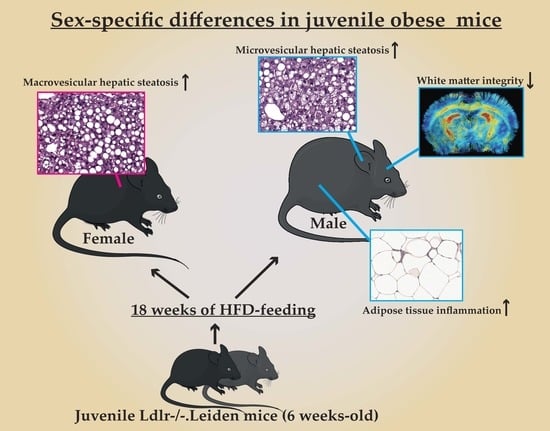
Sex-Specific Differences in Fat Storage, Development of Non-Alcoholic Fatty Liver Disease and Brain Structure in Juvenile HFD-Induced Obese Ldlr-/-.Leiden Mice
Abstract
1. Introduction
2. Materials and Methods
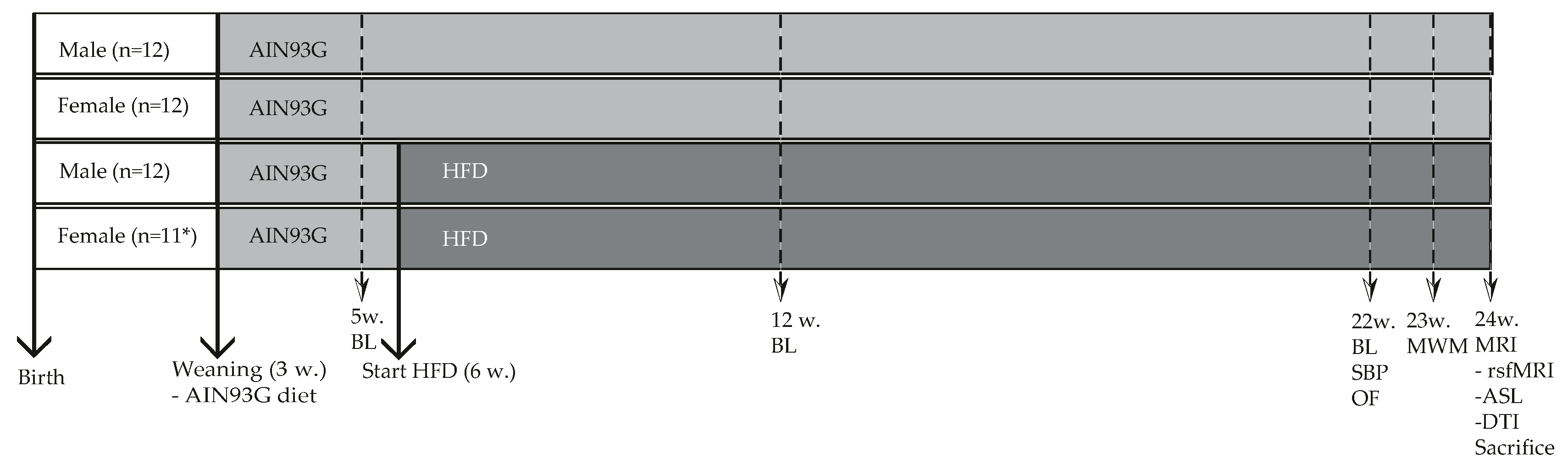
2.1. Animals, Diets and Study Design
2.2. Plasma Analyses
2.3. Sacrifice
2.4. Liver Analyses
2.5. Analyses in White Adipose Tissue
2.6. Behavioral and Cognitive Tests
2.6.1. Digitally Ventilated Cages
2.6.2. Systolic Blood Pressure Measurements
2.6.3. Open Field
2.6.4. Morris Water Maze
2.6.5. MRI Experiments
2.6.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
3.1. Sex-Specific Differences in Metabolically Active Tissues
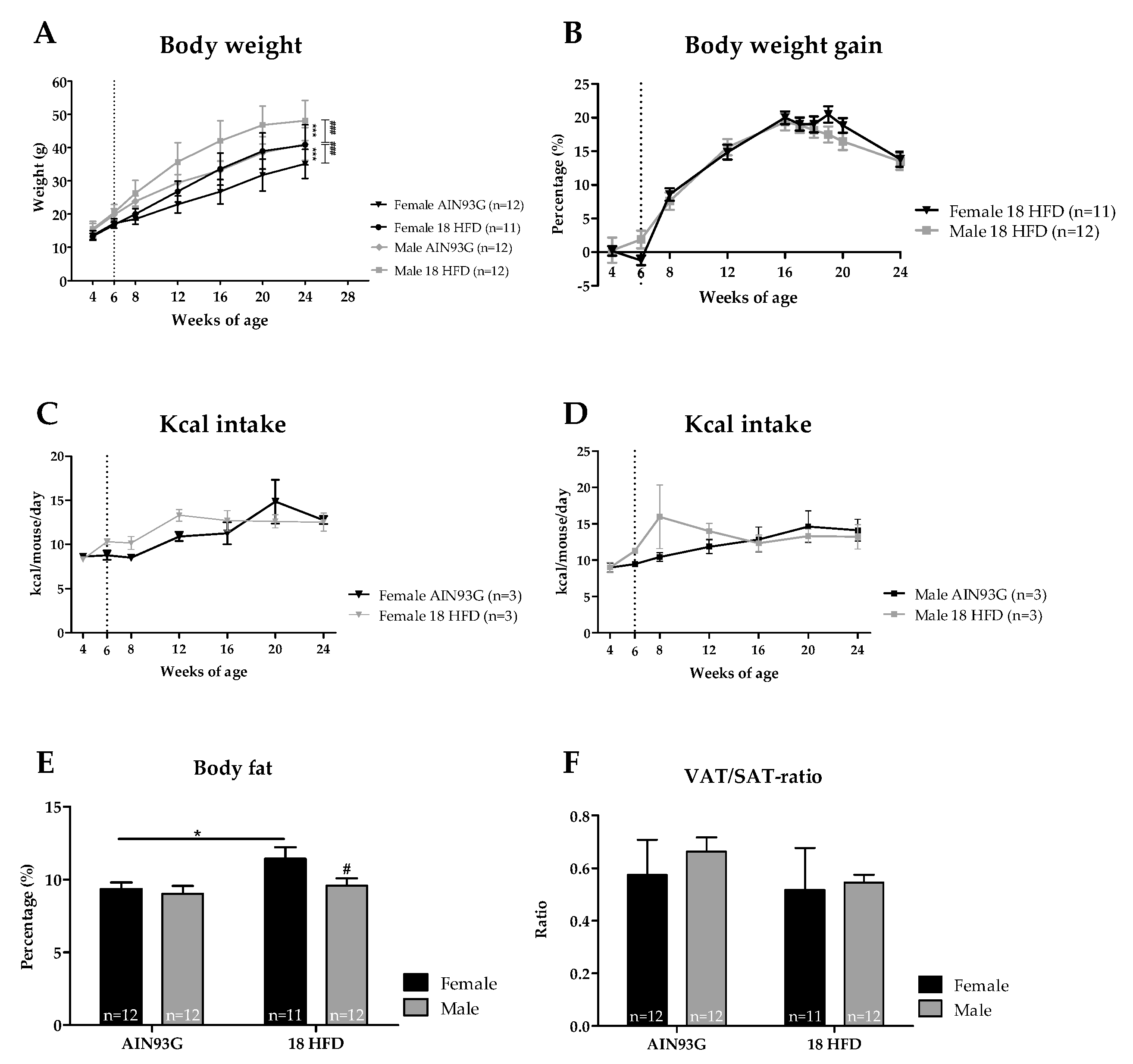
3.1.1. Male Mice Were Significantly Heavier than Female Mice, but no Differences Were Observed in HFD-Induced Body Weight Gain
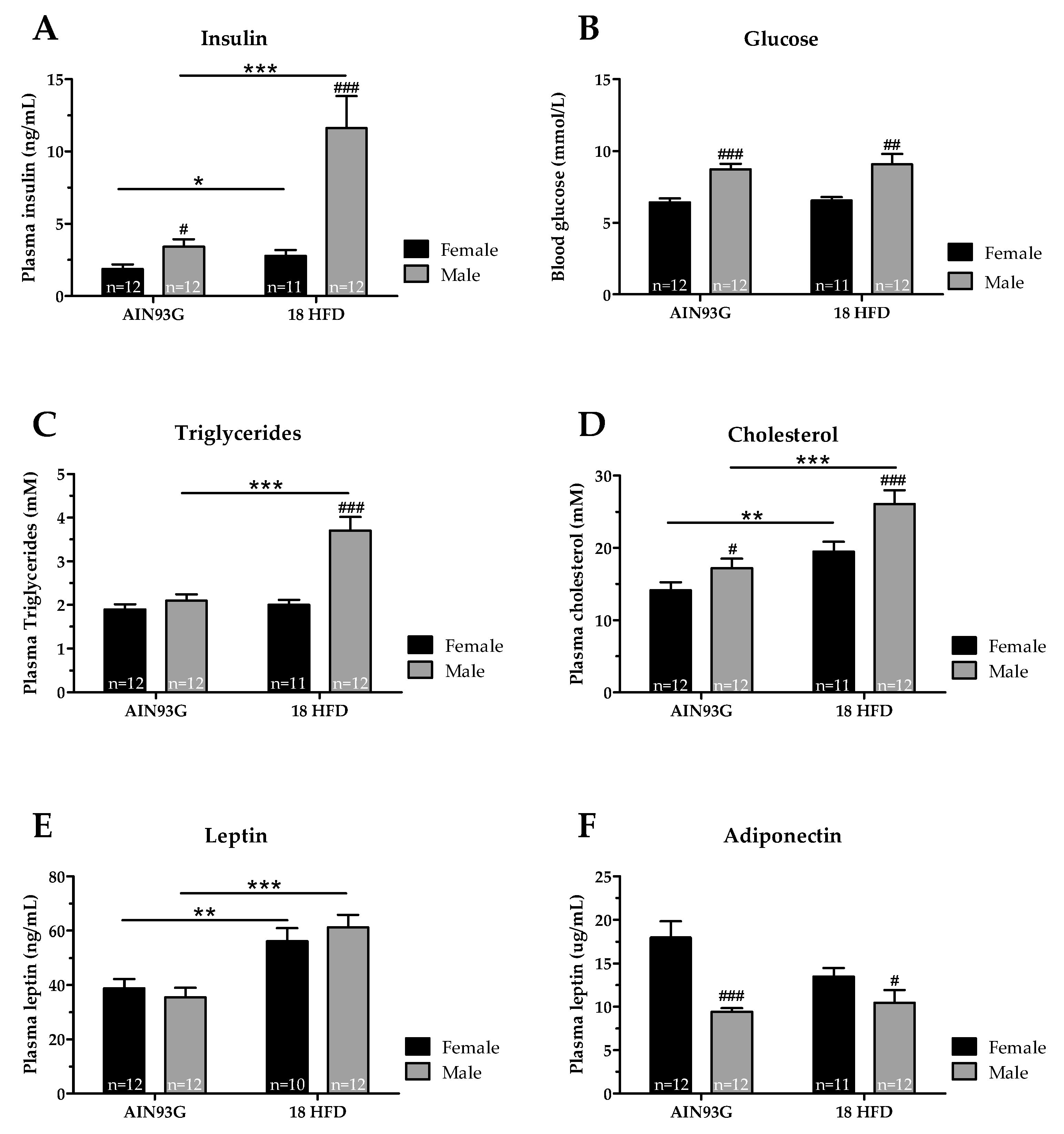
3.1.2. Female Mice Had Lower Glucose and Adiponectin Levels, and upon HFD Feeding Lower Insulin, Cholesterol and Triglyceride Levels than Male Mice
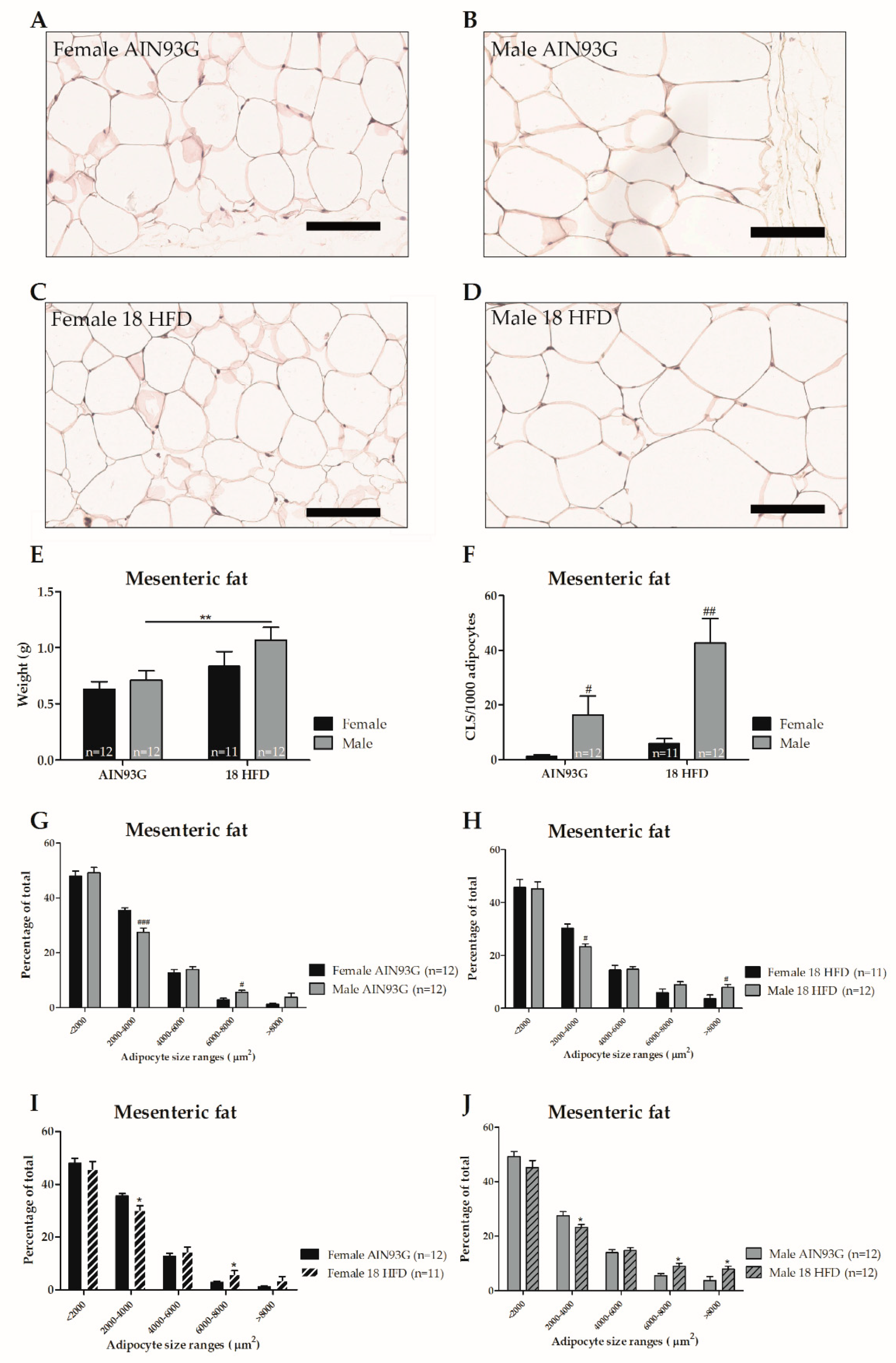
3.1.3. Female Mice on HFD Show a Predominant Fat Storage within the Perigonadal Fat Depot, and Exert a Lower Degree of Adipose Tissue Inflammation
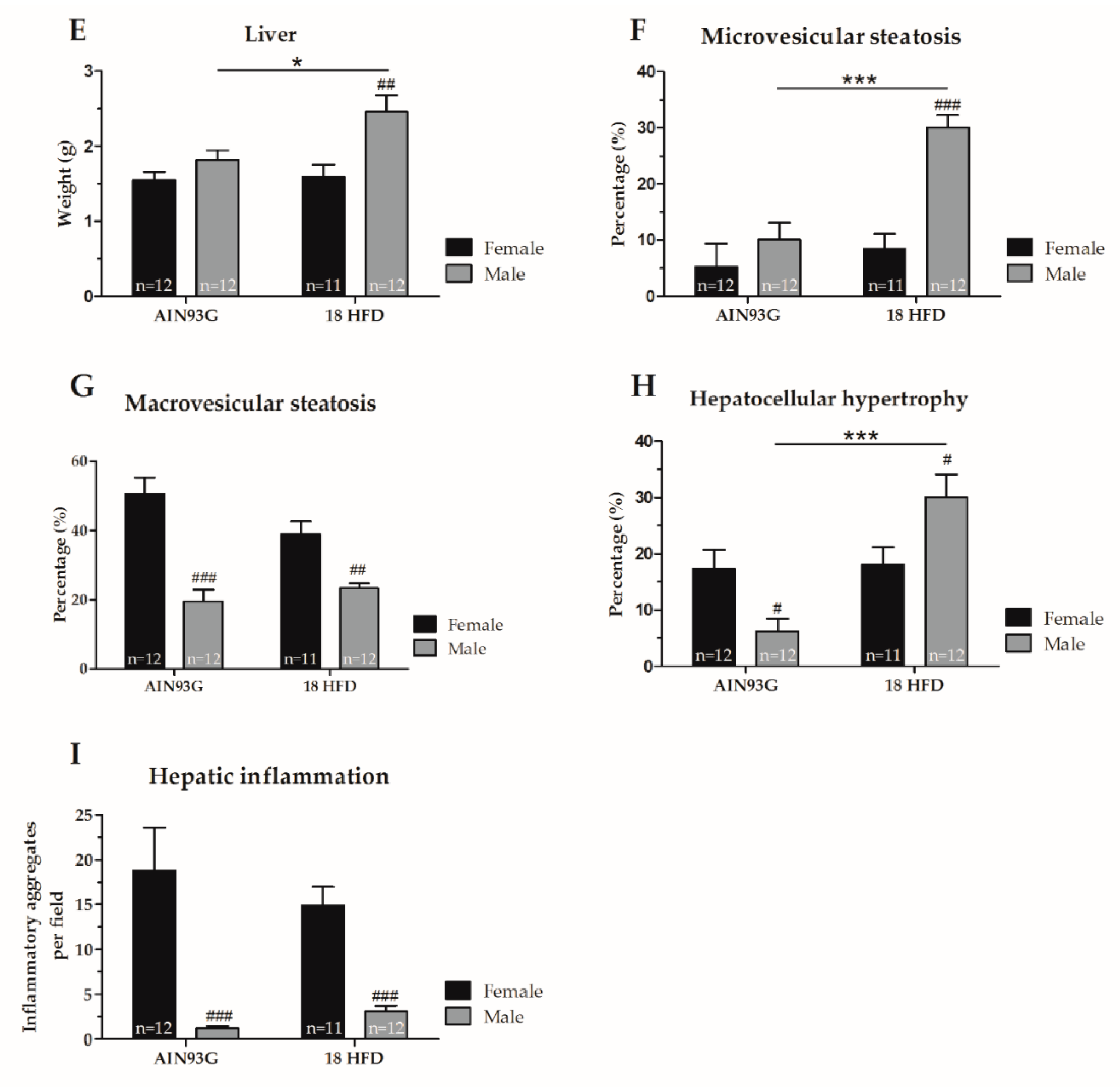
3.1.4. Increased Macrovesicular Steatosis and Hepatic Inflammation in HFD Fed Female Mice
3.2. Sex-Specific Alterations and HFD-Indued Juvenile Obesity in Brain Structure, Function and Behavior
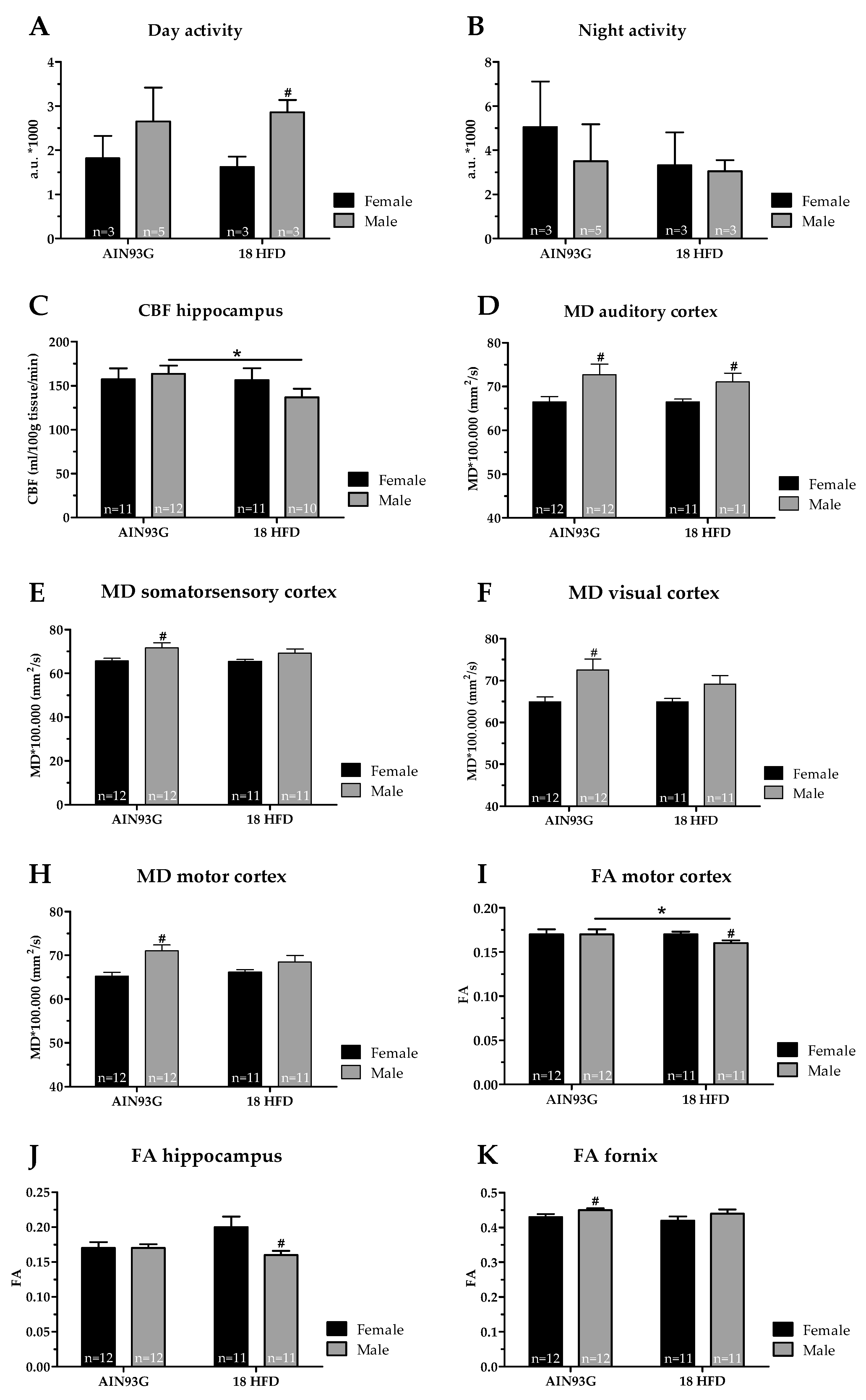
Male Mice Fed a HFD Have a Lower Cerebral Blood Flow and Decreased White Matter Integrity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Link, J.C.; Hasin-Brumshtein, Y.; Cantor, R.M.; Chen, X.; Arnold, A.P.; Lusis, A.J.; Reue, K. Diet, Gonadal Sex, and Sex Chromosome Complement Influence White Adipose Tissue Mirna Expression. BMC Genom. 2017, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Isensee, J.; Noppinger, P.R. Sexually Dimorphic Gene Expression in Mammalian Somatic Tissue. Gender Med. 2007, 4, S75–S95. [Google Scholar] [CrossRef]
- Morrison, C.D.; Pistell, P.J.; Ingram, D.K.; Johnson, W.D.; Liu, Y.; Fernandez-Kim, S.O.; White, C.L.; Purpera, M.N.; Uranga, R.M.; Bruce-Keller, A.J.; et al. High Fat Diet Increases Hippocampal Oxidative Stress and Cognitive Impairment in Aged Mice: Implications for Decreased Nrf2 Signaling. J. Neurochem. 2010, 114, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Aou, S.; Oomura, Y.; Hori, N.; Fukunaga, K.; Hori, T. Impairment of Long-Term Potentiation and Spatial Memory in Leptin Receptor-Deficient Rodents. Neuroscience 2002, 113, 607–615. [Google Scholar] [CrossRef]
- Gerges, N.Z.; Aleisa, A.M.; Alkadhi, K.A. Impaired Long-Term Potentiation in Obese Zucker Rats: Possible Involvement of Presynaptic Mechanism. Neuroscience 2003, 120, 535–539. [Google Scholar] [CrossRef]
- Beery, A.K.; Zucker, I. Sex Bias In Neuroscience and Biomedical Research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Boitard, C.; Cavaroc, A.; Sauvant, J.; Aubert, A.; Castanon, N.; Laye, S.; Ferreira, G. Impairment of Hippocampal-Dependent Memory Induced by Juvenile High-Fat Diet Intake Is Associated with Enhanced Hippocampal Inflammation in Rats. Brain Behav. Immun. 2014, 40, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Boitard, C.; Etchamendy, N.; Sauvant, J.; Aubert, A.; Tronel, S.; Marighetto, A.; Laye, S.; Ferreira, G. Juvenile, But Not Adult Exposure to High-Fat Diet Impairs Relational Memory and Hippocampal Neurogenesis in Mice. Hippocampus 2012, 22, 2095–2100. [Google Scholar] [CrossRef]
- Boitard, C.; Maroun, M.; Tantot, F.; Cavaroc, A.; Sauvant, J.; Marchand, A.; Laye, S.; Capuron, L.; Darnaudery, M.; Castanon, N.; et al. Juvenile Obesity Enhances Emotional Memory and Amygdala Plasticity through Glucocorticoids. J. Neurosci. 2015, 35, 4092–4103. [Google Scholar] [CrossRef]
