Metabolic Endotoxemia: A Potential Underlying Mechanism of the Relationship between Dietary Fat Intake and Risk for Cognitive Impairments in Humans?
Abstract
1. Introduction
2. Methods
3. Results
3.1. Dietary Fat and Endotoxemia
3.1.1. Role of Dietary Fat Interventions on Postprandial Metabolic Endotoxemia
Dietary Fats
Amount of Fats
Quality of Fats
Structure of Fats
Supplementation with Healthy Food Components
Metabolic Endotoxemia among Different Disease Phenotypes
3.1.2. Associations between Long-Term Dietary Interventions and Circulating LPS
3.1.3. Associations between Lifestyle Dietary Patterns and Circulating LPS (Table 2)
3.2. LPS, Cognitive Disorders and Dementia
3.2.1. LPS Injection and Short-Term Cognitive Function Assessment in Interventional Studies
3.2.2. Endotoxemia and HIV-Associated Neurocognitive Disorders in Observational Studies
3.2.3. Endotoxemia and Alzheimer’s Disease or Dementia Diagnosis in Observational Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013, 9, 63–75.e2. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Perier, M.-C.; Gaye, B.; Proust-Lima, C.; Helmer, C.; Dartigues, J.-F.; Berr, C.; Tzourio, C.; Empana, J.-P. Association of Cardiovascular Health Level in Older Age with Cognitive Decline and Incident Dementia. JAMA 2018, 320, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Canevelli, M.; Lucchini, F.; Quarata, F.; Bruno, G.; Cesari, M. Nutrition and Dementia: Evidence for Preventive Approaches? Nutrients 2016, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Kalmijn, S.; Launer, L.J.; Ott, A.; Witteman, J.C.; Hofman, A.; Breteler, M.M. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann. Neurol. 1997, 42, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Lourida, I.; Soni, M.; Thompson-Coon, J.; Purandare, N.; Lang, I.A.; Ukoumunne, O.C.; Llewellyn, D.J. Mediterranean diet, cognitive function, and dementia: A systematic review. Epidemiology 2013, 24, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C. Dietary fat composition and dementia risk. Neurobiol. Aging 2014, 35 (Suppl. 2), S59–S64. [Google Scholar] [CrossRef] [PubMed]
- Melo, H.M.; Santos, L.E.; Ferreira, S.T. Diet-Derived Fatty Acids, Brain Inflammation, and Mental Health. Front Neurosci 2019, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, T. Diabetes mellitus and dementia. Curr. Diab. Rep. 2014, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- Dye, L.; Boyle, N.B.; Champ, C.; Lawton, C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 2017, 76, 443–454. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, H.I.; Cha, H.Y.; Yang, Y.J.; Kwon, S.H.; Yang, S.J. Diabetes and Alzheimer’s Disease: Mechanisms and Nutritional Aspects. Clin. Nutr. Res. 2018, 7, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Malcomson, F.C.; Mathers, J.C. Nutrition and Ageing. Subcell. Biochem. 2018, 90, 373–424. [Google Scholar] [PubMed]
- Gardener, S.L.; Rainey-Smith, S.R.; Martins, R.N. Diet and Inflammation in Alzheimer’s Disease and Related Chronic Diseases: A Review. J. Alzheimers Dis. 2016, 50, 301–334. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016, 74, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Catorce, M.N.; Gevorkian, G. LPS-induced Murine Neuroinflammation Model: Main Features and Suitability for Pre-clinical Assessment of Nutraceuticals. Curr. Neuropharmacol. 2016, 14, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.C. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 1977, 31, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Hailman, E.; Lichenstein, H.S.; Wurfel, M.M.; Miller, D.S.; Johnson, D.A.; Kelley, M.; Busse, L.A.; Zukowski, M.M.; Wright, S.D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J. Exp. Med. 1994, 179, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Stoll, L.L.; Denning, G.M.; Weintraub, N.L. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.I. Nutritional Management of Metabolic Endotoxemia: A Clinical Review. Altern. Ther. Health Med. 2017, 23, 42–54. [Google Scholar]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Laugerette, F.; Vors, C.; Géloën, A.; Chauvin, M.-A.; Soulage, C.; Lambert-Porcheron, S.; Peretti, N.; Alligier, M.; Burcelin, R.; Laville, M.; et al. Emulsified lipids increase endotoxemia: Possible role in early postprandial low-grade inflammation. J. Nutr. Biochem. 2011, 22, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, S.; Witta, J.; Zhong, J.; de Villiers, W.; Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009, 50, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Netto Candido, T.L.; Bressan, J.; Alfenas R de, C.G. Dysbiosis and metabolic endotoxemia induced by high-fat diet. Nutr. Hosp. 2018, 35, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Laugerette, F.; Vors, C.; Peretti, N.; Michalski, M.-C. Complex links between dietary lipids, endogenous endotoxins and metabolic inflammation. Biochimie 2011, 93, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, T.; Rose, J.L.; Stevens, R.L.; Hoyt, D.G. Sensitivity of mice to lipopolysaccharide is increased by a high saturated fat and cholesterol diet. J. Inflamm. 2007, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Eikelenboom, P.; van Exel, E.; Hoozemans, J.J.M.; Veerhuis, R.; Rozemuller, A.J.M.; van Gool, W.A. Neuroinflammation—An early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegener. Dis. 2010, 7, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Brugg, B.; Dubreuil, Y.L.; Huber, G.; Wollman, E.E.; Delhaye-Bouchaud, N.; Mariani, J. Inflammatory processes induce beta-amyloid precursor protein changes in mouse brain. Proc. Natl. Acad. Sci. USA 1995, 92, 3032–3035. [Google Scholar] [CrossRef] [PubMed]
