The Metabolic Benefits of Menopausal Hormone Therapy Are Not Mediated by Improved Nutritional Habits. The OsteoLaus Cohort
Abstract
1. Introduction
2. Material and Methods
2.1. Setting
2.2. Participants
2.3. Dietary Data
2.4. Covariates
2.5. Statistical Analysis
3. Results
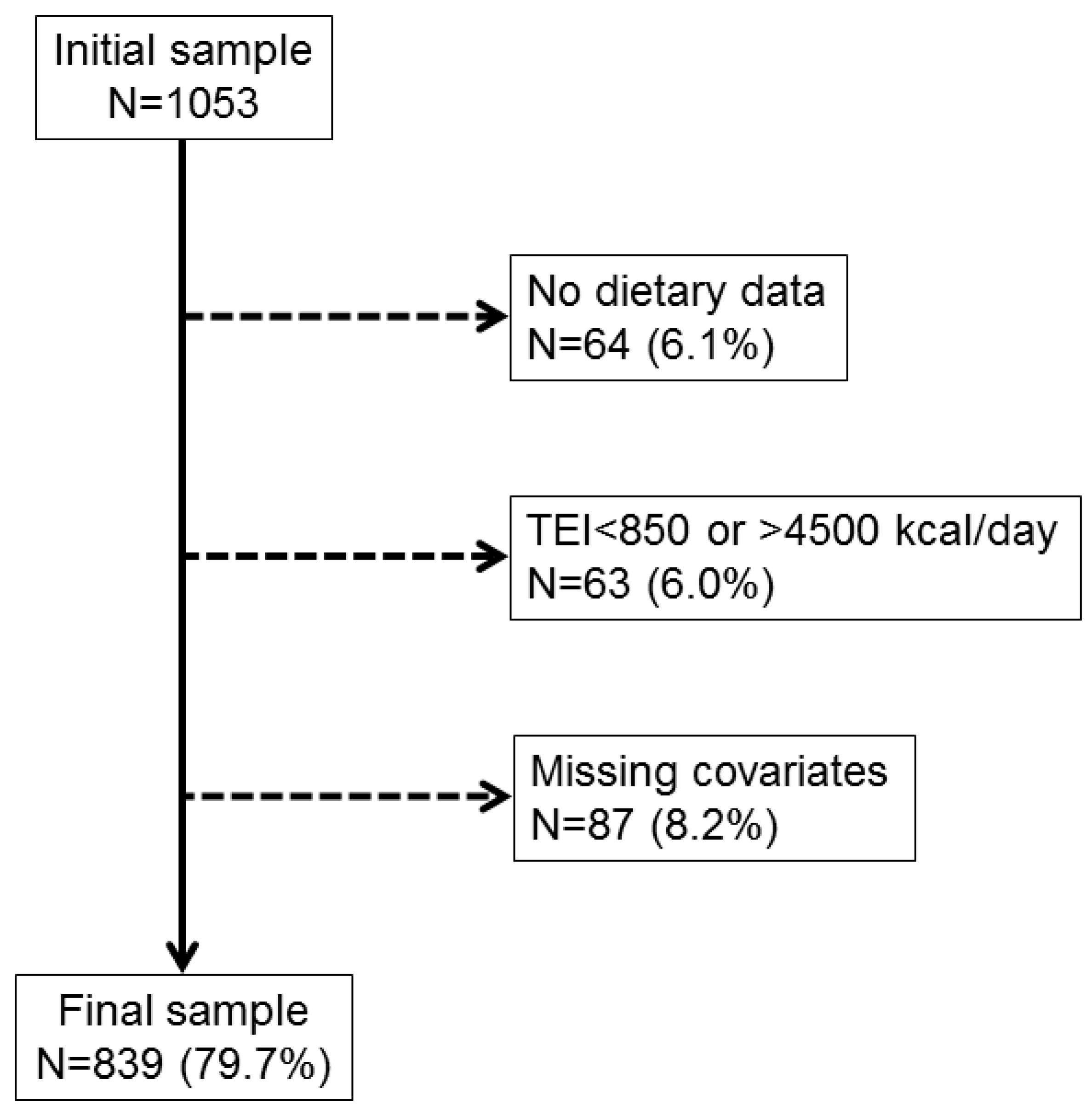
3.1. Selection of Participants and Characteristics of the Final Sample
3.2. Menopausal Hormone Therapy and Dietary Intake
3.3. Menopause Hormone Therapy and Dietary Patterns
3.4. Menopause Hormone Therapy and Dietary Scores
3.5. Menopause Hormone Therapy and Dietary Recommendations
4. Discussion
4.1. Menopause Hormone Therapy, Weight Change and Caloric Intake
4.2. Menopause Hormone Therapy and Diet Quality (Dietary Patterns, Scores, Recommendations)
4.3. Study Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burger, H.G.; Dudley, E.C.; Hopper, J.L.; Shelley, J.M.; Green, A.; Smith, A.; Dennerstein, L.; Morse, C. The endocrinology of the menopausal transition: A cross-sectional study of a population-based sample. J. Clin. Endocrinol. Metab. 1995, 80, 3537–3545. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.C.; Schleinitz, M.D.; Col, N.F. Revisiting the duration of vasomotor symptoms of menopause: A meta-analysis. J. Gen. Intern. Med. 2008, 23, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Sowers, M.; Han, W.; Huang, M.H.; Finkelstein, J.S.; Crandall, C.J.; Lee, J.S.; Karlamangla, A.S. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: Results from the Study of Women’s Health Across the Nation (SWAN). J. Bone Miner. Res. 2012, 27, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hassager, C.; Ravn, P.; Wang, S.; Christiansen, C. Total and regional body-composition changes in early postmenopausal women: Age-related or menopause-related? Am. J. Clin. Nutr. 1994, 60, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Sternfeld, B.; Huang, M.; Han, W.; Karvonen-Gutierrez, C.; Ruppert, K.; Cauley, J.A.; Finkelstein, J.S.; Jiang, S.F.; Karlamangla, A.S. Changes in body composition and weight during the menopause transition. JCI Insight 2019, 4, 124865. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, G.E.; Hans, D.; Rodriguez, E.G.; Vollenweider, P.; Waeber, G.; Marques-Vidal, P.; Lamy, O. Menopausal Hormone Therapy Is Associated with Reduced Total and Visceral Adiposity: The OsteoLaus Cohort. J. Clin. Endocrinol. Metab. 2018, 103, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.B.; Kristensen, K.; Hermann, P.A.; Katzenellenbogen, J.A.; Richelsen, B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J. Clin. Endocrinol. Metab. 