- Gustafson, D.; Rothenberg, E.; Blennow, K.; Steen, B.; Skoog, I. An 18-Year Follow-Up of Overweight and Risk of Alzheimer Disease. Arch. Intern. Med. 2003, 163, 1524–1528. [Google Scholar] [CrossRef]
- Hwang, L.L.; Wang, C.H.; Li, T.L.; Chang, S.D.; Lin, L.C.; Chen, C.P.; Chen, C.T.; Liang, K.C.; Ho, I.K.; Yang, W.S.; et al. Sex Differences in High-Fat Diet-Induced Obesity, Metabolic Alterations and Learning, and Synaptic Plasticity Deficits in Mice. Obesity 2010, 18, 463–469. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight. Available online: Http://Www.Who.Int/Mediacentre/Factsheets/Fs311/En/Index.Html (accessed on 28 May 2018).
- Pulit, S.L.; Karaderi, T.; Lindgren, C.M. Sexual Dimorphisms in Genetic Loci Linked to Body Fat Distribution. Biosci. Rep. 2017, 37, BSR20160184. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Frige, F.; Paganelli, M.; Folli, F. In Morbid Obesity Metabolic Abnormalities and Adhesion Molecules Correlate with Visceral Fat; Effect of Weight Loss Through Surgery. Diabetes 2008, 57, A482. [Google Scholar] [CrossRef]
- Fantuzzi, G.; Mazzone, T. Adipose Tissue and Atherosclerosis—Exploring the Connection. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 996–1003. [Google Scholar] [CrossRef]
- White, U.A.; Tchoukalova, Y.D. Sex Dimorphism and Depot Differences in Adipose Tissue Function. Biochim. Biophys. Acta 2014, 1842, 377–392. [Google Scholar] [CrossRef]
- Shi, H.; Clegg, D.J. Sex Differences in the Regulation of Body Weight. Physiol. Behav. 2009, 97, 199–204. [Google Scholar] [CrossRef]
- Cahill, L. Why Sex Matters for Neuroscience. Nat. Rev. Neurosci. 2006, 7, 477–484. [Google Scholar] [CrossRef]
- Hausmann, M. Why Sex Hormones Matter for Neuroscience: A Very Short Review on Sex, Sex Hormones, and Functional Brain Asymmetries. J. Neurosci. Res. 2017, 95, 40–49. [Google Scholar] [CrossRef]
- Jazin, E.; Cahill, L. Sex Differences in Molecular Neuroscience: From Fruit Flies to Humans. Nat. Rev. Neurosc. 2010, 11, 9–17. [Google Scholar] [CrossRef]
- Camargos, A.C.R.; Mendonca, V.A.; De Andrade, C.A.; Oliveira, K.S.C.; Lacerda, A.C.R. Overweight and Obese Infants Present Lower Cognitive and Motor Development Scores Than Normal-Weight Peers. Res. Dev. Disabil. 2016, 59, 410–416. [Google Scholar] [CrossRef]
- Morrison, M.C.; Mulder, P.; Salic, K.; Verheij, J.; Liang, W.; Van Duyvenvoorde, W.; Menke, A.; Kooistra, T.; Kleemann, R.; Wielinga, P.Y. Intervention with a Caspase-1 Inhibitor Reduces Obesity-Associated Hyperinsulinemia, Non-Alcoholic Steatohepatitis and Hepatic Fibrosis in Ldlr-/-.Leiden Mice. Int. J. Obes. 2016, 40, 1416–1423. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. Ain-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the Ain-76a Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Toyoda, A.; Shimonishi, H.; Sato, M.; Usuda, K.; Ohsawa, N.; Nagaoka, K. Effects of Non-Purified and Semi-Purified Commercial Diets on Behaviors, Plasma Corticosterone Levels, and Cecum Microbiome in C57bl/6j Mice. Neurosci. Lett. 2018, 670, 36–40. [Google Scholar] [CrossRef]
- Morrison, M.C.; Verschuren, L.; Salic, K.; Verheij, J.; Menke, A.; Wielinga, P.Y.; Iruarrizaga-Lejarreta, M.; Gole, L.; Yu, W.M.; Turner, S.; et al. Obeticholic Acid Modulates Serum Metabolites and Gene Signatures Characteristic of Human Nash and Attenuates Inflammation and Fibrosis Progression in Ldlr-/-.Leiden Mice. Hepatol. Commun. 2018, 2, 1513–1532. [Google Scholar] [CrossRef]
- Gart, E.; Souto Lima, E.; Schuren, F.; De Ruiter, C.G.F.; Attema, J.; Verschuren, L.; Keijer, J.; Salic, K.; Morrison, M.C.; Kleemann, R. Diet-Independent Correlations Between Bacteria and Dysfunction of Gut, Adipose Tissue, and Liver: A Comprehensive Microbiota Analysis in Feces and Mucosa of the Ileum and Colon in Obese Mice with Nafld. Int. J. Mol. Sci. 2018, 20, 1. [Google Scholar] [CrossRef]