- Medina-Fernández, F.J.; Luque, E.; Aguilar-Luque, M.; Agüera, E.; Feijóo, M.; García-Maceira, F.I.; Escribano, B.M.; Pascual-Leone, Á.; Drucker-Colín, R.; Túnez, I. Transcranial magnetic stimulation modifies astrocytosis, cell density and lipopolysaccharide levels in experimental autoimmune encephalomyelitis. Life Sci. 2017, 169, 20–26. [Google Scholar] [CrossRef]
- Miklossy, J.; Kis, A.; Radenovic, A.; Miller, L.; Forro, L.; Martins, R.; Reiss, K.; Darbinian, N.; Darekar, P.; Mihaly, L.; et al. Beta-amyloid deposition and Alzheimer’s type changes induced by Borrelia spirochetes. Neurobiol. Aging 2006, 27, 228–236. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflammation 2008, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Deopurkar, R.; Ghanim, H.; Friedman, J.; Abuaysheh, S.; Sia, C.L.; Mohanty, P.; Viswanathan, P.; Chaudhuri, A.; Dandona, P. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care 2010, 33, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Erridge, C.; Attina, T.; Spickett, C.M.; Webb, D.J. A high-fat meal induces low-grade endotoxemia: Evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 2007, 86, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Petry, N.; Walther, B.; Bütikofer, U.; Luginbühl, W.; Gille, D.; Chollet, M.; McTernan, P.G.; Gijs, M.A.M.; Vionnet, N.; et al. Inflammatory and metabolic responses to high-fat meals with and without dairy products in men. Br. J. Nutr. 2015, 113, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Schwander, F.; Kopf-Bolanz, K.A.; Buri, C.; Portmann, R.; Egger, L.; Chollet, M.; McTernan, P.G.; Piya, M.K.; Gijs, M.A.M.; Vionnet, N.; et al. A dose-response strategy reveals differences between normal-weight and obese men in their metabolic and inflammatory responses to a high-fat meal. J. Nutr. 2014, 144, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Vors, C.; Pineau, G.; Drai, J.; Meugnier, E.; Pesenti, S.; Laville, M.; Laugerette, F.; Malpuech-Brugère, C.; Vidal, H.; Michalski, M.-C. Postprandial Endotoxemia Linked with Chylomicrons and Lipopolysaccharides Handling in Obese Versus Lean Men: A Lipid Dose-Effect Trial. J. Clin. Endocrinol. Metab. 2015, 100, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Lyte, J.M.; Gabler, N.K.; Hollis, J.H. Postprandial serum endotoxin in healthy humans is modulated by dietary fat in a randomized, controlled, cross-over study. Lipids Health Dis. 2016, 15, 186. [Google Scholar] [CrossRef]
- Vors, C.; Drai, J.; Pineau, G.; Laville, M.; Vidal, H.; Laugerette, F.; Michalski, M.-C. Emulsifying dietary fat modulates postprandial endotoxemia associated with chylomicronemia in obese men: A pilot randomized crossover study. Lipids Health Dis. 2017, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.P.B.; Teixeira, T.F.S.; Alves, R.D.M.; Peluzio, M.C.G.; Costa, N.M.B.; Bressan, J.; Mattes, R.; Alfenas, R.C.G. Effect of a high-fat meal containing conventional or high-oleic peanuts on post-prandial lipopolysaccharide concentrations in overweight/obese men. J. Hum. Nutr. Diet 2016, 29, 95–104. [Google Scholar] [CrossRef]
- Ghanim, H.; Abuaysheh, S.; Sia, C.L.; Korzeniewski, K.; Chaudhuri, A.; Fernandez-Real, J.M.; Dandona, P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: Implications for insulin resistance. Diabetes Care 2009, 32, 2281–2287. [Google Scholar] [CrossRef]
- Ghanim, H.; Batra, M.; Abuaysheh, S.; Green, K.; Makdissi, A.; Kuhadiya, N.D.; Chaudhuri, A.; Dandona, P. Antiinflammatory and ROS Suppressive Effects of the Addition of Fiber to a High-Fat High-Calorie Meal. J. Clin. Endocrinol. Metab. 2017, 102, 858–869. [Google Scholar] [PubMed]
- Milan, A.M.; Pundir, S.; Pileggi, C.A.; Markworth, J.F.; Lewandowski, P.A.; Cameron-Smith, D. Comparisons of the Postprandial Inflammatory and Endotoxaemic Responses to Mixed Meals in Young and Older Individuals: A Randomised Trial. Nutrients 2017, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Harte, A.L.; Varma, M.C.; Tripathi, G.; McGee, K.C.; Al-Daghri, N.M.; Al-Attas, O.S.; Sabico, S.; O’Hare, J.P.; Ceriello, A.; Saravanan, P.; et al. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care 2012, 35, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Postigo, M.; Queipo-Ortuño, M.I.; Murri, M.; Boto-Ordoñez, M.; Perez-Martinez, P.; Andres-Lacueva, C.; Cardona, F.; Tinahones, F.J. Endotoxin increase after fat overload is related to postprandial hypertriglyceridemia in morbidly obese patients. J. Lipid Res. 2012, 53, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Al-Disi, D.A.; Al-Daghri, N.M.; Khan, N.; Alfadda, A.A.; Sallam, R.M.; Alsaif, M.; Sabico, S.; Tripathi, G.; McTernan, P.G. Postprandial Effect of a High-Fat Meal on Endotoxemia in Arab Women with and without Insulin-Resistance-Related Diseases. Nutrients 2015, 7, 6375–6389. [Google Scholar] [CrossRef] [PubMed]
- Laugerette, F.; Alligier, M.; Bastard, J.-P.; Drai, J.; Chanséaume, E.; Lambert-Porcheron, S.; Laville, M.; Morio, B.; Vidal, H.; Michalski, M.-C. Overfeeding increases postprandial endotoxemia in men: Inflammatory outcome may depend on LPS transporters LBP and sCD14. Mol. Nutr. Food Res. 2014, 58, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moreno, J.; Garcia-Carpintero, S.; Gomez-Delgado, F.; Jimenez-Lucena, R.; Vals-Delgado, C.; Alcala-Diaz, J.F.; Roncero-Ramos, I.; Rangel-Zuñiga, O.A.; Yubero-Serrano, E.M.; Malagon, M.M.; et al. Endotoxemia is modulated by quantity and quality of dietary fat in older adults. Exp. Gerontol. 2018, 109, 119–125. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, J.; García-Carpintero, S.; Jimenez-Lucena, R.; Haro, C.; Rangel-Zúñiga, O.A.; Blanco-Rojo, R.; Yubero-Serrano, E.M.; Tinahones, F.J.; Delgado-Lista, J.; Pérez-Martínez, P.; et al. Effect of Dietary Lipids on Endotoxemia Influences Postprandial Inflammatory Response. J. Agric. Food Chem. 2017, 65, 7756–7763. [Google Scholar] [CrossRef] [PubMed]