2004, 89, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Kew, K.A.; Ryan, T.E.; Pennington, E.R.; Lin, C.T.; Buddo, K.A.; Fix, A.M.; Smith, C.A.; Gilliam, L.A.; Karvinen, S.; et al. 17beta-Estradiol Directly Lowers Mitochondrial Membrane Microviscosity and Improves Bioenergetic Function in Skeletal Muscle. Cell Metab. 2018, 27, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Van Pelt, R.E.; Gavin, K.M.; Kohrt, W.M. Regulation of Body Composition and Bioenergetics by Estrogens. Endocrinol. Metab. Clin. N. Am. 2015, 44, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Leeners, B.; Geary, N.; Tobler, P.N.; Asarian, L. Ovarian hormones and obesity. Hum. Reprod. Update 2017, 23, 300–321. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Asarian, L.; Geary, N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1215–R1267. [Google Scholar] [CrossRef] [PubMed]
- Geiselman, P.J.; Martin, J.R.; Vanderweele, D.A.; Novin, D. Dietary self-selection in cycling and neonatally ovariectomized rats. Appetite 1981, 2, 87–101. [Google Scholar] [CrossRef]
- Sullivan, E.L.; Daniels, A.J.; Koegler, F.H.; Cameron, J.L. Evidence in female rhesus monkeys (Macaca mulatta) that nighttime caloric intake is not associated with weight gain. Obes. Res. 2005, 13, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nedungadi, T.P.; Zhu, L.; Sobhani, N.; Irani, B.G.; Davis, K.E.; Zhang, X.; Zou, F.; Gent, L.M.; Hahner, L.D.; et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011, 14, 453–465. [Google Scholar] [CrossRef]
- Firmann, M.; Mayor, V.; Vidal, P.M.; Bochud, M.; Pecoud, A.; Hayoz, D.; Paccaud, F.; Preisig, M.; Song, K.S.; Yuan, X.; et al. The CoLaus study: A population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc. Disord. 2008, 8, 6. [Google Scholar] [CrossRef]
- Shevroja, E.; Marques-Vidal, P.; Aubry-Rozier, B.; Hans, G.; Rivadeneira, F.; Lamy, O.; Hans, D. Cohort profile: The OsteoLaus study. Int. J. Epidemiol. 2018. [Google Scholar] [CrossRef]
- Papadakis, G.E.; Lamy, O. Effects of menopausal hormone therapy on bone and body composition. Osteologie 2019, 28, 122–130. [Google Scholar] [CrossRef]
- Bernstein, M.; Morabia, A.; Costanza, M.C.; Landis, J.R.; Ross, A.; Flandre, P.; Luong, B.L.; Kumanyika, S.; Sorenson, A.; Localio, R. Nutritional balance of the diet of the adult residents of Geneva. Soz. Praventivmed. 1994, 39, 333–344. [Google Scholar] [CrossRef]