- Morrison, M.C.; Kleemann, R.; Van Koppen, A.; Hanemaaijer, R.; Verschuren, L. Key Inflammatory Processes in Human Nash Are Reflected in Ldlr(-/-).Leiden Mice: A Translational Gene Profiling Study. Front. Physiol. 2018, 9, 132. [Google Scholar] [CrossRef]
- Arnoldussen, I.A.C.; Wiesmann, M.; Pelgrim, C.E.; Wielemaker, E.M.; Van Duyvenvoorde, W.; Amaral-Santos, P.L.; Verschuren, L.; Keijser, B.J.F.; Heerschap, A.; Kleemann, R.; et al. Butyrate Restores Hfd-Induced Adaptations in Brain Function and Metabolism in Mid-Adult Obese Mice. Int. J. Obes. 2017, 41, 935–944. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The Arrive Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Wielinga, P.Y.; Harthoorn, L.F.; Verschuren, L.; Schoemaker, M.H.; Jouni, Z.E.; Van Tol, E.A.; Kleemann, R.; Kooistra, T. Arachidonic Acid/Docosahexaenoic Acid-Supplemented Diet in Early Life Reduces Body Weight Gain, Plasma Lipids, and Adiposity in Later Life in Apoe*3leiden Mice. Mol. Nutr. Food Res. 2012, 56, 1081–1089. [Google Scholar] [CrossRef]
- Schoemaker, M.H.; Kleemann, R.; Morrison, M.C.; Verheij, J.; Salic, K.; Van Tol, E.A.F.; Kooistra, T.; Wielinga, P.Y. A Casein Hydrolysate Based Formulation Attenuates Obesity and Associated Non-Alcoholic Fatty Liver Disease and Atherosclerosis in Ldlr-/-.Leiden Mice. PLoS ONE 2017, 12, e0180648. [Google Scholar] [CrossRef]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; Van Den Hoek, A.M. Establishment of A General Nafld Scoring System for Rodent Models and Comparison to Human Liver Pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef]
- Zimmer, M.; Bista, P.; Benson, E.L.; Lee, D.Y.; Liu, F.; Picarella, D.; Vega, R.B.; Vu, C.B.; Yeager, M.; Ding, M.; et al. Cat-2003: A Novel Sterol Regulatory Element-Binding Protein Inhibitor That Reduces Steatohepatitis, Plasma Lipids, and Atherosclerosis in Apolipoprotein E*3-Leiden Mice. Hepatol. Commun. 2017, 1, 311–325. [Google Scholar] [CrossRef]
- Galarraga, M.; Campion, J.; Munoz-Barrutia, A.; Boque, N.; Moreno, H.; Martinez, J.A.; Milagro, F.; Ortiz-De-Solorzano, C. Adiposoft: Automated Software for the Analysis of White Adipose Tissue Cellularity in Histological Sections. J. Lipid Res. 2012, 53, 2791–2796. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih Image to Imagej: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Mulder, P.; Morrison, M.C.; Wielinga, P.Y.; Van Duyvenvoorde, W.; Kooistra, T.; Kleemann, R. Surgical Removal of Inflamed Epididymal White Adipose Tissue Attenuates the Development of Non-Alcoholic Steatohepatitis in Obesity. Int. J. Obes. 2016, 40, 675–684. [Google Scholar] [CrossRef]
- Giles, J.M.; Whitaker, J.W.; Moy, S.S.; Fletcher, C.A. Effect of Environmental Enrichment on Aggression in Balb/Cj And Balb/Cbyj Mice Monitored by Using An Automated System. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 236–243. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Van Der Zee, C.E.; Dederen, P.J.; Brouwer, K.M.; Reijmer, Y.D.; Van Groen, T.; Broersen, L.M.; Lutjohann, D.; Heerschap, A.; Kiliaan, A.J. Dha And Cholesterol Containing Diets Influence Alzheimer-Like Pathology, Cognition And Cerebral Vasculature in Appswe/Ps1de9 Mice. Neurobiol. Dis. 2009, 33, 482–498. [Google Scholar] [CrossRef]
- Streijger, F.; Oerlemans, F.; Ellenbroek, B.A.; Jost, C.R.; Wieringa, B.; Van Der Zee, C.E.E.M. Structural And Behavioural Consequences of Double Deficiency for Creatine Kinases Bck And Ubckmit. Behav. Brain Res. 2005, 157, 219–234. [Google Scholar] [CrossRef]
- Janssen, C.I.F.; Zerbi, V.; Mutsaers, M.P.C.; De Jong, B.S.W.; Wiesmann, M.; Arnoldussen, I.A.C.; Geenen, B.; Heerschap, A.; Muskiet, F.A.J.; Jouni, Z.E.; et al. Impact of Dietary N-3 Polyunsaturated Fatty Acids on Cognition, Motor Skills And Hippocampal Neurogenesis in Developing C57bl/6j Mice. J. Nutr. Biochem. 2015, 26, 24–35. [Google Scholar] [CrossRef]