- Pendyala, S.; Walker, J.M.; Holt, P.R. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 2012, 142, 1100–1101.e2. [Google Scholar] [CrossRef] [PubMed]
- Breusing, N.; Lagerpusch, M.; Engstler, A.J.; Bergheim, I.; Mueller, M.J.; Bosy-Westphal, A. Influence of Energy Balance and Glycemic Index on Metabolic Endotoxemia in Healthy Men. J. Am. Coll. Nutr. 2017, 36, 72–79. [Google Scholar] [CrossRef]
- Amar, J.; Burcelin, R.; Ruidavets, J.B.; Cani, P.D.; Fauvel, J.; Alessi, M.C.; Chamontin, B.; Ferriéres, J. Energy intake is associated with endotoxemia in apparently healthy men. Am. J. Clin. Nutr. 2008, 87, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Kallio, K.A.E.; Hätönen, K.A.; Lehto, M.; Salomaa, V.; Männistö, S.; Pussinen, P.J. Endotoxemia, nutrition, and cardiometabolic disorders. Acta Diabetol. 2015, 52, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Röytiö, H.; Mokkala, K.; Vahlberg, T.; Laitinen, K. Dietary intake of fat and fibre according to reference values relates to higher gut microbiota richness in overweight pregnant women. Br. J. Nutr. 2017, 118, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Ahola, A.J.; Lassenius, M.I.; Forsblom, C.; Harjutsalo, V.; Lehto, M.; Groop, P.-H. Dietary patterns reflecting healthy food choices are associated with lower serum LPS activity. Sci. Rep. 2017, 7, 6511. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Carnevale, R.; Nocella, C.; Novo, M.; Santulli, M.; Cammisotto, V.; Menichelli, D.; Pignatelli, P.; Violi, F. Gut-Derived Serum Lipopolysaccharide is Associated with Enhanced Risk of Major Adverse Cardiovascular Events in Atrial Fibrillation: Effect of Adherence to Mediterranean Diet. J. Am. Heart Assoc. 2017, 6, e005784. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; Wilcockson, D.C.; Campion, S.; Lunnon, K.; Perry, V.H. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J. Neurosci. 2005, 25, 9275–9284. [Google Scholar] [CrossRef] [PubMed]
- Reichenberg, A.; Yirmiya, R.; Schuld, A.; Kraus, T.; Haack, M.; Morag, A.; Pollmächer, T. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry 2001, 58, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.; Reichenberg, A.; Perry, C.; Ginzberg, D.; Pollmächer, T.; Soreq, H.; Yirmiya, R. Endotoxin-induced changes in human working and declarative memory associate with cleavage of plasma “readthrough” acetylcholinesterase. J. Mol. Neurosci. 2003, 21, 199–212. [Google Scholar] [CrossRef]
- Krabbe, K.S.; Reichenberg, A.; Yirmiya, R.; Smed, A.; Pedersen, B.K.; Bruunsgaard, H. Low-dose endotoxemia and human neuropsychological functions. Brain Behav. Immun. 2005, 19, 453–460. [Google Scholar] [CrossRef]
- Van den Boogaard, M.; Ramakers, B.P.; van Alfen, N.; van der Werf, S.P.; Fick, W.F.; Hoedemaekers, C.W.; Verbeek, M.M.; Schoonhoven, L.; van der Hoeven, J.G.; Pickkers, P. Endotoxemia-induced inflammation and the effect on the human brain. Crit. Care 2010, 14, R81. [Google Scholar] [CrossRef]
- Grigoleit, J.-S.; Oberbeck, J.R.; Lichte, P.; Kobbe, P.; Wolf, O.T.; Montag, T.; del Rey, A.; Gizewski, E.R.; Engler, H.; Schedlowski, M. Lipopolysaccharide-induced experimental immune activation does not impair memory functions in humans. Neurobiol. Learn. Mem. 2010, 94, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Grigoleit, J.-S.; Kullmann, J.S.; Wolf, O.T.; Hammes, F.; Wegner, A.; Jablonowski, S.; Engler, H.; Gizewski, E.; Oberbeck, R.; Schedlowski, M. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS ONE 2011, 6, e28330. [Google Scholar] [CrossRef] [PubMed]
- Moieni, M.; Irwin, M.R.; Jevtic, I.; Breen, E.C.; Eisenberger, N.I. Inflammation impairs social cognitive processing: A randomized controlled trial of endotoxin. Brain Behav. Immun. 2015, 48, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, J.S.; Grigoleit, J.-S.; Wolf, O.T.; Engler, H.; Oberbeck, R.; Elsenbruch, S.; Forsting, M.; Schedlowski, M.; Gizewski, E.R. Experimental human endotoxemia enhances brain activity during social cognition. Soc. Cogn. Affect. Neurosci. 2014, 9, 786–793. [Google Scholar] [CrossRef]
- Vassallo, M.; Dunais, B.; Durant, J.; Carsenti-Dellamonica, H.; Harvey-Langton, A.; Cottalorda, J.; Ticchioni, M.; Laffon, M.; Lebrun-Frenay, C.; Dellamonica, P.; et al. Relevance of lipopolysaccharide levels in HIV-associated neurocognitive impairment: The Neuradapt study. J. Neurovirol. 2013, 19, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.L.; Uno, H.; Ancuta, P.; Kamat, A.; Moore, D.J.; Singer, E.J.; Morgello, S.; Gabuzda, D. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J. Acquir. Immune Defic. Syndr. 2011, 57, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, S.; Pedersen, K.K.; Anesten, B.; Zetterberg, H.; Fuchs, D.; Gisslén, M.; Hagberg, L.; Trøseid, M.; Nielsen, S.D. Soluble CD14 in cerebrospinal fluid is associated with markers of inflammation and axonal damage in untreated HIV-infected patients: A retrospective cross-sectional study. BMC Infect. Dis. 2016, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Stamova, B.; Jin, L.-W.; DeCarli, C.; Phinney, B.; Sharp, F.R. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 2016, 87, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jaber, V.; Lukiw, W.J. Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer’s Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Front. Cell. Infect. Microbiol. 2017, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Ancuta, P.; Kamat, A.; Kunstman, K.J.; Kim, E.-Y.; Autissier, P.; Wurcel, A.; Zaman, T.; Stone, D.; Mefford, M.; Morgello, S.; et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE 2008, 3, e2516. [Google Scholar] [CrossRef]
