Pre-Conception Maternal Food Intake and the Association with Childhood Allergies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Population
2.2. Maternal Data Collection
2.3. Maternal Dietary Intake
2.4. Child Allergy Outcomes
2.5. Statistical Methods
3. Results
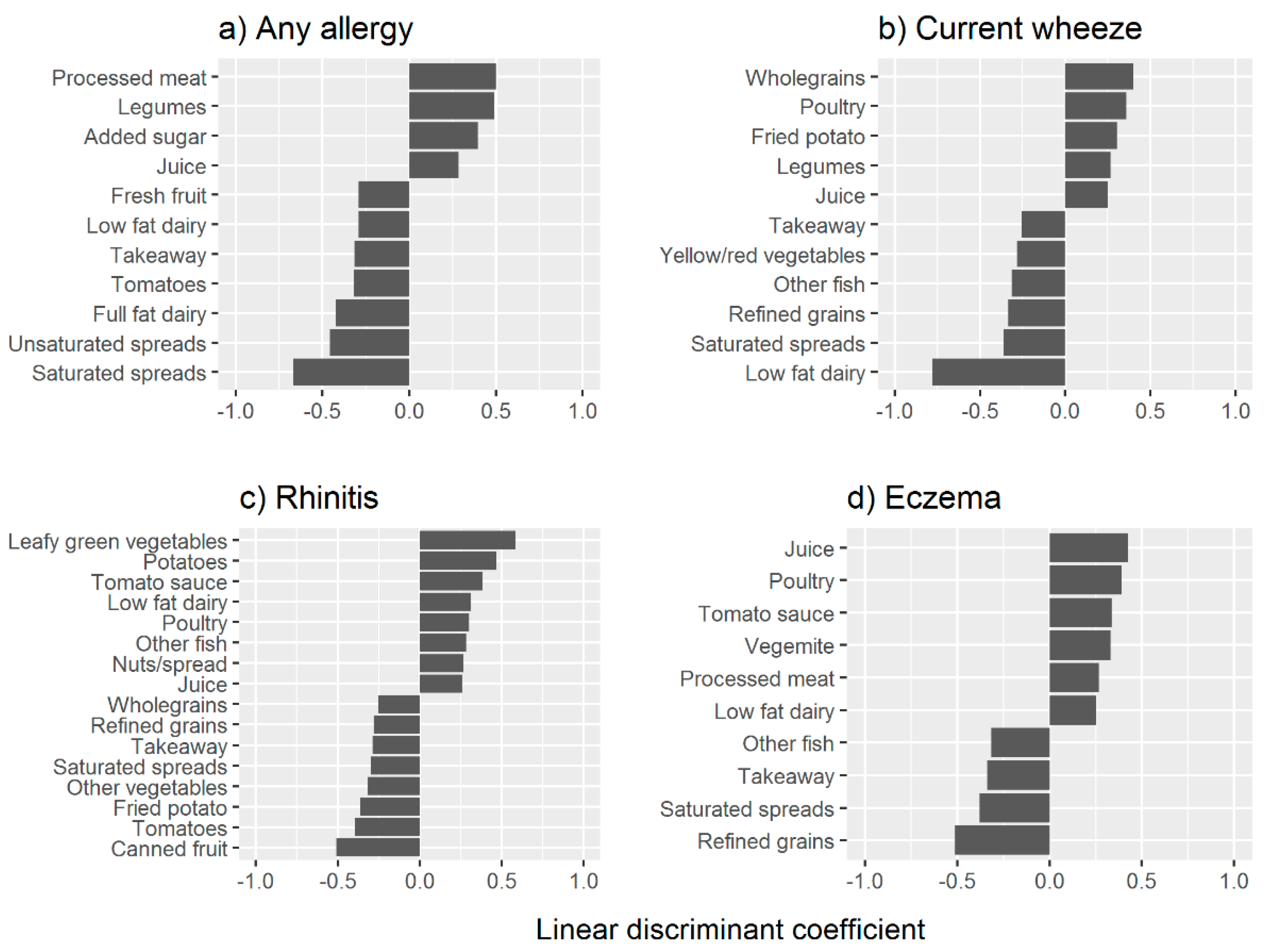
3.1. Food Components and Their Association with Allergy
3.2. Prediction of Food Components and Allergy Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pearce, N.; Ait-Khaled, N.; Beasley, R.; Mallol, J.; Keil, U.; Mitchell, E.; Robertson, C.; ISAAC Phase Three Study Group. Worldwide trends in the prevalence of asthma symptoms: Phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2007, 62, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.K.; Beasley, R.; Crane, J.; Foliaki, S.; Shah, J.; Weiland, S.; International Study of Asthma and Allergies in Childhood Phase Three Study Group. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009, 64, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare (AIHW). Australian Government. Asthma in Australian Children Findings from Growing Up in Australia, the Longitudinal Study of Australian Children. 2009. Available online: https://www.aihw.gov.au/getmedia/4e1c453a-d2dd-41c2-8320-6efc2abfc498/acm-17-10771.pdf.aspx?inline=true (accessed on 14 June 2019).