- Zerbi, V.; Jansen, D.; Wiesmann, M.; Fang, X.; Broersen, L.M.; Veltien, A.; Heerschap, A.; Kiliaan, A.J. Multinutrient Diets Improve Cerebral Perfusion And Neuroprotection in A Murine Model Of Alzheimer’s Disease. Neurobiol. Aging 2014, 35, 600–613. [Google Scholar] [CrossRef]
- Zerbi, V.; Wiesmann, M.; Emmerzaal, T.L.; Jansen, D.; Van Beek, M.; Mutsaers, M.P.; Beckmann, C.F.; Heerschap, A.; Kiliaan, A.J. Resting-State Functional Connectivity Changes in Aging Apoe4 And Apoe-Ko Mice. J. Neurosci. 2014, 34, 13963–13975. [Google Scholar] [CrossRef]
- Coventry, B.J.; Bradley, J.; Skinner, J.M. Differences Between Standard And High-Sensitivity Immunohistology in Tissue Sections—Comparison of Immunoperoxidase Staining Methods Using Computerized Video Image Analysis Techniques. Pathology 1995, 27, 221–223. [Google Scholar] [CrossRef]
- Grove, K.L.; Fried, S.K.; Greenberg, A.S.; Xiao, X.Q.; Clegg, D.J. A Microarray Analysis of Sexual Dimorphism of Adipose Tissues in High-Fat-Diet-Induced Obese Mice. Int. J. Obes. 2010, 34, 989–1000. [Google Scholar] [CrossRef]
- Mulder, P.; Liang, W.; Y Wielinga, P.; Verschuren, L.; Toet, K.; M Havekes, L.; Van Den Hoek, A.; Kleemann, R. Macrovesicular Steatosis Is Associated with Development of Lobular Inflammation And Fibrosis in Diet-Induced Non-Alcoholic Steatohepatitis (Nash). Inflamm. Cell Signal. 2015, 2, e804. [Google Scholar] [CrossRef]
- Mulder, P.; Morrison, M.C.; Verschuren, L.; Liang, W.; Van Bockel, J.H.; Kooistra, T.; Wielinga, P.Y.; Kleemann, R. Reduction of Obesity-Associated White Adipose Tissue Inflammation by Rosiglitazone Is Associated with Reduced Non-Alcoholic Fatty Liver Disease in Ldlr-Deficient Mice. Sci. Rep. 2016, 6, 31542. [Google Scholar] [CrossRef]
- Zwamborn, R.A.; Slieker, R.C.; Mulder, P.C.; Zoetemelk, I.; Verschuren, L.; Suchiman, H.E.; Toet, K.H.; Droog, S.; Slagboom, P.E.; Kooistra, T.; et al. Prolonged High-Fat Diet Induces Gradual And Fat Depot-Specific DNA Methylation Changes in Adult Mice. Sci. Rep. 2017, 7, 43261. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Sames, C.; Swisher, L.; Swick, A.G.; Ploutz-Snyder, L.L.; Steppan, C.M.; Sagendorf, K.S.; Feiglin, D.; Jaynes, E.B.; Meyer, R.A.; et al. Abdominal Fat Distribution in Pre- And Postmenopausal Women: The Impact of Physical Activity, Age, And Menopausal Status. Metabolism 2001, 50, 976–982. [Google Scholar] [CrossRef]
- Nilsson, S.; Gustafsson, J.-Å. Estrogen Receptors: Their Actions and Functional Roles in Health and Disease. In Nuclear Receptors; Springer: Berlin, Germany, 2010; pp. 91–141. [Google Scholar]
- Jones, M.E.E.; Thorburn, A.W.; Britt, K.L.; Hewitt, K.N.; Wreford, N.G.; Proietto, J.; Oz, O.K.; Leury, B.J.; Robertson, K.M.; Yao, S.G.; et al. Aromatase-Deficient (Arko) Mice Have A Phenotype of Increased Adiposity. Proc. Natl. Acad. Sci. USA 2000, 97, 12735–12740. [Google Scholar] [CrossRef]
- Takeda, K.; Toda, K.; Saibara, T.; Nakagawa, M.; Saika, K.; Onishi, T.; Sugiura, T.; Shizuta, Y. Progressive Development of Insulin Resistance Phenotype in Male Mice with Complete Aromatase (Cyp19) Deficiency. J. Endocrinol. 2003, 176, 237–246. [Google Scholar] [CrossRef]
- Bruun, J.M.; Nielsen, C.B.; Pedersen, S.B.; Flyvbjerg, A.; Richelsen, B. Estrogen Reduces Pro-Inflammatory Cytokines in Rodent Adipose Tissue: Studies in Vivo And in Vitro. Horm. Metab. Res. 2003, 35, 142–146. [Google Scholar] [CrossRef]
- Carmiel-Haggai, M.; Cederbaum, A.I.; Nieto, N. A High-Fat Diet Leads to the Progression of Non-Alcoholic Fatty Liver Disease in Obese Rats. FASEB J. 2005, 19, 136–138. [Google Scholar] [CrossRef]