- Zhang, R.; Miller, R.G.; Gascon, R.; Champion, S.; Katz, J.; Lancero, M.; Narvaez, A.; Honrada, R.; Ruvalcaba, D.; McGrath, M.S. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J. Neuroimmunol. 2009, 206, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Cândido, F.G.; Valente, F.X.; Grześkowiak, Ł.M.; Moreira, A.P.B.; Rocha, D.M.U.P.; Alfenas R de, C.G. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: Mechanisms and clinical implications on obesity. Int. J. Food Sci. Nutr. 2018, 69, 125–143. [Google Scholar] [CrossRef]
- Graham, C.; Mullen, A.; Whelan, K. Obesity and the gastrointestinal microbiota: A review of associations and mechanisms. Nutr. Rev. 2015, 73, 376–385. [Google Scholar] [CrossRef]
- Arora, T.; Bäckhed, F. The gut microbiota and metabolic disease: Current understanding and future perspectives. J. Intern. Med. 2016, 280, 339–349. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Parlesak, A.; Schäfer, C.; Schütz, T.; Bode, J.C.; Bode, C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J. Hepatol. 2000, 32, 742–747. [Google Scholar] [CrossRef]
- La Rosa, F.; Clerici, M.; Ratto, D.; Occhinegro, A.; Licito, A.; Romeo, M.; Iorio, C.D.; Rossi, P. The Gut-Brain Axis in Alzheimer’s Disease and Omega-3. A Critical Overview of Clinical Trials. Nutrients 2018, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Freeswick, P.D.; Khemlani, L.S.; Kispert, P.H.; Wang, S.C.; Su, G.L.; Billiar, T.R. Role of lipopolysaccharide (LPS), interleukin-1, interleukin-6, tumor necrosis factor, and dexamethasone in regulation of LPS-binding protein expression in normal hepatocytes and hepatocytes from LPS-treated rats. Infect. Immun. 1995, 63, 2435–2442. [Google Scholar]
- Yao, Z.; Mates, J.M.; Cheplowitz, A.M.; Hammer, L.P.; Maiseyeu, A.; Phillips, G.S.; Wewers, M.D.; Rajaram, M.V.S.; Robinson, J.M.; Anderson, C.L.; et al. Blood-Borne Lipopolysaccharide Is Rapidly Eliminated by Liver Sinusoidal Endothelial Cells via High-Density Lipoprotein. J. Immunol. 2016, 197, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Bollen, J.; Trick, L.; Llewellyn, D.; Dickens, C. The effects of acute inflammation on cognitive functioning and emotional processing in humans: A systematic review of experimental studies. J. Psychosom. Res. 2017, 94, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Anderson, I.; Adinolfi, C.; Doctrow, S.; Huffman, K.; Joy, K.A.; Malfroy, B.; Soden, P.; Rupniak, H.T.; Barnes, J.C. Oxidative signalling and inflammatory pathways in Alzheimer’s disease. Biochem. Soc. Symp. 2001, 67, 141–149. [Google Scholar] [CrossRef]
- Russell, R.M. Changes in gastrointestinal function attributed to aging. Am. J. Clin. Nutr. 1992, 55, 1203S–1207S. [Google Scholar] [CrossRef]
- Osipova, E.D.; Komleva, Y.K.; Morgun, A.V.; Lopatina, O.L.; Panina, Y.A.; Olovyannikova, R.Y.; Vais, E.F.; Salmin, V.V.; Salmina, A.B. Designing in vitro Blood-Brain Barrier Models Reproducing Alterations in Brain Aging. Front. Aging Neurosci. 2018, 10, 234. [Google Scholar] [CrossRef] [PubMed]
| Clinical Trials | |||||
|---|---|---|---|---|---|
| Reference | Sample Characteristics | Endotoxemia Assessment | Nutritional Characteristics | Analysis Design | Results |
| Lyte JM. et al., Lipids Health Dis, 2016 [37] | 20 healthy subjects (mean age 25 y, 60% men) | Serum LPS quantified by LAL | Four meals (35% fat provided from n-3 PUFA (fish oil), 35% fat provided from n-6 PUFA (grapeseed oil), 35% fat provided from SFA (coconut oil) and 20% fat (olive oil, control diet)) | Randomized single-blind crossover study | 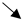 LPS until 5 h after n-3 PUFA meal compared to SFA meal LPS until 5 h after n-3 PUFA meal compared to SFA meal |
| Al-Disi DA. et al., Nutrients, 2015 [45] | 92 lean controls (mean age 24 y), 24 overweight or obese (mean age 32 y) and 50 T2DM (mean age 42 y) subjects, all women | Serum LPS quantified by LAL | High-fat meal (whipping cream with 75 g fat, 5 g carbohydrate and 6 g protein per m² body surface) | Randomized controlled trial |  LPS 3 h after high-fat meal in the lean (compared to baseline) and T2DM (compared to 1 h postprandial) groups LPS 3 h after high-fat meal in the lean (compared to baseline) and T2DM (compared to 1 h postprandial) groups |
| Vors C. et al., J Clin Endocrinol Metab, 2015 [36] | 8 normal-weight (mean age 29 y) and 8 obese (mean age 31 y) subjects, all men | Plasma LPS quantified by LAL | Mixed meals containing 10 or 40 g fat (69% SFA, 28% MUFA and 3% PUFA) | Randomized crossover study |  LPS only in obese subjects after 40 g fat compared to 10 g fat LPS only in obese subjects after 40 g fat compared to 10 g fat |
| Schwander F. et al., J Nutr, 2014 [35] | 19 normal-weight (mean age 41 y) and 18 obese (mean age 44 y) subjects, all men | Plasma LPS quantified by LAL | 500, 1000 and 1500 kcal of a high-fat meal (61% fat) | Randomized crossover study |  LPS with energy intake in both groups LPS with energy intake in both groups |
| No difference in the dose-response of postprandial LPS between normal-weight and obese | |||||