- Loh, W.; Tang, M.L.K. The Epidemiology of Food Allergy in the Global Context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [PubMed]
- Australian Centre for Asthma Monitoring (ACAM). Asthma in Australia 2011. AIHW Asthma Series No. 4. Cat. No. ACM 22; Australian Institute of Health and Welfare: Canberra, Australia, 2011.
- Almqvist, C.; Pershagen, G.; Wickman, M. Low socioeconomic status as a risk factor for asthma, rhinitis and sensitization at 4 years in a birth cohort. Clin. Exp. Allergy 2005, 35, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Erkkola, M.; Lumia, M.; Kronberg-Kippila, C.; Ahonen, S.; Kaila, M.; Ilonen, J.; Simell, O.; Knip, M.; Veijola, R.; et al. Maternal intake of fatty acids during pregnancy and allergies in the offspring. Br. J. Nutr. 2012, 108, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Torrent, M.; Garcia-Esteban, R.; Ferrer, C.; Ribas-Fito, N.; Anto, J.M.; Sunyer, J. Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin. Exp. Allergy 2007, 37, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.T.; Li, Y.F.; Langholz, B.; Gilliland, F.D. Maternal fish consumption during pregnancy and risk of early childhood asthma. J. Asthma 2005, 42, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Sausenthaler, S.; Koletzko, S.; Schaaf, B.; Lehmann, I.; Borte, M.; Herbarth, O.; von Berg, A.; Wichmann, H.E.; Heinrich, J.; Group, L.S. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am. J. Clin. Nutr. 2007, 85, 530–537. [Google Scholar]
- Venter, C.; Meyer, R.W.; Nwaru, B.I.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; Akdis, C.A.; Bischoff, S.; et al. EAACI position paper: Influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy 2019. [Google Scholar] [CrossRef]
- Viljoen, K.; Segurado, R.; O’Brien, J.; Murrin, C.; Mehegan, J.; Kelleher, C.C.; DMed on behalf of the Lifeways Cross Generation Cohort Study Steering Group. Pregnancy diet and offspring asthma risk over a 10-year period: The Lifeways Cross Generation Cohort Study, Ireland. BMJ Open 2018, 8, e017013. [Google Scholar] [CrossRef]
- Willers, S.M.; Devereux, G.; Craig, L.C.; McNeill, G.; Wijga, A.H.; Abou El-Magd, W.; Turner, S.W.; Helms, P.J.; Seaton, A. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax 2007, 62, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Garcia, R.; Roumeliotaki, T.; Basterrechea, M.; Begiristain, H.; Iniguez, C.; Vioque, J.; Kogevinas, M.; Sunyer, J.; INMA study group; et al. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br. J. Nutr. 2013, 110, 2058–2068. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Maternal consumption of dairy products, calcium, and vitamin D during pregnancy and infantile allergic disorders. Ann. Allergy Asthma Immunol. 2014, 113, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur. Respir. J. 2010, 35, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Larsen, V.; Ierodiakonou, D.; Jarrold, K.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Ruparelia, A.; Devani, P.; Trivella, M.; et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: A systematic review and meta-analysis. PLoS Med. 2018, 15, e1002507. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lei, F.; Zhang, R.; Liu, D.; Qu, P.; Cheng, Y.; Liu, X.; Chen, F.; Dang, S.; Yan, H. Socioeconomic disparity in the diet quality of pregnant women in Northwest China. Asia Pac. J. Clin. Nutr. 2019, 28, 330–340. [Google Scholar] [PubMed]
- Wilson, J.E.; Blizzard, L.; Gall, S.L.; Magnussen, C.G.; Oddy, W.H.; Dwyer, T.; Venn, A.J.; Smith, K.J. An age-and sex-specific dietary guidelines index is a valid measure of diet quality in an Australian cohort during youth and adulthood. Nutr. Res. 2019, 65, 43–53. [Google Scholar] [CrossRef]
- Grieger, J.A.; Wood, L.G.; Clifton, V.L. Improving asthma during pregnancy with dietary antioxidants: The current evidence. Nutrients 2013, 5, 3212–3234. [Google Scholar] [CrossRef]
- Grieger, J.A.; Grzeskowiak, L.E.; Wood, L.G.; Clifton, V.L. Asthma control in pregnancy is associated with pre-conception dietary patterns. Public Health Nutr. 2016, 19, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Tuck, A.; Grzeskowiak, L.; Osei-Kumah, A.; Saif, Z.; Edwards, S.M.; Tai, A.; Prescott, S.L.; Tulic, M.; Saffery, R.; Clifton, V.L. Distinct Sex-Specific Gene Expression Changes in the Placenta in Association with Childhood Allergy. Int. J. Respir. Pulm. Med. 2015, 2. [Google Scholar] [CrossRef]