- Allen, L.; Ramalingam, L.; Menikdiwela, K.; Scoggin, S.; Shen, C.L.; Tomison, M.D.; Kaur, G.; Dufour, J.M.; Chung, E.; Kalupahana, N.S.; et al. Effects of Delta-Tocotrienol on Obesity-Related Adipocyte Hypertrophy, Inflammation And Hepatic Steatosis in High-Fat-Fed Mice. J. Nutr. Biochem. 2017, 48, 128–137. [Google Scholar] [CrossRef]
- Fromenty, B.; Berson, A.; Pessayre, D. Microvesicular Steatosis And Steatohepatitis: Role of Mitochondrial Dysfunction And Lipid Peroxidation. J. Hepatol. 1997, 26 (Suppl. 1), 13–22. [Google Scholar] [CrossRef]
- Fromenty, B.; Pessayre, D. Inhibition of Mitochondrial Beta-Oxidation as a Mechanism of Hepatotoxicity. Pharmacol. Ther. 1995, 67, 101–154. [Google Scholar] [CrossRef]
- Hautekeete, M.L.; Degott, C.; Benhamou, J.P. Microvesicular Steatosis of the Liver. Acta. Clin. Belg. 1990, 45, 311–326. [Google Scholar] [CrossRef]
- Liang, W.; Verschuren, L.; Mulder, P.; Van Der Hoorn, J.W.; Verheij, J.; Van Dam, A.D.; Boon, M.R.; Princen, H.M.; Havekes, L.M.; Kleemann, R.; et al. Salsalate Attenuates Diet Induced Non-Alcoholic Steatohepatitis in Mice by Decreasing Lipogenic And Inflammatory Processes. Br. J. Pharmacol. 2015, 172, 5293–5305. [Google Scholar] [CrossRef]
- Tomizawa, M.; Kawanabe, Y.; Shinozaki, F.; Sato, S.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Sueishi, M. Triglyceride Is Strongly Associated with Nonalcoholic Fatty Liver Disease Among Markers of Hyperlipidemia And Diabetes. Biomed. Rep. 2014, 2, 633–636. [Google Scholar] [CrossRef]
- Mulder, P.; Van Den Hoek, A.M.; Kleemann, R. The Ccr2 Inhibitor Propagermanium Attenuates Diet-Induced Insulin Resistance, Adipose Tissue Inflammation And Non-Alcoholic Steatohepatitis. PLoS ONE 2017, 12, e0169740. [Google Scholar] [CrossRef]
- Mirea, A.M.; Tack, C.J.; Chavakis, T.; Joosten, L.A.B.; Toonen, E.J.M. Il-1 Family Cytokine Pathways Underlying Nafld: Towards New Treatment Strategies. Trends Mol. Med. 2018, 24, 458–471. [Google Scholar] [CrossRef]
- Brunt, E.M. Histopathology of Non-Alcoholic Fatty Liver Disease. Clin. Liver Dis. 2009, 13, 533–544. [Google Scholar] [CrossRef]
- Van De Poll, S.W.; Romer, T.J.; Volger, O.L.; Delsing, D.J.; Bakker Schut, T.C.; Princen, H.M.; Havekes, L.M.; Jukema, J.W.; Van Der Laarse, A.; Puppels, G.J. Raman Spectroscopic Evaluation of the Effects of Diet And Lipid-Lowering Therapy on Atherosclerotic Plaque Development in Mice. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1630–1635. [Google Scholar] [CrossRef]
- Zadelaar, S.; Kleemann, R.; Verschuren, L.; De Vries-Van Der Weij, J.; Van Der Hoorn, J.; Princen, H.M.; Kooistra, T. Mouse Models for Atherosclerosis And Pharmaceutical Modifiers. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1706–1721. [Google Scholar] [CrossRef]
- Van De Steeg, E.; Kleemann, R.; Jansen, H.T.; Van Duyvenvoorde, W.; Offerman, E.H.; Wortelboer, H.M.; Degroot, J. Combined Analysis of Pharmacokinetic And Efficacy Data of Preclinical Studies with Statins Markedly Improves Translation of Drug Efficacy to Human Trials. J. Pharmacol. Exp. Ther. 2013, 347, 635–644. [Google Scholar] [CrossRef]
- Dewey, F.E.; Gusarova, V.; Dunbar, R.L.; O’Dushlaine, C.; Schurmann, C.; Gottesman, O.; Mccarthy, S.; Van Hout, C.V.; Bruse, S.; Dansky, H.M.; et al. Genetic And Pharmacologic Inactivation of Angptl3 And Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 211–221. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, W.C.; Shin, K.S.; Roh, J.K.; Cho, D.H.; Cho, K. Large Interrelated Clusters of Repetitive Elements (Res) And Re Arrays Predominantly Represent Reference Mouse Chromosome Y. Chromosome Res. Int. J. Mol. Supramol. Evol. Asp. Chromosome Biol. 2013, 21, 15–26. [Google Scholar] [CrossRef][Green Version]
- Paria, N.; Raudsepp, T.; Pearks Wilkerson, A.J.; O’Brien, P.C.; Ferguson-Smith, M.A.; Love, C.C.; Arnold, C.; Rakestraw, P.; Murphy, W.J.; Chowdhary, B.P. A Gene Catalogue of the Euchromatic Male-Specific Region of the Horse Y Chromosome: Comparison with Human And Other Mammals. PLoS ONE 2011, 6, e21374. [Google Scholar] [CrossRef]