| Harte AL. et al., Diabetes Care, 2012 [43] | 9 non-obese (mean age 40 y, 50% men), 15 obese (mean age 44 y, 50% men), 12 impaired glucose tolerance (IGT, mean age 42 y, 58% men) and 18 T2DM (mean age 45 y, 61% men) subjects | Serum LPS quantified by LAL | High-fat meal (whipping cream with 75 g fat, 5 g carbohydrate and 6 g protein per m² body surface) | Controlled trial |  LPS after high-fat diet in all four groups compared to baseline LPS after high-fat diet in all four groups compared to baseline |
 LPS by 20% in obese and IGT and by 125% in T2DM subjects compared to non-obese participants LPS by 20% in obese and IGT and by 125% in T2DM subjects compared to non-obese participants | |||||
| Milan AM. et al., Nutrients, 2017 [42] | 15 young adults (mean age 23 y, 40% men) and 15 elderly subjects (mean age 67 y, 40% men) | Plasma LPS quantified by LAL | High-fat (32% fat) or low-fat (11.5% fat) meals | Randomized crossover study | No difference in LPS levels after high-fat diet compared to baseline |
| Schmid A. et al., Br J Nutr, 2015 [34] | 19 healthy subjects (mean age 42 y, all men) | Plasma LPS quantified by LAL | Three meals (High-fat dairy meal, High-fat non-dairy meal supplemented with milk or not, all 60% fat) | Randomized crossover study |  LPS after high-fat meal compared to baseline LPS after high-fat meal compared to baseline |
| No difference in postprandial LPS levels between the three meals | |||||
| Moreira AP. et al., J Hum Nutr Diet, 2016 [39] | 65 overweight and obese subjects (mean age 27 y, all men) | Plasma LPS quantified by LAL | Three meals (shake with conventional peanuts CVP, high-oleic peanuts HOP or control biscuit CT, all 49% fat) | Controlled trial |  LPS in the CT diet 3 h after ingestion compared to CVP and HOP meals LPS in the CT diet 3 h after ingestion compared to CVP and HOP meals |
| Clemente-Postigo M. et al., J Lipid Res, 2012 [44] | 10 subjects with HOMA-IR ≤ 5 and TG < 80 mg/dL (group 1, mean age 40 y), 10 subjects with HOMA-IR > 8 and TG < 80 mg/dL (group 2, mean age 39 y), 10 subjects with HOMA-IR ≤ 5 and TG > 80 mg/dL (group 3, mean age 43 y) and 10 subjects with HOMA-IR > 8 and TG > 80 mg/dL (group 4, mean age 42 y), all morbidly obese subjects | Serum LPS quantified by LAL | High-fat meal (50% fat) | Controlled trial |  LPS after high-fat meal in groups 3 and 4 (TG > 80 mg/dL) compared to baseline LPS after high-fat meal in groups 3 and 4 (TG > 80 mg/dL) compared to baseline |
| No difference in LPS levels after high-fat diet between groups | |||||
| Deopurkar R. et al., Diabetes Care, 2010 [32] | 48 healthy subjects (range age 25–47 y) | Plasma LPS quantified by LAL | 33 g dairy cream (70% SFA, no carbohydrate), 75 g glucose-based drink, orange juice or water | Controlled trial |  LPS after ingestion of dairy cream compared to baseline LPS after ingestion of dairy cream compared to baseline |
| No difference in LPS levels after ingestion of glucose-based drink, orange juice or water | |||||
| Ghanim H. et al., Diabetes Care, 2009 [40] | 20 healthy subjects (range age 20–50 y, 75% men) | Plasma LPS quantified by LAL | High-fat high cholesterol HFHC (42% fat) or American Heart Association (AHA)-recommended (27% fat)) meals | Controlled trial |  LPS after HFHC meal compared to baseline LPS after HFHC meal compared to baseline |
| No difference in LPS levels after AHA diet compared to baseline | |||||
| Ghanim H. et al., J Clin Endocrinol Metab, 2017 [41] | 10 healthy subjects (mean age 33 y, 60% men) | Plasma LPS quantified by LAL | High-fat high-carbohydrate HFHC meal (42% fat) with or without additional 30 g of fibre | Randomized crossover study |  LPS after HFHC meal LPS after HFHC meal |
| No difference in postprandial LPS levels with additional 30 g of fibre to the HFHC | |||||
| Erridge C. et al., Am J Clin Nutr, 2007 [33] | 12 healthy subjects (mean age 32 y), all men | Plasma LPS quantified by LAL | High-fat meal (toast with 50 g butter) | Randomized crossover study | LPS after high-fat meal |
| Laugerette F. et al., J Nutr Biochem, 2011 [21] | 12 healthy subjects (mean age 27 y, all men) | Plasma LPS quantified by LAL | Mixed meal (33% fat) | Controlled trial |  LPS after mixed meal compared to baseline LPS after mixed meal compared to baseline |
| Vors C. et al., Lipids Health Dis, 2017 [38] | 8 normal-weight (mean age 29 y) and 8 obese (mean age 31 y) subjects, all men | Plasma LPS quantified by LAL | Meals with emulsified or spread fat | Randomized crossover study |  LPS after emulsified fat meal in obese subjects compared to (i) spread fat and (ii) emulsified or spread fat in normal-weight subjects LPS after emulsified fat meal in obese subjects compared to (i) spread fat and (ii) emulsified or spread fat in normal-weight subjects |
| Clinical Trials | |||||
|---|---|---|---|---|---|
| Reference | Sample Characteristics at Baseline | Endotoxemia Assessment | Nutritional Assessment | Analysis Design | Results |
| Laugerette F. et al., Mol Nutr Food Res, 2014 [46] | 18 healthy subjects (mean age 31 y, all men) | Plasma LPS quantified by LAL | Overfeeding with +70 g of lipids to the usual daily diet with 46.3% from saturated fatty acids during 8 weeks | Controlled trial |  Postprandial levels of LPS compared to baseline Postprandial levels of LPS compared to baseline |
| No difference in fasting levels of LPS after overfeeding period compared to baseline | |||||
