Yeh Hsu, Hsiao-Hsien Rau, Li-Yin Lin, and Jane C-J Chao. 2019. "Inflammatory Dietary Pattern Predicts Dyslipidemia and Anemia in Middle-Aged and Older Taiwanese Adults with Declined Kidney Function: A Cross-Sectional Population Study from 2008 to 2010" Nutrients 11, no. 9: 2052. https://doi.org/10.3390/nu11092052
APA StyleKurniawan, A. L., Hsu, C.-Y., Rau, H.-H., Lin, L.-Y., & Chao, J. C.-J. (2019). Inflammatory Dietary Pattern Predicts Dyslipidemia and Anemia in Middle-Aged and Older Taiwanese Adults with Declined Kidney Function: A Cross-Sectional Population Study from 2008 to 2010. Nutrients, 11(9), 2052. https://doi.org/10.3390/nu11092052