- Meyer, C.E.; Kurth, F.; Lepore, S.; Gao, J.L.; Johnsonbaugh, H.; Oberoi, M.R.; Sawiak, S.J.; Mackenzie-Graham, A. In Vivo Magnetic Resonance Images Reveal Neuroanatomical Sex Differences Through the Application of Voxel-Based Morphometry in C57bl/6 Mice. Neuroimage 2017, 163, 197–205. [Google Scholar] [CrossRef]
- Koolschijn, P.C.; Crone, E.A. Sex Differences And Structural Brain Maturation from Childhood to Early Adulthood. Dev. Cogn. Neurosci. 2013, 5, 106–118. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.L.; Patel, V.R.; De Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the Circadian Clock by Nutritional Challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef]
- Selim, M.; Jones, R.; Novak, P.; Zhao, P.; Novak, V. The Effects of Body Mass Index on Cerebral Blood Flow Velocity. Clin. Auton. Res. 2008, 18, 331–338. [Google Scholar] [CrossRef]
- Birdsill, A.C.; Carlsson, C.M.; Willette, A.A.; Okonkwo, O.C.; Johnson, S.C.; Xu, G.F.; Oh, J.M.; Gallagher, C.L.; Koscik, R.L.; Jonaitis, E.M.; et al. Low Cerebral Blood Flow Is Associated with Lower Memory Function in Metabolic Syndrome. Obesity 2013, 21, 1313–1320. [Google Scholar] [CrossRef]
- Hong, Y.J.; Yoon, B.; Lim, S.C.; Shim, Y.S.; Kim, J.Y.; Ahn, K.J.; Han, I.W.; Yang, D.W. Microstructural Changes in the Hippocampus And Posterior Cingulate in Mild Cognitive Impairment And Alzheimer’s Disease: A Diffusion Tensor Imaging Study. Neurol. Sci. 2013, 34, 1215–1221. [Google Scholar] [CrossRef]
- Song, S.K.; Kim, J.H.; Lin, S.J.; Brendza, R.P.; Holtzman, D.M. Diffusion Tensor Imaging Detects Age-Dependent White Matter Changes in a Transgenic Mouse Model with Amyloid Deposition. Neurobiol. Dis. 2004, 15, 640–647. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsumoto, Y.; Ota, H.; Sugimura, K.; Takahashi, J.; Ito, K.; Miyata, S.; Furukawa, K.; Arai, H.; Fukumoto, Y.; et al. Hippocampal Blood Flow Abnormality Associated with Depressive Symptoms And Cognitive Impairment in Patients with Chronic Heart Failure. Circ. J. 2016, 80, 1773–1780. [Google Scholar] [CrossRef]
- Cabrera-Pastor, A.; Llansola, M.; Montoliu, C.; Malaguarnera, M.; Balzano, T.; Taoro-Gonzalez, L.; Garcia-Garcia, R.; Mangas-Losada, A.; Izquierdo-Altarejos, P.; Arenas, Y.M.; et al. Peripheral Inflammation Induces Neuroinflammation That Alters Neurotransmission And Cognitive And Motor Function in Hepatic Encephalopathy: Underlying Mechanisms And Therapeutic Implications. Acta Physiol. 2019, 226, e13270. [Google Scholar] [CrossRef]
- Garcia-Martinez, R.; Cordoba, J. Liver-Induced Inflammation Hurts the Brain. J. Hepatol. 2012, 56, 515–517. [Google Scholar] [CrossRef]


| AIN93G | HFD | |||
|---|---|---|---|---|
| gm% | kcal% | gm% | kcal% | |
| Protein | 20 | 20.3 | 24 | 20 |
| Carbohydrate | 64 | 63.9 | 41 | 35 |
| Fat | 7 | 15.8 | 24 | 45 |
| Total | 100 | 100 | ||
| Kcal/gm | 3.9 | 4.73 | ||
| Ingredient | ||||
| Casein, 30 Mesh | 200 | 800 | 200 | 800 |
| L-Cystine | 3 | 12 | 3 | 12 |
| Corn starch | 397 | 1590 | 72.8 | 291 |
| Maltodextrin | 132 | 528 | 100 | 400 |
| Sucrose | 100 | 400 | 172.8 | 691 |
| Cellulose | 50 | 0 | 50 | 0 |
| Soybean oil | 70 | 630 | 25 | 225 |
| Lard | 177.5 | 1598 | ||
| t-Butylhydroquinone | 0.0014 | 0 | ||
| Di-calcium phosphate | 13 | 0 | ||
| Calcium carbonate | 5.5 | 0 | ||
| Potassium citrate | 16.5 | 0 | ||
| Choline bitartrate | 2.5 | 0 | 2.0 | 0 |
| Mineral mix S10022G | 35 | 0 | ||
| Mineral mix S10026 | 10 | 0 | ||
| Vitamin mix V10037 | 10 | 40 | ||
| Vitamin mix V10001 | 10 | 40 | ||
| FD&C red dye #40 | 0.05 | 0 | ||
| Total | 1000 | 4000 | 858.15 | 4057 |
| Anatomical T2 * w | ASL | DTI | rsfMRI | |
|---|---|---|---|---|