| Breusing N. et al., J Am Coll Nutr, 2017 [50] | 15 healthy subjects (age 20–29 y, all men) | Plasma LPS quantified by LAL | Overfeeding (+50% of the energy requirement) during 1 week, caloric restriction (−50% of the energy requirement, 3.5% fat) during 3 weeks and hyper-caloric refeeding (+50% of the energy requirement) with either low- or high-glycemic index diet during 2 weeks | Randomized crossover study |  Fasting levels of LPS after overfeeding period (+30.8% compared to baseline) Fasting levels of LPS after overfeeding period (+30.8% compared to baseline) |
| Normalization of fasting levels of LPS levels with the caloric restriction diet | |||||
 Fasting levels of LPS after hyper-caloric refeeding period (+24.7% compared to baseline) Fasting levels of LPS after hyper-caloric refeeding period (+24.7% compared to baseline) | |||||
| López-Moreno J. et al., J Agric Food Chem, 2017 [48] | 75 subjects with metabolic syndrome (mean age 56 y) | Plasma LPS quantified by LAL | Four diets (High-saturated-fatty acids diet (HSFA, 38% fat with 16% SFA, 12% MUFA and 6% PUFA), High MUFA (HMUFA, 38% fat with 8% SFA, 20% MUFA and 6% PUFA), Low-fat high complex carbohydrate (LFHCC, 28% fat) and LFHCC n-3 supplemented with n-3 PUFA) during 12 weeks | Randomized controlled trial |  Postprandial levels of LPS after HSFA diet compared to baseline Postprandial levels of LPS after HSFA diet compared to baseline |
| No difference in postprandial LPS levels after HMUFA, LFHCC and LFHCC n-3 diets | |||||
| No difference in fasting levels of LPS between all 4 groups of diet after intervention | |||||
| López-Moreno J. et al., Exp Gerontol, 2018 [47] | 20 healthy subjects (mean age 67 y, 50% men) | Plasma LPS quantified by LAL | Mediterranean diet enriched in MUFA with virgin olive oil (38% fat) or SFA-rich diet (38% fat) or low-fat high-carbohydrate diet enriched in n-3 PUFA (CHO-PUFA diet, 30% fat) during 3 weeks | Randomized crossover study |  Postprandial levels of LPS after CHO-PUFA Postprandial levels of LPS after CHO-PUFA |
| No difference in postprandial levels of LPS after Mediterranean diets enriched in MUFA or SFA | |||||
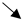 Fasting levels of LPS after CHO-PUFA diet compared to Mediterranean diets enriched in MUFA or SFA Fasting levels of LPS after CHO-PUFA diet compared to Mediterranean diets enriched in MUFA or SFA | |||||
| Pendyala S. et al., Gastroenterology, 2012 [49] | 8 healthy subjects (mean age 60 y, 38% men) hospitalized for the study | Plasma LPS quantified by a neutrophil priming method | Western-type diet (40% fat with 20.8% from saturated fat, 20% protein and 40% carbohydrates) or Prudent-type diet (20% with 5.8% from saturated fat, 20% protein and 60% carbohydrates) during 1 month | Randomized crossover study |  Fasting levels of LPS after Western-type diet (+71% compared to baseline) Fasting levels of LPS after Western-type diet (+71% compared to baseline) |
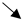 Fasting levels of LPS after prudent-type diet (−38% compared to baseline) Fasting levels of LPS after prudent-type diet (−38% compared to baseline) | |||||
| Observational studies | |||||
| Reference | Sample Characteristics at Baseline | Endotoxemia Assessment | Nutritional Assessment | Analysis Design | Results |
| Amar J. et al., Am J Clin Nutr, 2008 [51] | 130 subjects below the LPS detection threshold (mean age 55 y), 44 subjects between 9–39 U/mL (mean age 54 y) and 27 subjects under 39 U/mL (mean age 53 y), all healthy men | Plasma LPS quantified by Kinetic-QCL TM test | 3 days of food-record diary | Cross-sectional |  Fasting levels of LPS with fat and total energy intake Fasting levels of LPS with fat and total energy intake |
| Kallio KA. et al., Acta Diabetol, 2015 [52] | 2452 subjects (mean age 52 y) | Serum LPS quantified by LAL | 24 h dietary recall | Cross-sectional |  Fasting levels of LPS with total energy intake in lean healthy subjects Fasting levels of LPS with total energy intake in lean healthy subjects |
| No significant association between fasting levels of LPS and fat intake, and among subjects with obesity, metabolic syndrome, diabetes or coronary heart disease | |||||
| Röytiö H. et al., Br J Nutr, 2017 [53] | 88 overweight pregnant women (mean age 30 y) | Serum LPS quantified by LAL | Three groups based on 3 days of food-record diary (low fibre (<25 g/j) and moderate fat intake (25–40%) n = 57, high fibre (>=25 g/j) and moderate fat intake (25–40%) n=18 and low fibre (<25 g/j) and high fat intake (>=40%) n = 13) | Cross-sectional | No significant association between fasting levels of LPS and fat intake |
| No difference in fasting levels of LPS levels among the three diet groups | |||||
| Ahola AJ. Et al., Sci Rep, 2017 [54] | 668 patients with type 1 diabetes (mean age 45 y, 44% men) | Serum LPS quantified by LAL | Food frequency questionnaire and3 days of food-records diary | Cross-sectional | 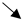 Fasting levels of LPS with higher adherence score of fish, healthy snack and modern diets Fasting levels of LPS with higher adherence score of fish, healthy snack and modern diets |
| No difference in fasting levels of LPS levels for sweet, cheese, vegetable or traditional diets | |||||
| No difference in fasting levels of LPS levels for energy, macronutrients and fibre intake | |||||
| Pastori D. et al., J Am Heart Assoc, 2017 [55] | 704 patients with nonvalvular atrial fibrillation treated by vitamin K antagonists (mean age 74 y, 57% men) | Serum LPS quantified by ELISA | Short food frequency questionnaire | Cross-sectional |  Fasting levels of LPS with higher adherence to a Mediterranean diet Fasting levels of LPS with higher adherence to a Mediterranean diet |