- Marques-Vidal, P.; Gaspoz, J.M.; Theler, J.M.; Guessous, I. Twenty-year trends in dietary patterns in French-speaking Switzerland: Toward healthier eating. Am. J. Clin. Nutr. 2017, 106, 217–224. [Google Scholar] [CrossRef]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Fahimi, S.; Lim, S.; Andrews, K.G.; Engell, R.E.; Powles, J.; Ezzati, M.; Mozaffarian, D.; et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. BMJ 2014, 348, g2272. [Google Scholar] [CrossRef] [PubMed]
- Marques-Vidal, P.; Waeber, G.; Vollenweider, P.; Guessous, I. Socio-demographic and lifestyle determinants of dietary patterns in French-speaking Switzerland, 2009-2012. BMC Public Health 2018, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Vormund, K.; Braun, J.; Rohrmann, S.; Bopp, M.; Ballmer, P.; Faeh, D. Mediterranean diet and mortality in Switzerland: An alpine paradox? Eur. J. Nutr. 2015, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Guenther, P.M.; Kirkpatrick, S.I.; Reedy, J.; Krebs-Smith, S.M.; Buckman, D.W.; Dodd, K.W.; Casavale, K.O.; Carroll, R.J. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J. Nutr. 2014, 144, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Marques-Vidal, P.; Waeber, G.; Vollenweider, P.; Bochud, M.; Stringhini, S.; Guessous, I. Sociodemographic and Behavioural Determinants of a Healthy Diet in Switzerland. Ann. Nutr. Metab. 2015, 67, 87–95. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, D.; Guessous, I.; Vaucher, J.; Preisig, M.; Waeber, G.; Vollenweider, P.; Marques-Vidal, P. Low compliance with dietary recommendations for food intake among adults. Clin. Nutr. 2013, 32, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.; Sloutskis, D.; Kumanyika, S.; Sparti, A.; Schutz, Y.; Morabia, A. Data-based approach for developing a physical activity frequency questionnaire. Am. J. Epidemiol. 1998, 147, 147–154. [Google Scholar] [CrossRef]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. Lond 2008, 32, 949–958. [Google Scholar] [CrossRef]
- Macdonald, H.M.; New, S.A.; Campbell, M.K.; Reid, D.M. Longitudinal changes in weight in perimenopausal and early postmenopausal women: Effects of dietary energy intake, energy expenditure, dietary calcium intake and hormone replacement therapy. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 669–676. [Google Scholar] [CrossRef]
- Sites, C.K.; L’Hommedieu, G.D.; Toth, M.J.; Brochu, M.; Cooper, B.C.; Fairhurst, P.A. The effect of hormone replacement therapy on body composition, body fat distribution, and insulin sensitivity in menopausal women: A randomized, double-blind, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2005, 90, 2701–2707. [Google Scholar] [CrossRef] [PubMed]