| Imaging method | GE | FAIR-ASL | 4-shot spin-echo PI | Spin-echo EPI |
| Echo time (ms) | 7.35 | 10.8 | 21.0 | 10.0 |
| Repetition time (s) | 0.86 | 12.0 | 21.0 | 1.85 |
| Image matrix (pixel × pixel) | 512 × 512 | 128 × 96 | 128 × 128 | 96 × 96 |
| Field-of-view (mm) | 40 × 40 | 25 × 25 | 20 × 20 | 25 × 25 |
| Spatial resolution (µm/pixel) | 78 × 78 × 500 | 195 × 260 × 1000 | 156 × 156 × 500 | 260 × 260 × 500 |
| Number of slices | 20 × 3 | 16 | 20 | 20 |
| Total acquisition time (min) | 8 | 12 | 35 | 11 |
| Parameters | AIN93G | HFD |
|---|---|---|
| Female Versus Male | Female Versus Male | |
| Body weight | ↓ p < 0.001, F(1,22) = 25.98 | ↓ p < 0.001, F(1,22) = 17.22 |
| Body weight gain | = | = |
| Food intake | = | = |
| Percentage of body fat | = | ↑ p < 0.050, F(1,21) = 4.19 |
| VAT/SAT-ratio | = | = |
| Fasting plasma levels | ||
| Glucose | ↓ p < 0.001, F(1,22) = 24.43 | ↓ p = 0.010, F(1,20) = 7.98 |
| Insulin | ↓ p = 0.015, F(1,22) = 6.97 | ↓ p < 0.001, F(1,20) = 25.50 |
| Cholesterol | = | ↓ p = 0.018, F(1,20) = 6.65 |
| Triglycerides | = | ↓ p < 0.001, F(1,20) = 30.20 |
| Adiponectin | ↑ p < 0.001, F(1, 22) = 33.51 | ↑ p = 0.020, F(1,20) = 6.34 |
| Adipose tissue | ||
| Mesenteric weight | = | = |
| Mesenteric adipocyte size | ||
| Averaged | = | = |
| Distribution, <2000 µm2 | = | = |
| 2000–4000 µm2 | ↑ p < 0.001, F(1,22) = 19.90 | ↑ p = 0.001, F(1,21) = 13.98 |
| 4000–6000 µm2 | = | = |
| 6000–8000 µm2 | ↓ p < 0.010, F(1, 22) = 9.50 | = |
| >8000 µm2 | = | ↓p = 0.036, F(1,21) = 5.01 |
| Mesenteric CLS | ↓ p = 0.012, F(1,22) = 7.48 | ↓ p < 0.001, F(1,21) = 17.44 |
| Perigonadal weight | ↓ p = 0.020, F(1,22) = 5.84 | ↑ p = 0.007, F(1,21) = 9.08 |
| Perigonadal adipocyte size | ||
| Averaged in µm2 | = | ↑ p = 0.026, F(1,21) = 5.70 |
| Distribution, <2000 µm2 | = | ↓ p = 0.001, F(1,21) = 13.98 |
| 2000–4000 µm2 | = | = |
| 4000–6000 µm2 | ↑ p < 0.013, F(1, 22) = 7.21 | = |
| 6000–8000 µm2 | ↑ p < 0.005, F(1, 22) = 9.70 | ↑ p = 0.003, F(1,21) = 10.85 |
| >8000 µm2 | = | = |
| Perigonadal CLS | = | ↓ p = 0.050, F(1,21) = 4.34 |
| Inguinal weight | ↓ p = 0.002, F(1,22) = 11.86 | = |
| Inguinal adipocyte size | ||
| Averaged in µm2 | = | = |
| Distribution in µm2 | = | = |
| Inguinal CLS | ↓ p = 0.027, F(1,22) = 5.63 | ↓ p = 0.034, F(1,21) = 5.13 |
| >8000 µm2 | ||
| Liver | ||
| Liver weight | = | ↑ p = 0.022, F(1,22) = 6.05 |
| Microvesicular steatosis | = | ↓ p < 0.001, F(1,18) = 31.61 |
| Macrovesicular steatosis | ↑ p < 0.001, F(1,21) = 27.31 | ↑ p = 0.002, F(1,18) = 13.13 |
| Inflammation | ↑ p < 0.001, F(1,21) = 48.59 | ↑ p < 0.001, F(1,18) = 38.65 |
| Hypertrophy | = | ↓ p = 0.043, F(1,18) = 4.74 |
| Cognition | ||
| Open field | = | = |
| Morris water maze | = | = |
| DVC | = | ↓ p = 0.027, F(1,4) = 11.72 |
| SBP | = | = |
| MRI | ||
| Blood vessels (GLUT-1) | = | = |
| Cerebral blood flow | = | = |
| Neurogenesis (DCX) | = | = |
| Neuroinflammation (IBA-1) | = | = |
| Functional connectivity | = | = |
| DTI, mean diffusivity | ||
| Auditory cortex | ↓ p = 0.030, F(1,22) = 5.37 | ↓ p = 0.041, F(1,20) = 4.77 |
| Somatosensory cortex | ↓ p = 0.038, F(1,22) = 4.86 | = |
| Visual cortex | ↓ p = 0.013, F(1,22) = 7.22 | = |
| Motor cortex | ↓ p = 0.002, F(1,22) = 12.60 | = |
| DTI, fractional anisotropy | ||
| Hippocampus | = | ↑ p = 0.033, F(1,20) = 5.24 |
| Motor cortex | = | ↑ p = 0.013, F(1,20) = 7.42 |
| Parameters | Female | Male |
|---|---|---|
| HFD versus AIN93G | HFD versus AIN93G | |
| Body weight | ↑ p = 0.004, F(1,21) = 10.31 | ↑ p = 0.002, F(1,22) = 12.11 |
| Food intake | = | = |
| Percentage of body fat | ↑ p = 0.026, F(1,21) = 5.69 | = |
| VAT/SAT-ratio | = | = |
| Fasting plasma levels | ||
| Glucose | = | = |
| Insulin | ↑ p = 0.035, F(1,20) = 5.11 | ↑ p < 0.001, F(1,22) = 19.72 |
| Cholesterol | ↑ p = 0.007, F(1,20) = 8.88 | ↑ p = 0.001, F(1,22) = 14.83 |