- Ambrosini, G.L.; Oddy, W.H.; Robinson, M.; O’Sullivan, T.A.; Hands, B.P.; de Klerk, N.H.; Silburn, S.R.; Zubrick, S.R.; Kendall, G.E.; Stanley, F.J.; et al. Adolescent dietary patterns are associated with lifestyle and family psycho-social factors. Public Health Nutr. 2009, 12, 1807–1815. [Google Scholar] [CrossRef]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W.; et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer-Verlag: New York, NY, USA, 2002. [Google Scholar]
- Ronto, R.; Wu, J.H.; Singh, G.M. The global nutrition transition: trends, disease burdens and policy interventions. Public Health Nutr. 2018, 21, 2267–2270. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.; Galletly, C.; Semmler-Booth, T.; Dekker, G. Antenatal psychosocial risk factors and depression among women living in socioeconomically disadvantaged suburbs in Adelaide, South Australia. Aust. N. Z. J. Psychiatry 2008, 42, 45–50. [Google Scholar] [CrossRef]
- Glover, J.; Hetzel, D.; Glover, L.; Tennant, S.; Page, A. A Social Health Atlas of South Australia, 3rd ed.; The University of Adelaide: Adelaide, Australia, March 2006. [Google Scholar]
- Grech, A.; Sui, Z.; Siu, H.Y.; Zheng, M.; Allman-Farinelli, M.; Rangan, A. Socio-Demographic Determinants of Diet Quality in Australian Adults Using the Validated Healthy Eating Index for Australian Adults (HEIFA-2013). Healthcare 2017, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.C.K.; Sichieri, R.; Junior, E.V.; Boccolini, C.S.; de Moura Souza, A.; Cunha, D.B. Trends in obesity prevalence among Brazilian adults from 2002 to 2013 by educational level. BMC Public Health 2019, 19, 965. [Google Scholar] [CrossRef] [PubMed]
- Asghari, G.; Mirmiran, P.; Yuzbashian, E.; Azizi, F. A systematic review of diet quality indices in relation to obesity. Br. J. Nutr. 2017, 117, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.P. Obesity and atopy. Clin. Exp. Allergy 2015, 45, 75–86. [Google Scholar] [CrossRef]
- Amarasekera, M.; Prescott, S.L.; Palmer, D.J. Nutrition in early life, immune-programming and allergies: The role of epigenetics. Asian Pac. J. Allergy Immunol. 2013, 31, 175–182. [Google Scholar]
- Baiz, N.; Just, J.; Chastang, J.; Forhan, A.; de Lauzon-Guillain, B.; Magnier, A.M.; Annesi-Maesano, I.; the EDEN Mother-Child Cohort Study Group. Maternal diet before and during pregnancy and risk of asthma and allergic rhinitis in children. Allergy Asthma Clin. Immunol. 2019, 15, 40. [Google Scholar] [CrossRef]
- Hallit, S.; Raherison, C.; Abou Abdallah, R.; Hallit, R.; Salameh, P. Correlation of types of food and asthma diagnosis in childhood: A case-control study. J. Asthma 2018, 55, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Varela, M.M.; Alvarez, L.G.; Kogan, M.D.; Ferreira, J.C.; Martinez Gimeno, A.; Aguinaga Ontoso, I.; Gonzalez Diaz, C.; Arnedo Pena, A.; Dominguez Aurrecoechea, B.; Busquets Monge, R.M.; et al. Diet and prevalence of atopic eczema in 6 to 7-year-old schoolchildren in Spain: ISAAC phase III. J. Investig. Allergol. Clin. Immunol. 2010, 20, 469–475. [Google Scholar] [PubMed]
- Maslova, E.; Halldorsson, T.I.; Strom, M.; Olsen, S.F. Low-fat yoghurt intake in pregnancy associated with increased child asthma and allergic rhinitis risk: A prospective cohort study. J. Nutr. Sci. 2012, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adolfsson, O.; Meydani, S.N.; Russell, R.M. Yogurt and gut function. Am. J. Clin. Nutr. 2004, 80, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.J. Aspartame as a cause of allergic reactions, including anaphylaxis. Arch. Intern. Med. 1996, 156, 1027–1028. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Seo, J.; Cho, K.H. Aspartame-fed zebrafish exhibit acute deaths with swimming defects and saccharin-fed zebrafish have elevation of cholesteryl ester transfer protein activity in hypercholesterolemia. Food Chem. Toxicol. 2011, 49, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council and Department of Health. Australian Dietary Guidelines; Australian Government: Canberra, Australia, 2013; pp. 1–5. Available online: https://www.eatforhealth.gov.au/guidelines/australian-dietary-guidelines-1-5 (accessed on 3 July 2019).