 Fasting levels of LPS with higher consumption of fruits and legumes Fasting levels of LPS with higher consumption of fruits and legumes | |||||
| No difference in fasting levels of LPS levels for the consumption of olive oil, vegetables, fish, wine, meat and bread | |||||
| Clinical Trials | |||||
|---|---|---|---|---|---|
| Reference | Sample Characteristics at Baseline | Endotoxemia Assessment | Outcomes | Study Design | Results |
| Krabbe KS. et al., Brain Behav Immun, 2005 [59] | 12 healthy subjects (mean age 26 y, all men) | Injection of LPS E. coli 0.2 ng/kg or placebo | Memory and learning (Word-list memory test) Working memory (Digit Span backward and Letter–Number Sequencing) Attention (Digit span forward and Digit symbol) Executive functions (Trail Making Test A and B) Assessment before and at 1.5, 6 and 24 h post-injection | Randomized double-blind crossover study | Negative correlation between IL-6 at 4.5 and 6 h post-injection and the word-list learning performance LPS injection did not affect performance on other cognitive tests |
| Kullmann JS et al., Soc Cogn Affect Neurosci, 2014 [64] | 18 healthy subjects (mean age 26 y, all men) | Injection of LPS E. coli 0.4 ng/kg or placebo | Emotional/social processing (Reading the mind in the eye) Brain activation during the test Assessment at 2 h post-injection | Randomized double-blind crossover study | LPS injection did not affect the number of correct responses during the Reading the mind in the eye test Responses in the fusiform gyrus, temporo-parietal junction, superior temporal gyrus and precuneus regions compared to placebo Responses in the fusiform gyrus, temporo-parietal junction, superior temporal gyrus and precuneus regions compared to placebo |
| Grigoleit JS. et al., Neurobiol Learn Mem, 2010 [61] | 24 healthy subjects (mean age 25 y, all men) | Injection of LPS E. coli 0.4 ng/kg or placebo | Attention and executive functions (Color word stroop task (assessment before and 1.5 h post-injection)) Verbal, visual and delayed memory (Revised Wechsler Memory Scale (assessment at 3 h post-injection)) | Randomized double-blind controlled trial | LPS injection did not affect performance on cognitive tests |
| Grigoleit JS. et al., PLoS One, 2011 [62] | 18 healthy subjects in the low-dose group (LPS 0.4 ng/kg) and 16 subjects in the high-dose group (LPS 0.8 ng/kg), mean age 25 y | Injection of LPS E. coli 0.4 ng/kg or placebo, or LPS E. coli 0.8 ng/kg or placebo | Working memory (N-back task) Assessment at 1.75 and 3 h post-injection | Randomized double-blind crossover study |  Reaction time in N-back task in the high-dose group compared to placebo Reaction time in N-back task in the high-dose group compared to placeboLPS did not affect the accuracy in N-back task |
| Moieni M et al., Brain Behav Immun, 2015 [63] | 109 healthy subjects (mean age 24 y, 40% men): 58 subjects in the LPS injection group and 51 in the placebo group | Injection of LPS E. coli 0.8 ng/kg or placebo | Emotional/social processing (Reading the mind in the eye) Assessment at 1 h 40 post-injection | Randomized double-blind controlled trial |  Number of correct responses in the LPS group Number of correct responses in the LPS group |
| Reichenberg A. et al., Arch Gen Psychiatry, 2001 [57] | 20 healthy subjects (mean age 24 y, all men) | Injection of LPS Salmonella abortus equi 0.8 ng/kg or placebo | Declarative memory (Story recall, Figure recall and Word-list learning) Attention (Digit span forward, Digit symbol, Ruff 2 and 7 cancellation test, Simple reaction time test and Continuous performance test) Executive functions (Colored Trail Making Test A and B and Word fluency test) Assessment at 1–2, 3–4 and 9–10 h post-injection | Randomized double-blind crossover study |  Performance on story recall, figure recall and word-list learning tests compared to placebo at 1–2, 3–4 and 9–10 h post-injection Performance on story recall, figure recall and word-list learning tests compared to placebo at 1–2, 3–4 and 9–10 h post-injectionLPS injection did not affect performance on other cognitive tests |
| Cohen O. et al., J Mol Neurosci, 2003 [58] | 10 healthy subjects (sub-sample of the Reichenberg’s study [57]) | Injection of LPS Salmonella abortus equi 0.8 ng/kg or placebo | Declarative memory (Story recall) Working memory (Digit span backward) Attention (Digit span forward and Ruff 2 and 7 cancellation test) Assessment at 1–2, 3–4 and 9–10 h post-injection | Randomized double-blind crossover study |  Performance on story recall compared to placebo at 1–2, 3–4 and 9–10 h post-injection Performance on story recall compared to placebo at 1–2, 3–4 and 9–10 h post-injection Performance digit span backward test compared to placebo at 1–2, 3–4 and 9–10 h post-injection Performance digit span backward test compared to placebo at 1–2, 3–4 and 9–10 h post-injectionLPS injection did not affect performance on other cognitive tests |
| Van den Boogaard M. et al., Crit Care, 2010 [60] | 15 healthy subjects in the injection group (mean age 23 y, all men) and 10 healthy controls (mean age 25 y, all men) | Injection of LPS E. coli 2 ng/kg or no injection | Working memory (Digit span backward) Attention (Digit span forward, Color word stroop task and Paced Auditory Serial Addition Test (PASAT)) Psychomotor speed capacity and information processing ability (number of correct response on the Digit symbol test) Fine control motor (time required to finish the Grooved pegboard test) Assessment at 1–2, 3–4 and 9–10 h post-injection | Single-blind trial |  Performance on PASAT test compared to control groupLPS injection did not affect performance on other cognitive tests Performance on PASAT test compared to control groupLPS injection did not affect performance on other cognitive tests |