| Triglycerides | = | ↑ p < 0.001, F(1,22) = 25.47 |
| Adiponectin | = | = |
| Leptin | ↑ p = 0.006, F(1,20) = 9.59 | ↑ p < 0.001, F(1,22) = 19.75 |
| Adipose tissue | ||
| Mesenteric weight | = | ↑ p = 0.045, F(1,22) = 4.50 |
| Mesenteric adipocyte size | ||
| Averaged | = | = |
| Distribution, < 2000 µm2 | = | = |
| 2000–4000 µm2 | ↓ p = 0.008, F(1,21) = 8.59 | ↓ p = 0.036, F(1,22) = 4.98 |
| 4000–6000 µm2 | = | = |
| 6000–8000 µm2 | ↑ p = 0.036, F(1,21) = 5.00 | ↑ p = 0.016, F(1,22) = 6.88 |
| >8000 µm2 | = | = |
| Mesenteric CLS | = | ↑ p = 0.016, F(1,22) = 6.86 |
| Perigonadal weight | ↑ p = 0.003, F(1,21) = 11.78 | = |
| Perigonadal adipocyte size | ||
| Averaged | = | = |
| Distribution, <2000 µm2 | = | = |
| 2000–4000 µm2 | ↓ p = 0.047, F(1,21) = 84.43 | = |
| 4000–6000 µm2 | = | = |
| 6000–8000 µm2 | = | = |
| >8000 µm2 | = | = |
| Perigonadal CLS | = | = |
| Inguinal weight | ↑ p = 0.030, F(1,21) = 5.17 | ↑ p = 0.002, F(1,22) = 11.86 |
| Inguinal adipocyte size | ||
| Averaged | ↑ p < 0.001, F(1,21) = 22.82 | ↑ p = 0.002, F(1,22) = 12.80 |
| Distribution, < 2000 µm2 | ↓ p = 0.002, F(1,21) = 12.31 | ↓ p = 0.017, F(1,22) = 6.70 |
| 2000–4000 µm2 | ↓ p = 0.003, F(1,21) = 10.86 | |
| 4000–6000 µm2 | ↑ p = 0.008, F(1,21) = 8.67 | = |
| 6000–8000 µm2 | ↑ p < 0.000, F(1,21) = 19.43 | ↑ p = 0.032, F(1,22) = 5.22 |
| >8000 µm2 | = | ↑ p = 0.002, F(1,22) = 12.80 |
| Inguinal CLS | = | ↑ p = 0.016, F(1,22) = 6.82 |
| Liver | ||
| Liver weight | = | ↑ p = 0.022, F(1,22) = 6.05 |
| Microvesicular steatosis | = | ↑ p < 0.001, F(1,18) = 23.47 |
| Macrovesicular steatosis | = | = |
| Inflammation | = | ↑ p < 0.001, F(1,21) = 48.59 |
| Hypertrophy | = | ↑ p = 0.013, F(1,21) = 7.32 |
| Cognition | ||
| Open field | = | = |
| Morris water maze | = | = |
| Digital ventilated cages | = | = |
| Systolic blood pressure | = | = |
| MRI | ||
| Blood vessels (GLUT-1) | = | = |
| Cerebral blood flow | = | ↓ p = 0.010, F(1,17) = 8.44 |
| Neurogenesis (DCX) | = | = |
| Neuroinflammation (IBA-1) | = | = |
| Functional connectivity | = | = |
| DTI, mean diffusivity | = | = |
| DTI, fractional anisotropy | ||
| Motor cortex | = | ↓ p = 0.045, F(1,21) = 4.55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, S.A.H.; Gart, E.; Vreeken, D.; Franx, B.A.A.; Wekking, L.; Verweij, V.G.M.; Worms, N.; Schoemaker, M.H.; Gross, G.; Morrison, M.C.; et al. Sex-Specific Differences in Fat Storage, Development of Non-Alcoholic Fatty Liver Disease and Brain Structure in Juvenile HFD-Induced Obese Ldlr-/-.Leiden Mice. Nutrients 2019, 11, 1861. https://doi.org/10.3390/nu11081861
Jacobs SAH, Gart E, Vreeken D, Franx BAA, Wekking L, Verweij VGM, Worms N, Schoemaker MH, Gross G, Morrison MC, et al. Sex-Specific Differences in Fat Storage, Development of Non-Alcoholic Fatty Liver Disease and Brain Structure in Juvenile HFD-Induced Obese Ldlr-/-.Leiden Mice. Nutrients. 2019; 11(8):1861. https://doi.org/10.3390/nu11081861
Chicago/Turabian StyleJacobs, Sophie A.H., Eveline Gart, Debby Vreeken, Bart A.A. Franx, Lotte Wekking, Vivienne G.M. Verweij, Nicole Worms, Marieke H. Schoemaker, Gabriele Gross, Martine C. Morrison, and et al. 2019. "Sex-Specific Differences in Fat Storage, Development of Non-Alcoholic Fatty Liver Disease and Brain Structure in Juvenile HFD-Induced Obese Ldlr-/-.Leiden Mice" Nutrients 11, no. 8: 1861. https://doi.org/10.3390/nu11081861
APA StyleJacobs, S. A. H., Gart, E., Vreeken, D., Franx, B. A. A., Wekking, L., Verweij, V. G. M., Worms, N., Schoemaker, M. H., Gross, G., Morrison, M. C., Kleemann, R., Arnoldussen, I. A. C., & Kiliaan, A. J. (2019). Sex-Specific Differences in Fat Storage, Development of Non-Alcoholic Fatty Liver Disease and Brain Structure in Juvenile HFD-Induced Obese Ldlr-/-.Leiden Mice. Nutrients, 11(8), 1861. https://doi.org/10.3390/nu11081861