- Von Ehrenstein, O.S.; Aralis, H.; Flores, M.E.; Ritz, B. Fast food consumption in pregnancy and subsequent asthma symptoms in young children. Pediatr. Allergy Immunol. 2015, 26, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, P.; Asher, M.I.; Garcia-Marcos, L.; Williams, H.; Keil, U.; Robertson, C.; Nagel, G.; Group, I.P.I.S. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) phase three. Thorax 2013, 68, 351–360. [Google Scholar] [CrossRef]
- Fsadni, C.; Fsadni, P.; Montefort, S.; Fava, S. Food consumption and the risk of childhood allergy. Asia Pac. Allergy 2018, 8, e35. [Google Scholar] [CrossRef]
- Lumia, M.; Luukkainen, P.; Tapanainen, H.; Kaila, M.; Erkkola, M.; Uusitalo, L.; Niinisto, S.; Kenward, M.G.; Ilonen, J.; Simell, O.; et al. Dietary fatty acid composition during pregnancy and the risk of asthma in the offspring. Pediatr. Allergy Immunol. 2011, 22, 827–835. [Google Scholar] [CrossRef]
- Simpson, J.L.; Scott, R.; Boyle, M.J.; Gibson, P.G. Inflammatory subtypes in asthma: Assessment and identification using induced sputum. Respirology 2006, 11, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Garg, M.L.; Gibson, P.G. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J. Allergy Clin. Immunol. 2011, 127, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council and Department of Health. Nutrient Reference Values for Australia and New Zealand. Fats: Total Fat and Fatty Acids. 2013. Available online: https://www.nrv.gov.au/nutrients/fats-total-fat-fatty-acids (accessed on 3 July 2019).
- American Heart Association. The American Heart Association Diet and Lifestyle Recommendations. 2019. Available online: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/aha-diet-and-lifestyle-recommendations (accessed on 3 July 2019).
- Hooper, L.; Martin, N.; Abdelhamid, A.; Davey Smith, G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2015, 6, CD011737. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lim, I.Y.; Wu, Y.; Teh, A.L.; Chen, L.; Aris, I.M.; Soh, S.E.; Tint, M.T.; MacIsaac, J.L.; Morin, A.M.; et al. Developmental pathways to adiposity begin before birth and are influenced by genotype, prenatal environment and epigenome. BMC Med. 2017, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.M.; Matthews, S.M.; Hakim, E.A.; Stevens, M.; Arshad, S.H.; Hide, D.W. The prevalence of and risk factors for atopy in early childhood: A whole population birth cohort study. J. Allergy Clin. Immunol. 1998, 101, 587–593. [Google Scholar] [CrossRef]
- Hodge, A.; Patterson, A.J.; Brown, W.J.; Ireland, P.; Giles, G. The Anti-Cancer Council of Victoria FFQ: Relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust. N. Z. J. Public Health 2000, 24, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Hebden, L.; Kostan, E.; O’Leary, F.; Hodge, A.; Allman-Farinelli, M. Validity and reproducibility of a food frequency questionnaire as a measure of recent dietary intake in young adults. PLoS ONE 2013, 8, e75156. [Google Scholar] [CrossRef]
- Xinying, P.X.; Noakes, M.; Keogh, J. Can a food frequency questionnaire be used to capture dietary intake data in a 4 week clinical intervention trial? Asia Pac. J. Clin. Nutr. 2004, 13, 318–323. [Google Scholar]