- Reubinoff, B.E.; Wurtman, J.; Rojansky, N.; Adler, D.; Stein, P.; Schenker, J.G.; Brzezinski, A. Effects of hormone replacement therapy on weight, body composition, fat distribution, and food intake in early postmenopausal women: A prospective study. Fertil. Steril. 1995, 64, 963–968. [Google Scholar] [CrossRef]
- Reimer, R.A.; Debert, C.T.; House, J.L.; Poulin, M.J. Dietary and metabolic differences in pre- versus postmenopausal women taking or not taking hormone replacement therapy. Physiol. Behav. 2005, 84, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Vercambre, M.N.; Fournier, A.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Ringa, V.; Berr, C. Differential dietary nutrient intake according to hormone replacement therapy use: An underestimated confounding factor in epidemiologic studies? Am. J. Epidemiol. 2007, 166, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Klump, K.L.; Racine, S.E.; Hildebrandt, B.; Burt, S.A.; Neale, M.; Sisk, C.L.; Boker, S.; Keel, P.K. Ovarian Hormone Influences on Dysregulated Eating: A Comparison of Associations in Women with versus without Binge Episodes. Clin. Psychol. Sci. 2014, 2, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Arabshahi, S.; Lahmann, P.H.; Williams, G.M.; Marks, G.C.; van der Pols, J.C. Longitudinal change in diet quality in Australian adults varies by demographic, socio-economic, and lifestyle characteristics. J. Nutr. 2011, 141, 1871–1879. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Jinlin, F.; Binyou, W.; Terry, C. A new approach to the study of diet and risk of type 2 diabetes. J. Postgrad. Med. 2007, 53, 139–143. [Google Scholar]
- Richard, A.; Rohrmann, S.; Vandeleur, C.L.; Lasserre, A.M.; Strippoli, M.F.; Eichholzer, M.; Glaus, J.; Marques-Vidal, P.; Vollenweider, P.; Preisig, M. Adherence to dietary recommendations is not associated with depression in two Swiss population-based samples. Psychiatry Res. 2017, 252, 310–318. [Google Scholar] [CrossRef]
- Aguilar-Salinas, C.A.; Garcia-Garcia, E.; Gomez Perez, F.J.; Rull, J.A. The healthy women bias and hormone replacement therapy in women with type 2 diabetes. Diabetes Care 2002, 25, 246–247. [Google Scholar] [CrossRef][Green Version]
| Characteristic | Never | Current | Past | p-Value |
|---|---|---|---|---|
| Number | 432 | 180 | 227 | |
| Age (years) | 60.8 ± 7.5 | 62.5 ± 6.8 | 66.9 ± 6.2 | <0.001 |
| Educational level (%) | 0.177 | |||
| University | 71 (16.4) | 34 (18.9) | 36 (15.9) | |