| Observational studies | |||||
| Reference | Sample characteristics at baseline | Endotoxemia assessment | Outcomes | Study design | Results |
| Lyons JL. et al., J Acquir Immune Defic Syndr, 2011 [66] | 97 HIV-infected patients (mean age 47 y, 76% men) | Plasma LPS quantified by LAL | HIV-associated neurocognitive disorders (HAND) Global T test (motor skills, speed of information processing, attention (working memory), learning (memory encoding), memory (memory recall), language fluency, and executive function) | Cross-sectional | LPS levels did not differ according to the severity of HAND LPS levels did not differ in subjects with global impairment (Global T test < 40) compared to unimpaired subjects |
| Vassallo M. et al., J Neurovirol, 2013 [65] | 40 HIV-infected patients with HIV-associated neurocognitive disorders (HAND) (median age 46 y, 78% men) and 139 HIV-infected patients without HAND (median age 44 y, 72% men) | Plasma LPS quantified by LAL | HIV-associated neurocognitive disorders (HAND) z-score (learning and recall episodic memory, attention/concentration, working memory, executive functions, language, visual agnosia and motor/psychomotor speed) | Cross-sectional | LPS levels was higher in the HAND group compared to no-HAND group LPS levels did not differ according to the severity of HAND |
| Jespersen S. et al., BMC Infect Dis, 2016 [67] | 62 untreated HIV-infected patients without evidence of impaired cognitive function (mean age 39 y, 52% men) | Plasma and CSF LPS quantified by LAL | CSF neurofilament light chain protein (marker of CNS axonal damage) and CSF neopterin (marker of monocyte activation) | Cross-sectional | No association between plasma LPS and CSF neurofilament light chain protein or CSF neopterin LPS was not detectable in CSF |
| Alzheimer’s Disease Brain Samples | |||||
|---|---|---|---|---|---|
| Reference | Sample Characteristics at Baseline | Endotoxemia Assessment | Outcomes | Study Design | Results |
| Zhan X. et al., Neurology, 2016 [68] | 24 AD brains (mean age 77 y, 38% men, median Braak stage: 6) and 18 age-matched controls brains (mean age 81 y, 56% men, median Braak stage: 2) | LPS E. coli antibodies and E. coli K99 pili protein | Superior temporal gyrus from grey matter (GM) and frontal lobe from white matter (WM) | Cross-sectional |  Detection of E. coli K99 and LPS levels in GM and WM AD compared to control Detection of E. coli K99 and LPS levels in GM and WM AD compared to controlLPS colocalized with Ab1-40/42 in amyloid plaques and with Ab1-40/42 around blood in AD brains |
| Zhao Y. et al., Front Cell Infect Microbiol, 2017 [69] | 10 AD brains (mean age 74 y) and 8 controls brains (mean age 73 y), all women | LPS E. coli antibodies | Neocortex (temporal lobe) and hippocampus | Cross-sectional |  LPS on average over two-fold in AD neocortex compared to controls LPS on average over two-fold in AD neocortex compared to controls LPS on average over three-fold in AD hippocampus compared to controls (up to 26-fold in some advanced AD) LPS on average over three-fold in AD hippocampus compared to controls (up to 26-fold in some advanced AD) |
| Alzheimer’s disease and dementia diagnosis | |||||
| Zhang R. et al., J Neuroimmunol, 2009 [71] | 18 AD patients (mean age 79 y, 39% men) and 18 healthy controls (mean age 55 y, 67% men) | Plasma LPS quantified by LAL | Alzheimer’s disease | Cross-sectional |  LPS in AD patients (61 ρg/mL on average) compared to healthy controls (21 ρg/mL on average) LPS in AD patients (61 ρg/mL on average) compared to healthy controls (21 ρg/mL on average) |
| Ancuta P. et al., PLoS One, 2008 [70] | 119 HIV patients whose 32 with No-NCI, 28 with HIV-associated dementia, 25 with MCMD, 20 with NPI-O, 9 ANI and 5 unable to assign | Plasma LPS quantified by LAL | HIV-associated dementia (HAD, defined as the involvement of at least two cognitive domains and documented by a performance of two SD below the normative mean on neuropsychological tests, with marked interference in daily functioning) | Cross-sectional |  LPS in HAD compared to No-NCI HIV-patients LPS in HAD compared to No-NCI HIV-patientsLPS levels did not differ in MCMD, NPI-O or ANI compared to No-NCI patients |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, P.; Laugerette, F.; Féart, C. Metabolic Endotoxemia: A Potential Underlying Mechanism of the Relationship between Dietary Fat Intake and Risk for Cognitive Impairments in Humans? Nutrients 2019, 11, 1887. https://doi.org/10.3390/nu11081887
André P, Laugerette F, Féart C. Metabolic Endotoxemia: A Potential Underlying Mechanism of the Relationship between Dietary Fat Intake and Risk for Cognitive Impairments in Humans? Nutrients. 2019; 11(8):1887. https://doi.org/10.3390/nu11081887
Chicago/Turabian StyleAndré, Perrine, Fabienne Laugerette, and Catherine Féart. 2019. "Metabolic Endotoxemia: A Potential Underlying Mechanism of the Relationship between Dietary Fat Intake and Risk for Cognitive Impairments in Humans?" Nutrients 11, no. 8: 1887. https://doi.org/10.3390/nu11081887
APA StyleAndré, P., Laugerette, F., & Féart, C. (2019). Metabolic Endotoxemia: A Potential Underlying Mechanism of the Relationship between Dietary Fat Intake and Risk for Cognitive Impairments in Humans? Nutrients, 11(8), 1887. https://doi.org/10.3390/nu11081887