- Van Zyl, Z.; Maslin, K.; Dean, T.; Blaauw, R.; Venter, C. The accuracy of dietary recall of infant feeding and food allergen data. J. Hum. Nutr. Diet. 2016, 29, 777–785. [Google Scholar] [CrossRef]
| Maternal | Total (n = 234) |
|---|---|
| Age (years), mean (SD) | 26.9 (5.6) |
| Weight (kg), median (IQR) | 73 (61–85) |
| Body mass index (kg/m2), median (IQR) | 27.4 (23.2–32.0) |
| Body mass index category, n (%) | |
| <25 kg/m2 | 94 (40.3%) |
| 25–29 kg/m2 | 57 (24.5%) |
| ≥30 kg/m2 | 82 (35.2%) |
| Socioeconomic status, n (%) | |
| 5 (highest) | 11 (4.7%) |
| 4 | 18 (7.7%) |
| 3 | 8 (3.4%) |
| 2 | 66 (28.2%) |
| 1 (lowest) | 131 (56.0%) |
| Ethnicity, n (%) | |
| Caucasian | 217 (92.7%) |
| Non-Caucasian | 17 (7.3%) |
| Smoking status, n (%) | |
| Non-smoker/former smoker | 175 (74.8%) |
| Quit during pregnancy | 24 (10.3%) |
| Current smoker | 35 (15.0%) |
| Asthma status, n (%) | |
| Non-asthmatic | 108 (46.2%) |
| Asthmatic | 126 (53.8%) |
| Asthma exacerbations, n (%) | |
| Non-asthmatic | 108 (46.2%) |
| Controlled | 61 (26.1%) |
| Mild exacerbation | 33 (14.1%) |
| Severe exacerbation | 32 (13.7%) |
| Gravida, n (%) | |
| 0–1 | 66 (28.4%) |
| ≥2 | 166 (71.6%) |
| Parity, n (%) | |
| 0 | 92 (39.8%) |
| ≥1 | 139 (60.2%) |
| Neonatal | |
| Birthweight (g), mean (SD) | 3405 (564) |
| Length (cm), mean (SD) 1 | 49.8 (3.0) |
| Head Circumference (cm), median (IQR) 1 | 35 (34–36) |
| Sex, n (%) | |
| Male | 117 (50%) |
| Female | 117 (50%) |
| Gestation, weeks, median (IQR) | 39 (38–40) |
| Allergy status at 3 years, n (%) | |
| Any allergy/No allergy | 188 (80%)/46 (20%) |
| Eczema/No eczema | 105 (45%)/129 (55%) |
| Current wheeze/No current wheeze | 131 (56%)/103 (44%) |
| Rhinitis/No rhinitis | 93 (40%)/141 (60%) |
| Predicted Allergy Status | Actual Allergy Status | Sensitivity | Specificity | |
|---|---|---|---|---|
| Absent | Present | |||
| Any allergy | ||||
| Absent | 11 | 4 | 97.9% | 23.9% |
| Present | 35 | 184 | ||
| Eczema | ||||
| Absent | 99 | 53 | 49.5% | 76.7% |
| Present | 30 | 52 | ||
| Current wheeze | ||||
| Absent | 55 | 32 | 75.6% | 53.4% |
| Present | 48 | 99 | ||
| Rhinitis | ||||
| Absent | 121 | 63 | 32.3% | 85.8% |
| Present | 20 | 30 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grieger, J.A.; Pelecanos, A.M.; Hurst, C.; Tai, A.; Clifton, V.L. Pre-Conception Maternal Food Intake and the Association with Childhood Allergies. Nutrients 2019, 11, 1851. https://doi.org/10.3390/nu11081851
Grieger JA, Pelecanos AM, Hurst C, Tai A, Clifton VL. Pre-Conception Maternal Food Intake and the Association with Childhood Allergies. Nutrients. 2019; 11(8):1851. https://doi.org/10.3390/nu11081851
Chicago/Turabian StyleGrieger, Jessica A., Anita M. Pelecanos, Cameron Hurst, Andrew Tai, and Vicki L. Clifton. 2019. "Pre-Conception Maternal Food Intake and the Association with Childhood Allergies" Nutrients 11, no. 8: 1851. https://doi.org/10.3390/nu11081851
APA StyleGrieger, J. A., Pelecanos, A. M., Hurst, C., Tai, A., & Clifton, V. L. (2019). Pre-Conception Maternal Food Intake and the Association with Childhood Allergies. Nutrients, 11(8), 1851. https://doi.org/10.3390/nu11081851