| High school | 115 (26.6) | 47 (26.1) | 58 (25.6) | |
| Apprenticeship | 174 (40.3) | 77 (42.8) | 81 (35.7) | |
| Mandatory | 72 (16.7) | 22 (12.2) | 52 (22.9) | |
| Smoking categories (%) | 0.413 | |||
| Never | 178 (41.2) | 81 (45.0) | 108 (47.6) | |
| Former | 167 (38.7) | 69 (38.3) | 85 (37.4) | |
| Current | 87 (20.1) | 30 (16.7) | 34 (15.0) | |
| Body mass index (kg/m2) | 25.6 ± 4.1 | 24.7 ± 3.9 | 25.4 ± 4.1 | 0.032 |
| BMI categories (%) | 0.047 | |||
| Normal | 207 (47.9) | 101 (56.1) | 106 (46.7) | |
| Overweight | 151 (35.0) | 63 (35.0) | 91 (40.1) | |
| Obese | 74 (17.1) | 16 (8.9) | 30 (13.2) | |
| Sedentary status (%) | 279 (64.6) | 109 (60.6) | 155 (68.3) | 0.268 |
| Diabetes (%) | 24 (5.6) | 5 (2.8) | 11 (4.9) | 0.334 |
| Bivariate Analysis | Multivariable Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Never | Current | Past | p-Value | Never | Current | Past | p-Value | |
| Number | 432 | 180 | 227 | 432 | 180 | 227 | ||
| Total energy intake (kcal) | 1673 ± 546 | 1699 ± 565 | 1766 ± 578 | 0.121 | - | - | - | - |
| Macronutrients (g/day) | ||||||||
| Total protein | 64.0 ± 23.2 | 64.0 ± 23.0 | 64.9 ± 24.5 | 0.969 | 64.8 ± 0.6 | 64.2 ± 1.0 | 63.2 ± 0.9 | 0.358 |
| Vegetal protein | 20.4 ± 9.4 | 19.8 ± 8.5 | 21.5 ± 8.7 | 0.039 | 20.7 ± 0.3 | 19.9 ± 0.4 | 20.8 ± 0.4 | 0.201 |
| Animal protein | 43.6 ± 19.2 | 44.2 ± 19.5 | 43.3 ± 20 | 0.847 | 44.1 ± 0.7 | 44.3 ± 1.1 | 42.4 ± 1.1 | 0.356 |
| Carbohydrates | 200.7 ± 81.4 | 200.7 ± 83.1 | 212.2 ± 83.2 | 0.154 | 204.8 ± 2.0 | 201.7 ± 3.0 | 204.0 ± 2.8 | 0.699 |
| Disaccharides | 104.1 ± 48.3 | 108.2 ± 58.4 | 113.2 ± 56.5 | 0.232 | 106.2 ± 1.9 | 108.3 ± 2.9 | 109.0 ± 2.7 | 0.663 |
| Polysaccharides | 96.2 ± 52.8 | 92.1 ± 48.0 | 98.6 ± 46.8 | 0.254 | 98.1 ± 1.7 | 92.9 ± 2.6 | 94.5 ± 2.5 | 0.206 |
| Total fat | 63.7 ± 23.6 | 64.9 ± 24.5 | 66.4 ± 26.6 | 0.534 | 64.9 ± 0.7 | 65.0 ± 1.1 | 64.1 ± 1.0 | 0.778 |
| SFA | 23.2 ± 9.7 | 23.9 ± 11.0 | 23.7 ± 10.4 | 0.783 | 23.7 ± 0.3 | 23.9 ± 0.5 | 22.7 ± 0.5 | 0.152 |
| MUFA | 25.8 ± 10.3 | 26.4 ± 10.2 | 27.1 ± 12.3 | 0.581 | 26.2 ± 0.4 | 26.4 ± 0.6 | 26.3 ± 0.5 | 0.954 |
| PUFA | 8.9 ± 4.3 | 8.9 ± 3.6 | 9.4 ± 4.3 | 0.156 | 9.1 ± 0.2 | 8.9 ± 0.2 | 9.0 ± 0.2 | 0.875 |
| Fiber | 16.7 ± 8.7 | 16.6 ± 8.5 | 18.5 ± 9.5 | 0.016 | 17.0 ± 0.3 | 16.6 ± 0.5 | 17.9 ± 0.5 | 0.137 |
| Micronutrients | ||||||||
| Cholesterol (mg/day) | 275 ± 120 | 276 ± 133 | 271 ± 130 | 0.756 | 278 ± 5 | 277 ± 7 | 264 ± 7 | 0.231 |
| Calcium (mg/day) | 999 ± 490 | 1040 ± 541 | 1021 ± 503 | 0.891 | 1020 ± 20 | 1039 ± 30 | 986 ± 28 | 0.425 |
| Iron (mg/day) | 9.8 ± 3.4 | 9.8 ± 3.4 | 10.6 ± 3.6 | 0.033 | 9.9 ± 0.1 | 9.9 ± 0.1 | 10.3 ± 0.1 | 0.032 |
| Vitamin D (μg/day) | 2.5 ± 1.8 | 2.5 ± 1.8 | 2.5 ± 2.2 | 0.924 | 2.5 ± 0.1 | 2.5 ± 0.1 | 2.4 ± 0.1 | 0.901 |
| Specific foods (g/day) | ||||||||
| Dairy | 211 ± 190 | 228 ± 195 | 211 ± 164 | 0.725 | 217 ± 8 | 227 ± 13 | 199 ± 12 | 0.281 |
| Red meat | 38 ± 34 | 37 ± 34 | 39 ± 44 | 0.933 | 38 ± 2 | 37 ± 3 | 39 ± 2 | 0.911 |
| Processed meats | 10 ± 13 | 10 ± 13 | 9 ± 12 | 0.302 | 11 ± 1 | 10 ± 1 | 8 ± 1 | 0.119 |
| Wholegrain | 51 ± 56 | 50 ± 54 | 55 ± 53 | 0.133 | 53 ± 3 | 50 ± 4 | 54 ± 4 | 0.733 |
| Fruits a | 284 ± 245 | 298 ± 267 | 345 ± 303 | 0.040 | 289 ± 12 | 301 ± 18 | 333 ± 17 | 0.119 |
| Fruits b | 320 ± 262 | 335 ± 277 | 391 ± 317 | 0.016 | 325 ± 13 | 338 ± 19 | 378 ± 18 | 0.058 |
| Fruits c | 377 ± 289 | 396 ± 307 | 453 ± 339 | 0.012 | 382 ± 14 | 398 ± 21 | 442 ± 19 | 0.049 |
| Vegetables | 171 ± 110 | 171 ± 110 | 196 ± 148 | 0.043 | 171 ± 6 | 171 ± 8 | 194 ± 8 | 0.056 |
| Fish d | 30 ± 26 | 28 ± 22 | 31 ± 29 | 0.850 | 30 ± 1 | 28 ± 2 | 31 ± 2 | 0.569 |
| Fish e | 36 ± 28 | 34 ± 24 | 37 ± 33 | 0.578 | 36 ± 1 | 34 ± 2 | 37 ± 2 | 0.521 |
| Dietary scores | ||||||||
| Mediterranean f | 3.93 ± 1.48 | 4.04 ± 1.49 | 4.08 ± 1.45 | 0.476 | 3.94 ± 0.07 | 4.04 ± 0.11 | 4.06 ± 0.1 | 0.602 |
| Mediterranean g | 4.61 ± 1.86 | 4.84 ± 1.93 | 5.03 ± 1.87 | 0.038 | 4.63 ± 0.08 | 4.84 ± 0.13 | 5.00 ± 0.12 | 0.036 |
| AHEI | 33.3 ± 10.3 | 34.8 ± 9.9 | 35.0 ± 9.7 | 0.101 | 33.4 ± 0.5 | 34.7 ± 0.7 | 35.0 ± 0.7 | 0.104 |
| Dietary patterns score | ||||||||
| Meat and chips | −0.29 ± 1.22 | −0.45 ± 0.95 | −0.40 ± 1.00 | 0.378 | −0.29 ± 0.05 | −0.42 ± 0.08 | −0.42 ± 0.07 | 0.212 |
| Fruits and vegetables | 0.38 ± 1.55 | 0.50 ± 1.48 | 0.77 ± 1.71 | 0.017 | 0.43 ± 0.07 | 0.50 ± 0.10 | 0.68 ± 0.10 | 0.129 |
| Fat and sugar | 0.01 ± 1.44 | −0.05 ± 1.33 | 0.09 ± 1.38 | 0.484 | 0.10 ± 0.05 | −0.04 ± 0.08 | −0.07 ± 0.07 | 0.146 |
| Bivariate Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Never | Current | Past | p-Value | Never | Current | Past | |
| Guidelines | |||||||
| Fruits ≥ 2/day | 209 (48.4) | 102 (56.7) | 136 (59.9) | 0.011 | 1 (ref.) | 1.36 (0.93–1.97) | 1.35 (0.93–1.94) |
| Vegetables ≥ 3/day | 39 (9.0) | 13 (7.2) | 24 (10.6) | 0.504 | 1 (ref.) | 0.74 (0.37–1.49) | 1.06 (0.57–1.98) |
| Meat ≤ 5/week | 296 (68.5) | 128 (71.1) | 155 (68.3) | 0.788 | 1 (ref.) | 1.06 (0.71–1.59) | 0.92 (0.62–1.37) |
| Fish ≥ 1/week a | 293 (67.8) | 120 (66.7) | 161 (70.9) | 0.611 | 1 (ref.) | 1.02 (0.70–1.50) | 1.28 (0.88–1.88) |
| Fish ≥ 1/week b | 196 (45.4) | 80 (44.4) | 101 (44.5) | 0.966 | 1 (ref.) | 0.99 (0.69–1.43) | 1.04 (0.73–1.49) |
| Dairy ≥ 3/day | 40 (9.3) | 21 (11.7) | 22 (9.7) | 0.657 | 1 (ref.) | 1.14 (0.63–2.06) | 0.81 (0.44–1.49) |
| At least three guidelines a | 129 (29.9) | 64 (35.6) | 92 (40.5) | 0.020 | 1 (ref.) | 1.24 (0.84–1.82) | 1.40 (0.96–2.03) |
| At least three guidelines b | 97 (22.5) | 48 (26.7) | 69 (30.4) | 0.078 | 1 (ref.) | 1.20 (0.79–1.84) | 1.38 (0.92–2.07) |
| Dietary scores | |||||||
| Mediterranean c | 59 (13.7) | 29 (16.1) | 38 (16.7) | 0.516 | 1 (ref.) | 1.20 (0.73–1.96) | 1.38 (0.84–2.25) |
| Mediterranean d | 76 (17.6) | 38 (21.1) | 51 (22.5) | 0.281 | 1 (ref.) | 1.29 (0.80–2.08) | 1.42 (0.89–2.26) |
| AHEI | 88 (20.7) | 47 (26.1) | 59 (26.6) | 0.157 | 1 (ref.) | 1.32 (0.85–2.03) | 1.45 (0.94–2.24) |
| Dietary patterns | |||||||
| Meat and chips | 115 (27.3) | 37 (21.1) | 52 (23.6) | 0.245 | 1 (ref.) | 0.75 (0.47–1.18) | 0.81 (0.52–1.27) |
| Fruits and vegetables | 93 (22.1) | 43 (24.6) | 68 (30.9) | 0.049 | 1 (ref.) | 0.99 (0.61–1.60) | 1.23 (0.79–1.92) |
| Fat and sugar | 115 (27.3) | 36 (20.6) | 53 (24.1) | 0.209 | 1 (ref.) | 0.48 (0.28–0.82) | 0.47 (0.28–0.78) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadakis, G.E.; Hans, D.; Gonzalez Rodriguez, E.; Vollenweider, P.; Waeber, G.; Marques-Vidal, P.; Lamy, O. The Metabolic Benefits of Menopausal Hormone Therapy Are Not Mediated by Improved Nutritional Habits. The OsteoLaus Cohort. Nutrients 2019, 11, 1930. https://doi.org/10.3390/nu11081930
Papadakis GE, Hans D, Gonzalez Rodriguez E, Vollenweider P, Waeber G, Marques-Vidal P, Lamy O. The Metabolic Benefits of Menopausal Hormone Therapy Are Not Mediated by Improved Nutritional Habits. The OsteoLaus Cohort. Nutrients. 2019; 11(8):1930. https://doi.org/10.3390/nu11081930
Chicago/Turabian StylePapadakis, Georgios E., Didier Hans, Elena Gonzalez Rodriguez, Peter Vollenweider, Gerard Waeber, Pedro Marques-Vidal, and Olivier Lamy. 2019. "The Metabolic Benefits of Menopausal Hormone Therapy Are Not Mediated by Improved Nutritional Habits. The OsteoLaus Cohort" Nutrients 11, no. 8: 1930. https://doi.org/10.3390/nu11081930
APA StylePapadakis, G. E., Hans, D., Gonzalez Rodriguez, E., Vollenweider, P., Waeber, G., Marques-Vidal, P., & Lamy, O. (2019). The Metabolic Benefits of Menopausal Hormone Therapy Are Not Mediated by Improved Nutritional Habits. The OsteoLaus Cohort. Nutrients, 11(8), 1930. https://doi.org/10.3390/nu11081930





